Iron Oxide Colloidal Nanoclusters as Theranostic Vehicles and Their Interactions at the Cellular Level
Abstract
1. Introduction
2. Materials and Methods
2.1. Biodistribution Experiments
2.1.1. Radiolabeling
2.1.2. Stability Study
2.1.3. Animal Studies
- (a)
- Biodistribution
- (b)
- Scintigraphic imaging
2.2. Materials and Synthesis of Nanoclusters
2.3. In Vitro Experiments
2.3.1. Animal Models
2.3.2. Cell Cultures
- (a)
- Spleen cells
- (b)
- Macrophage and lymphocyte isolation
- (c)
- Internalization of nanoclusters
- (d)
- Cytokine production
- (e)
- Cell proliferation assay
2.4. Characterization Methods
2.4.1. TEM Imaging
- (a)
- TEM imaging of the inorganic nanomaterials
- (b)
- TEM imaging of the biological samples
- (c)
- SEM imaging and EDS analysis
2.4.2. Z-Potential and Dynamic Light Scattering (DLS) Analysis
2.4.3. Magnetic Measurements
2.4.4. Relaxivity Measurements and T1 & T2 Weighted Phantoms
3. Results
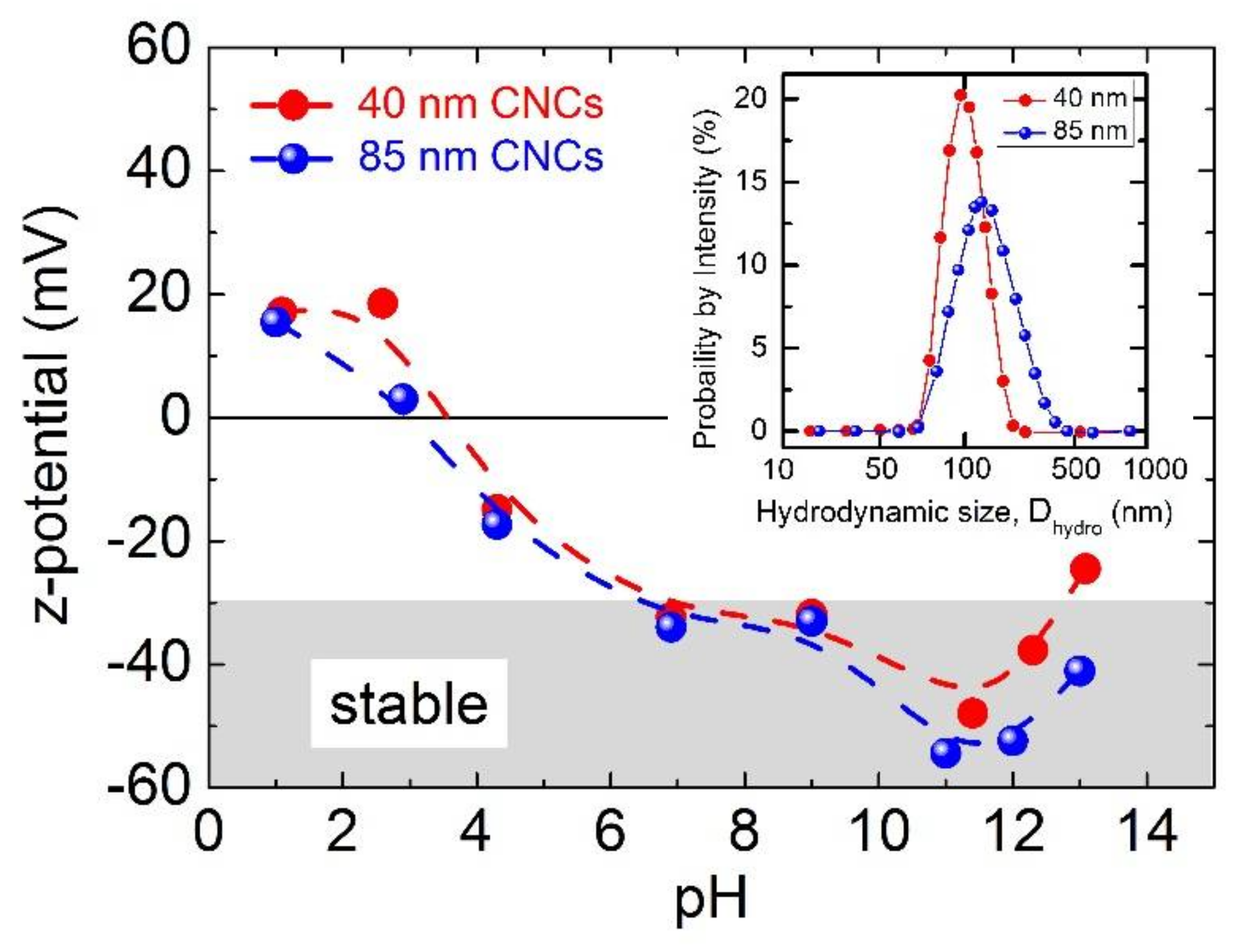
3.1. Functional Colloidal Maghemite Nanoclusters and their MRI Detection Capability
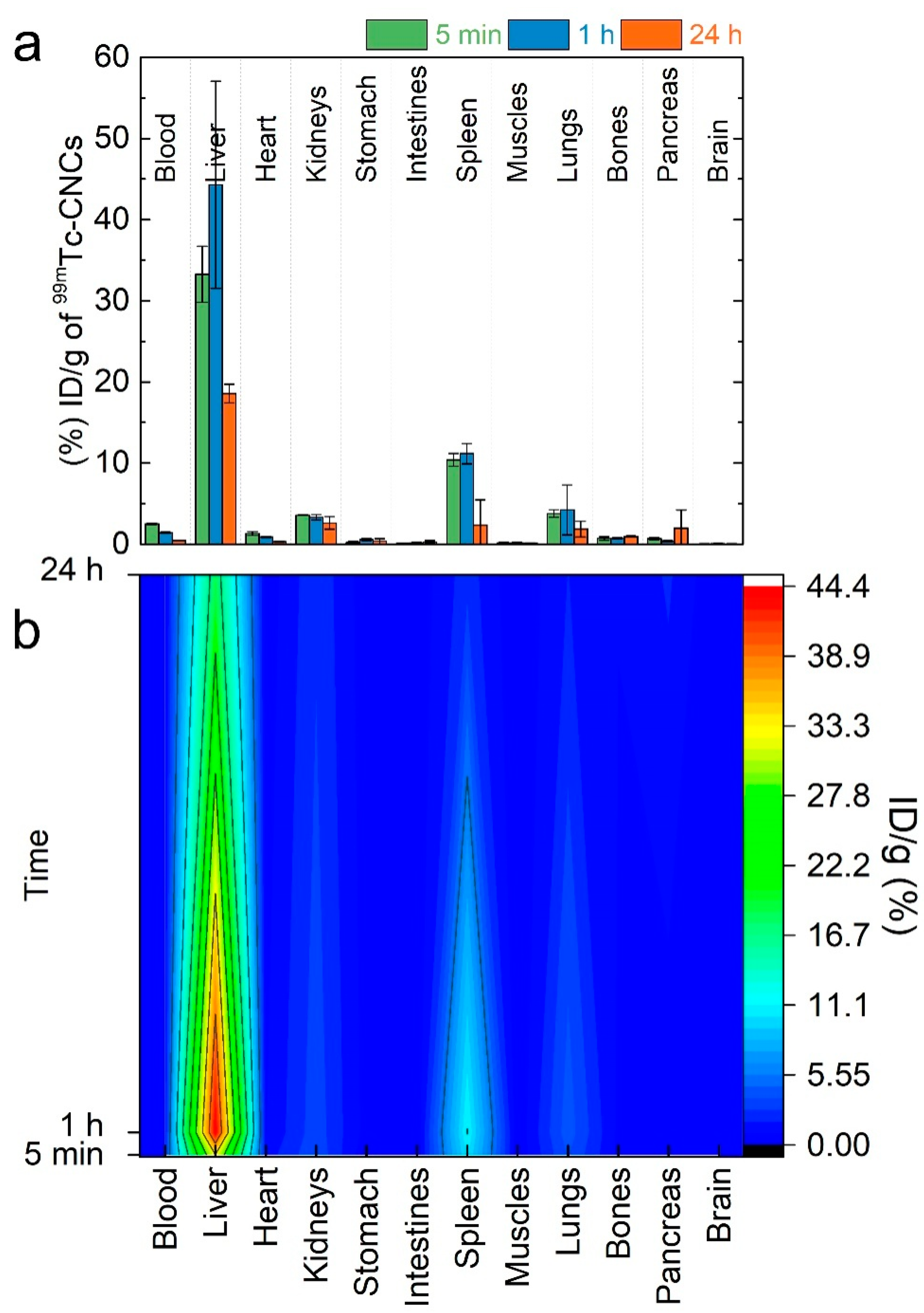
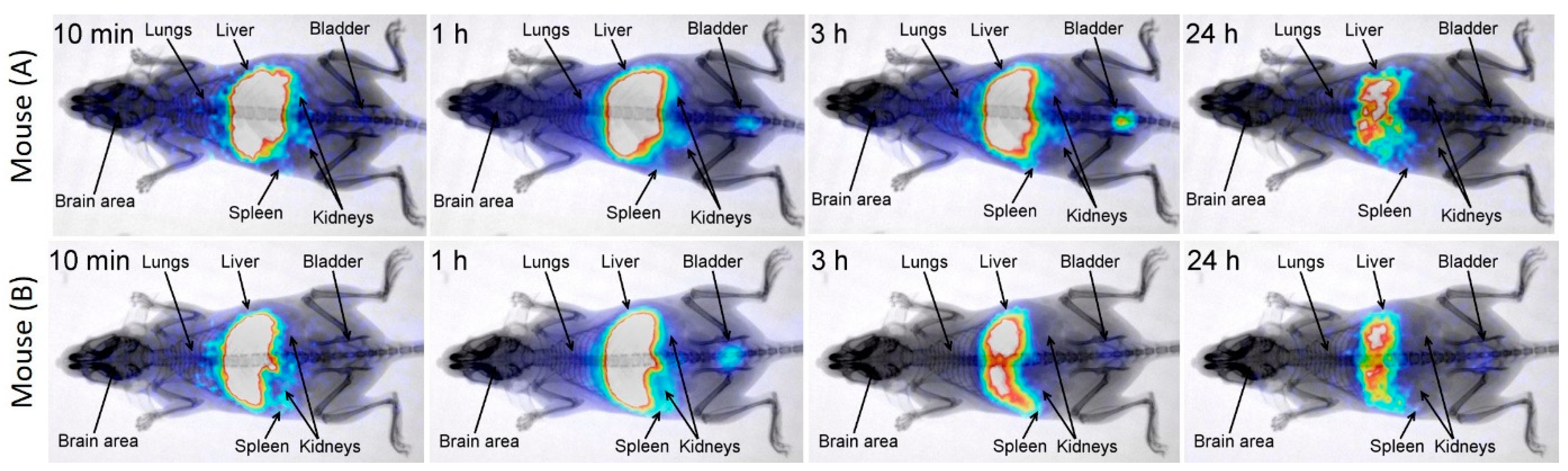
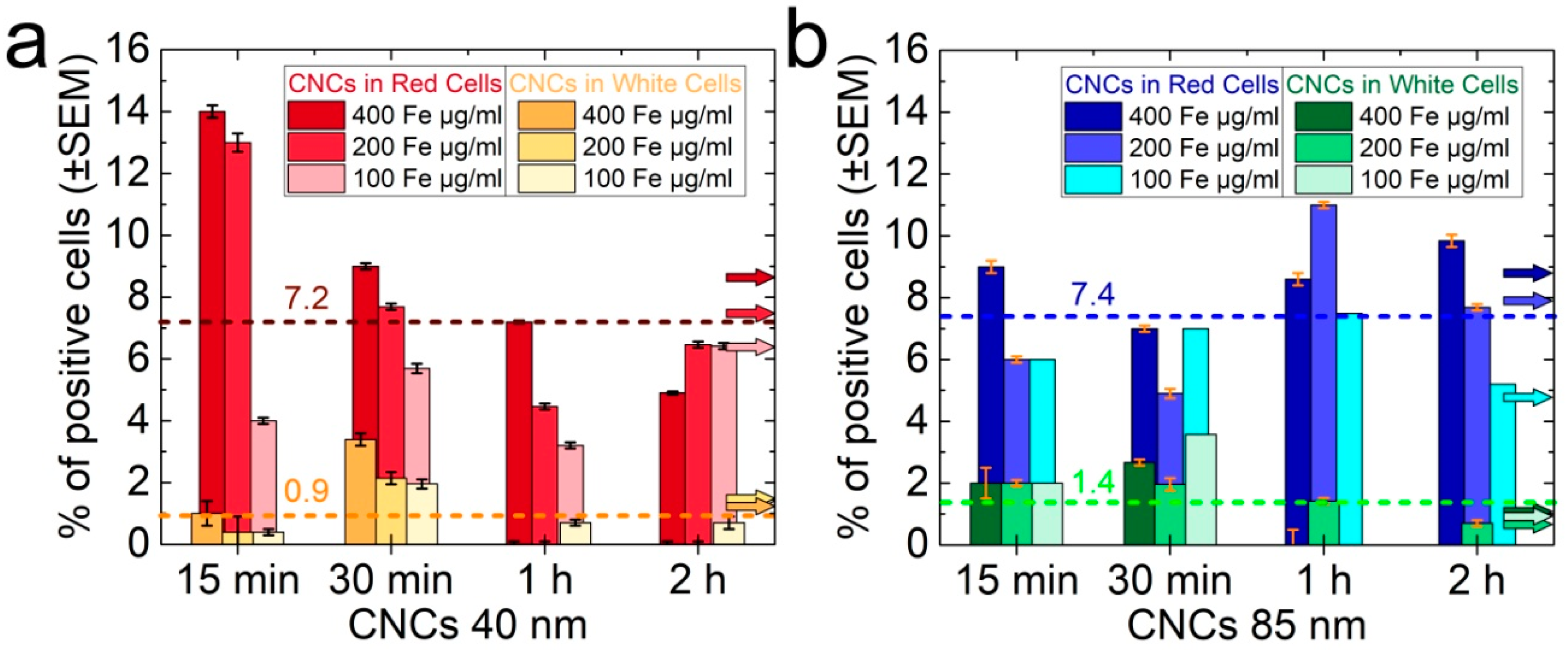
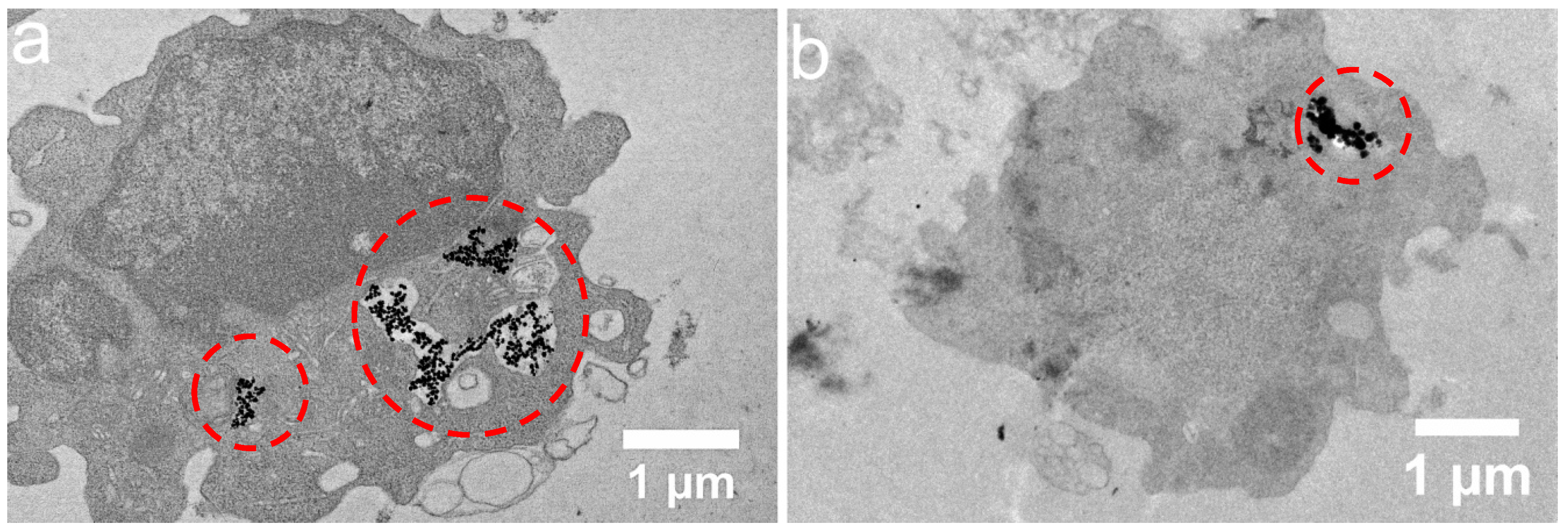
3.2. Time-Dependent Nanocluster Biodistribution and Their Excretion through Urine
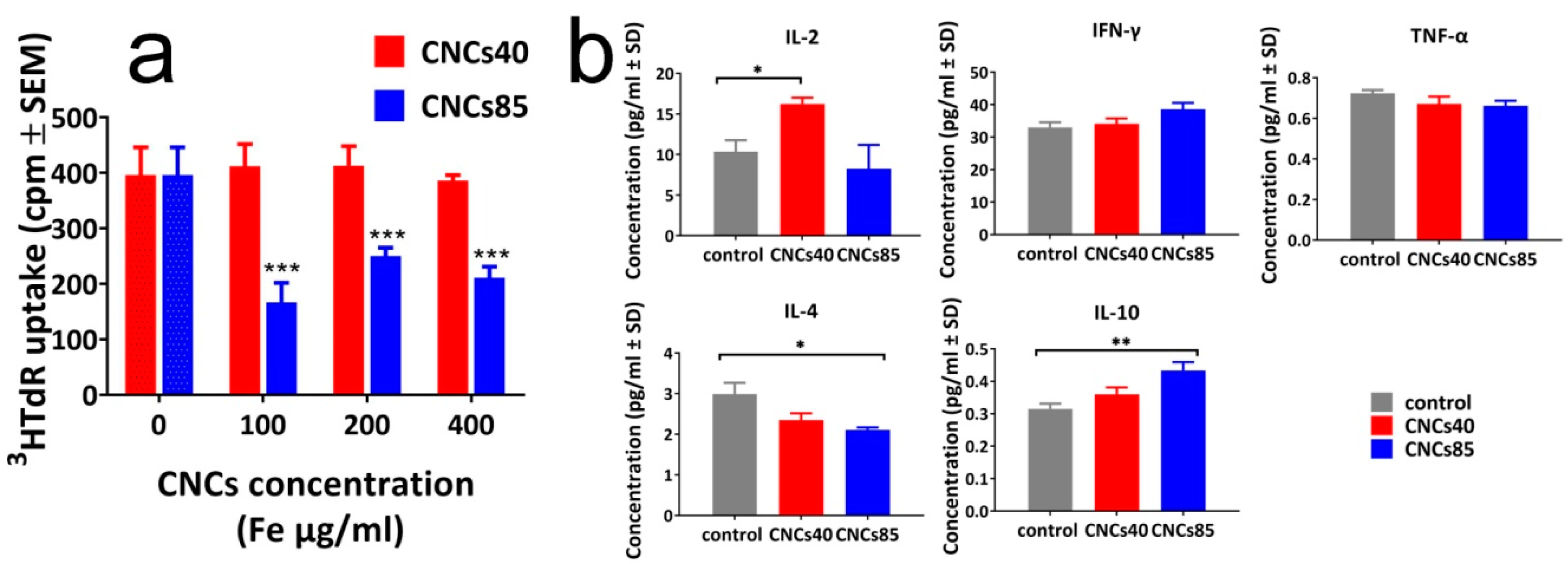
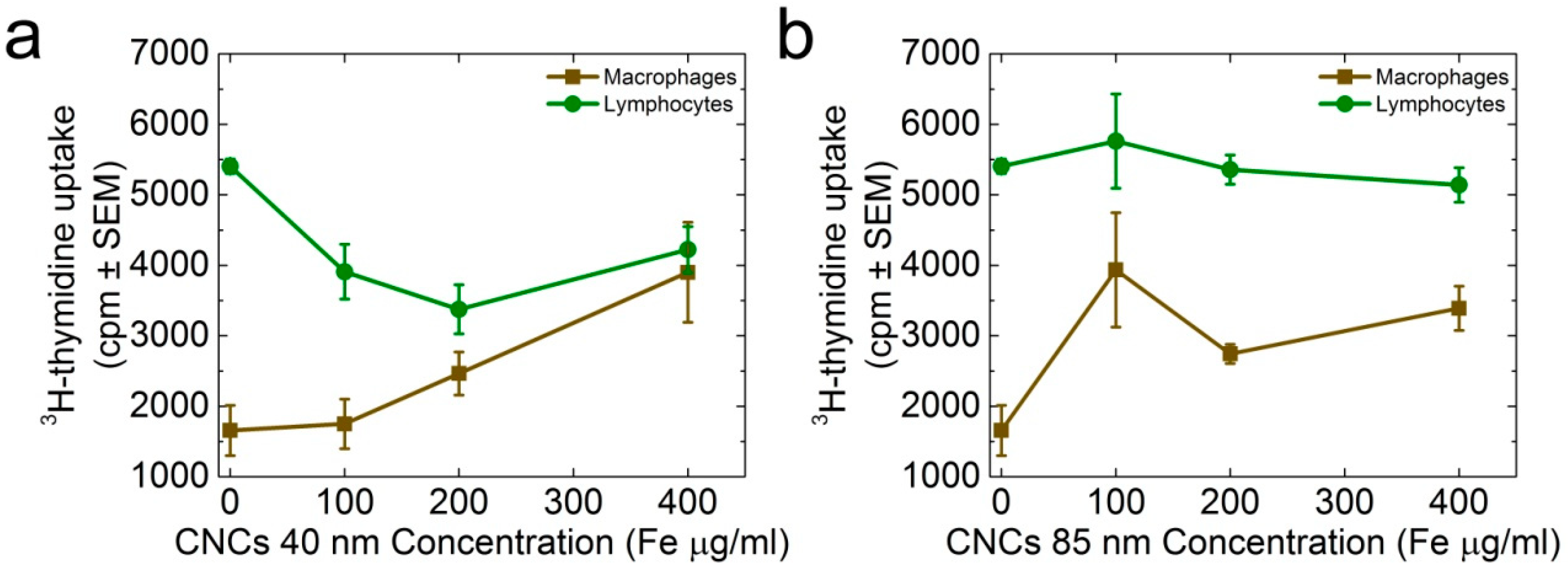
3.3. Size-Dependent Effect of the CNCs on Spleen Cell Proliferation and Cytokine Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzan, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef]
- Kostopoulou, A.; Lappas, A. Colloidal magnetic nanocrystal clusters: Variable length-scale interaction mechanisms, synergetic functionalities and technological advantages. Nanotechnol. Rev. 2015, 4, 595–624. [Google Scholar] [CrossRef]
- Rosa, L.; Blackledge, J.; Boretti, A. Nano-Magnetic Resonance Imaging (Nano-MRI) Gives Personalized Medicine a New Perspective. Biomedicines 2017, 5, 7. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Wilhelm, C.; Devaud, M.; Levitz, P.; Gazeau, F. How cellular processing of superparamagnetic nanoparticles affects their magnetic behavior and NMR relaxivity. Contrast Media Mol. Imaging 2012, 7, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Sheen, M.R.; Lizotte, P.H.; Toraya-Brown, S.; Fiering, S. Stimulating antitumor immunity with nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Corti, M.; Lascialfari, A.; Marinone, M.; Masotti, A.; Micotti, E.; Orsini, F.; Ortaggi, G.; Poletti, G.; Innocenti, C.; Sangregorio, C. Magnetic and relaxometric properties of polyethylenimine-coated superparamagnetic MRI contrast agents. J. Magn. Magn. Mater. 2008, 320, E316–E319. [Google Scholar] [CrossRef]
- Boni, A.; Marinone, M.; Innocenti, C.; Sangregorio, C.; Corti, M.; Lascialfari, A.; Mariani, M.; Orsini, F.; Poletti, G.; Casula, M.F. Magnetic and relaxometric properties of Mn ferrites. J. Phys. D Appl. Phys. 2008, 41, 13. [Google Scholar] [CrossRef]
- Corti, M.; Lascialfari, A.; Micotti, E.; Castellano, A.; Donativi, M.; Quarta, A.; Cozzoli, P.D.; Manna, L.; Pellegrino, T.; Sangregorio, C. Magnetic properties of novel superparamagnetic MRI contrast agents based on colloidal nanocrystals. J. Magn. Magn. Mater. 2008, 320, E320–E323. [Google Scholar] [CrossRef]
- Lartigue, L.; Innocenti, C.; Kalaivani, T.; Awwad, A.; Duque, M.D.S.; Guari, Y.; Larionova, J.; Guerin, C.; Montero, J.L.G.; Barragan-Montero, V.; et al. Water-Dispersible Sugar-Coated Iron Oxide Nanoparticles. An Evaluation of their Relaxometric and Magnetic Hyperthermia Properties. J. Am. Chem. Soc. 2011, 133, 10459–10472. [Google Scholar] [CrossRef] [PubMed]
- De, M.; Chou, S.S.; Joshi, H.M.; Dravid, V.P. Hybrid magnetic nanostructures (MNS) for magnetic resonance imaging applications. Adv. Drug Deliv. Rev. 2011, 63, 1282–1299. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.D.; Henriques, F.; Ferreira, L.P.; Godinho, M.; Cruz, M.M. Iron oxide nanoparticles: The Influence of synthesis method and size on composition and magnetic properties. J. Solid State Chem. 2013, 201, 144–152. [Google Scholar] [CrossRef]
- Chen, R.; Christiansen, M.G.; Sourakov, A.; Mohr, A.; Matsumoto, Y.; Okada, S.; Jasanoff, A.; Anikeeva, P. High-Performance Ferrite Nanoparticles through Nonaqueous Redox Phase Tuning. Nano Lett. 2016, 16, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.W.; Jacobs, P. Physical and Chemical-Properties of Superparamagnetic Iron-Oxide Mr Contrast Agents—Ferumoxides, Ferumoxtran, Ferumoxsil. Magn. Reson. Imaging 1995, 13, 661–674. [Google Scholar] [CrossRef]
- Jun, Y.W.; Huh, Y.M.; Choi, J.S.; Lee, J.H.; Song, H.T.; Kim, S.; Yoon, S.; Kim, K.S.; Shin, J.S.; Suh, J.S.; et al. Nanoscale size effect of magnetic nanocrystals and their utilization for cancer diagnosis via magnetic resonance imaging. J. Am. Chem. Soc. 2005, 127, 5732–5733. [Google Scholar] [CrossRef] [PubMed]
- Cheong, S.; Ferguson, P.; Feindel, K.W.; Hermans, I.F.; Callaghan, P.T.; Meyer, C.; Slocombe, A.; Su, C.H.; Cheng, F.Y.; Yeh, C.S.; et al. Simple Synthesis and Functionalization of Iron Nanoparticles for Magnetic Resonance Imaging. Angew. Chem. Int. Ed. 2011, 50, 4206–4209. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Choi, Y.; Lee, Y.; Park, M.; Moon, W.K.; Choi, S.H.; Hyeon, T. Water-Dispersible Ferrimagnetic Iron Oxide Nanocubes with Extremely High r(2) Relaxivity for Highly Sensitive in Vivo MRI of Tumors. Nano Lett. 2012, 12, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Billotey, C.; Garofalo, A.; Ulhaq-Bouillet, C.; Lefevre, C.; Taleb, J.; Laurent, S.; Vander Elst, L.; Muller, R.N.; Lartigue, L.; et al. Mastering the Shape and Composition of Dendronized Iron Oxide Nanoparticles To Tailor Magnetic Resonance Imaging and Hyperthermia. Chem. Mater. 2014, 26, 5252–5264. [Google Scholar] [CrossRef]
- Tong, S.; Hou, S.J.; Zheng, Z.L.; Zhou, J.; Bao, G. Coating Optimization of Superparamagnetic Iron Oxide Nanoparticles for High T-2 Relaxivity. Nano Lett. 2010, 10, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-Soluble Iron Oxide Nanocubes with High Values of Specific Absorption Rate for Cancer Cell Hyperthermia Treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Myrovali, E.; Maniotis, N.; Makridis, A.; Terzopoulou, A.; Ntomprougkidis, V.; Simeonidis, K.; Sakellari, D.; Kalogirou, O.; Samaras, T.; Salikhov, R.; et al. Arrangement at the nanoscale: Effect on magnetic particle hyperthermia. Sci. Rep. 2016, 6, 37934. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Quarta, A.; Espinosa, A.; Figuerola, A.; Wilhelm, C.; Garcia-Hernandez, M.; Genovese, A.; Falqui, A.; Alloyeau, D.; Buonsanti, R.; et al. Correlating Magneto-Structural Properties to Hyperthermia Performance of Highly Monodisperse Iron Oxide Nanoparticles Prepared by a Seeded-Growth Route. Chem. Mater. 2011, 23, 4170–4180. [Google Scholar] [CrossRef]
- Mertz, D.; Sandre, O.; Begin-Colin, S. Drug releasing nanoplatforms activated by alternating magnetic fields. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1617–1641. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Yang, Q.; Dong, Z.M.; Zhang, J.S.; Zhang, W.J.; Wang, Q.D.; Tan, S.N.; Smyth, H.D.C. Magnetically triggered drug release from nanoparticles and its applications in anti-tumor treatment. Drug Deliv. 2017, 24, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Ono, K.; Suzuki, H.; Sawada, M.; Moriya, M.; Sakamoto, W.; Yogo, T. High-Frequency, Magnetic-Field-Responsive Drug Release from Magnetic Nanoparticle/Organic Hybrid Based on Hyperthermic Effect. ACS Appl. Mater. Interfaces 2010, 2, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Ngobili, T.A.; Daniele, M.A. Nanoparticles and direct immunosuppression. Exp. Biol. Med. 2016, 241, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Incoronato, M.; Salvatore, M.; Soricelli, A. Nanoparticle-based strategies for cancer immunotherapy and immunodiagnostics. Nanomedicine 2017, 12, 2349–2365. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.; Sun, X.L.; Sun, S.H. Monodisperse Magnetic Nanoparticles for Theranostic Applications. Acc. Chem. Res. 2011, 44, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.H.; Shin, T.H.; Cheon, J. Theranostic Magnetic Nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Vamvakidis, K.; Mourdikoudis, S.; Makridis, A.; Paulidou, E.; Angelakeris, M.; Dendrinou-Samara, C. Magnetic hyperthermia efficiency and MRI contrast sensitivity of colloidal soft/hard ferrite nanoclusters. J. Colloid Interface Sci. 2018, 511, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Barick, K.C.; Aslam, M.; Lin, Y.P.; Bahadur, D.; Prasad, P.V.; Dravid, V.P. Novel and efficient MR active aqueous colloidal Fe3O4 nanoassemblies. J. Mater. Chem. 2009, 19, 7023–7029. [Google Scholar] [CrossRef]
- Yoon, T.J.; Lee, H.; Shao, H.L.; Hilderbrand, S.A.; Weissleder, R. Multicore Assemblies Potentiate Magnetic Properties of Biomagnetic Nanoparticles. Adv. Mater. 2011, 23, 4793–4797. [Google Scholar] [CrossRef] [PubMed]
- Mikhaylov, G.; Mikac, U.; Magaeva, A.A.; Itin, V.I.; Naiden, E.P.; Psakhye, I.; Babes, L.; Reinheckel, T.; Peters, C.; Zeiser, R.; et al. Ferri-liposomes as an MRI-visible drug-delivery system for targeting tumours and their microenvironment. Nat. Nanotechnol. 2011, 6, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Poselt, E.; Kloust, H.; Tromsdorf, U.; Janschel, M.; Hahn, C.; Masslo, C.; Weller, H. Relaxivity Optimization of a PEGylated Iron-Oxide-Based Negative Magnetic Resonance Contrast Agent for T-2-Weighted Spin-Echo Imaging. ACS Nano 2012, 6, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Hemery, G.; Keyes, A.C.; Garaio, E.; Rodrigo, I.; Garcia, J.A.; La, F.P.; Garanger, E.; Sandre, O. Tuning Sizes, Morphologies, and Magnetic Properties of Monocore Versus Multicore Iron Oxide Nanoparticles through the Controlled Addition of Water in the Polyol Synthesis. Inorg. Chem. 2017, 56, 8232–8243. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.H.; Wang, F.; Wang, Y.X.J.; Yu, J.C.; Leung, K.C.F. Facile synthesis of size-controllable monodispersed ferrite nanospheres. J. Mater. Chem. 2010, 20, 5086–5094. [Google Scholar] [CrossRef]
- Lu, Z.D.; Yin, Y.D. Colloidal nanoparticle clusters: Functional materials by design. Chem. Soc. Rev. 2012, 41, 6874–6887. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.J.; Cheng, C.M.; Chen, D.X.; Gu, H.C. Magnetite Nanocrystal Clusters with Ultra-High Sensitivity in Magnetic Resonance Imaging. Chemphyschem 2012, 13, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Hugounenq, P.; Alloyeau, D.; Clarke, S.P.; Levy, M.; Bacri, J.C.; Bazzi, R.; Brougham, D.F.; Wilhelm, C.; Gazeau, F. Cooperative Organization in Iron Oxide Multi-Core Nanoparticles Potentiates Their Efficiency as Heating Mediators and MRI Contrast Agents. ACS Nano 2012, 6, 10935–10949. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Tsiaoussis, I.; Lappas, A. Magnetic iron oxide nanoclusters with tunable optical response. Photonics Nanostruct. Fundam. Appl. 2011, 9, 201–206. [Google Scholar] [CrossRef]
- Fragouli, D.; Torre, B.; Villafiorita-Monteleone, F.; Kostopoulou, A.; Nanni, G.; Falqui, A.; Casu, A.; Lappas, A.; Cingolani, R.; Athanassiou, A. Nanocomposite Pattern-Mediated Magnetic Interactions for Localized Deposition of Nanomaterials. ACS Appl. Mater. Interfaces 2013, 5, 7253–7257. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Brintakis, K.; Vasilakaki, M.; Trohidou, K.N.; Douvalis, A.P.; Lascialfari, A.; Manna, L.; Lappas, A. Assembly-mediated interplay of dipolar interactions and surface spin disorder in colloidal maghemite nanoclusters. Nanoscale 2014, 6, 3764–3776. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Velu, S.K.P.; Thangavel, K.; Orsini, F.; Brintakis, K.; Psycharakis, S.; Ranella, A.; Bordonali, L.; Lappas, A.; Lascialfari, A. Colloidal assemblies of oriented maghemite nanocrystals and their NMR relaxometric properties. Dalton Trans. 2014, 43, 8395–8404. [Google Scholar] [CrossRef] [PubMed]
- Sakellari, D.; Brintakis, K.; Kostopoulou, A.; Myrovali, E.; Simeonidis, K.; Lappas, A.; Angelakeris, M. Ferrimagnetic nanocrystal assemblies as versatile magnetic particle hyperthermia mediators. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.J. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J. Gastroenterol. 2015, 21, 13400–13402. [Google Scholar] [CrossRef] [PubMed]
- Colloidal Superparamagnetic Ferumoxide Associated with Dextran for Intravenous (i.v.) Administration, also Known as Feridex®. It Was Manufactured by Advanced Magnetics, Inc., Cambridge, MA 02138 for Bayer HealthCare Pharmaceuticals Inc., but Its Production Was Discontinued by Late 2000’s due to Lack of Clinical Utility. Available online: http://mriquestions.com/index.html (accessed on 3 May 2018).
- Psimadas, D.; Bouziotis, P.; Georgoulias, P.; Valotassiou, V.; Tsotakos, T.; Loudos, G. Radiolabeling approaches of nanoparticles with Tc-99m. Contrast Media Mol. Imaging 2013, 8, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, M.; Fysikopoulos, E.; Mikropoulos, K.; Fragogeorgi, E.; Loudos, G. Characterization of “gamma-Eye”: A Low-Cost Benchtop Mouse-Sized Gamma Camera for Dynamic and Static Imaging Studies. Mol. Imaging Biol. 2017, 19, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Rouchota, M.; Georgiou, M.; Fysikopoulos, E.; Fragogeorgi, E.; Mikropoulos, K.; Papadimitroulas, P.; Kagadis, G.C.; Loudos, G. A prototype PET/SPET/X-rays scanner dedicated for whole body small animal studies. Hell. J. Nucl. Med. 2017, 20, 146–153. [Google Scholar] [PubMed]
- Potter, M. The Balb/C Mouse—Genetics and Immunology—Preface. Curr. Top. Microbiol. Immunol. 1985, 122, R5–R7. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Andujar, C.; Walter, A.; Cotin, G.; Bordeianu, C.; Mertz, D.; Felder-Flesch, D.; Begin-Colin, S. Design of iron oxide-based nanoparticles for MRI and magnetic hyperthermia. Nanomedicine 2016, 11, 1889–1910. [Google Scholar] [CrossRef] [PubMed]
- Vuong, Q.L.; Berret, J.F.; Fresnais, J.; Gossuin, Y.; Sandre, O. A Universal Scaling Law to Predict the Efficiency of Magnetic Nanoparticles as MRI T2-Contrast Agents. Adv. Healthc. Mater. 2012, 1, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Casula, M.F.; Floris, P.; Innocenti, C.; Lascialfari, A.; Marinone, M.; Corti, M.; Sperling, R.A.; Parak, W.J.; Sangregorio, C. Magnetic Resonance Imaging Contrast Agents Based on Iron Oxide Superparamagnetic Ferrofluids. Chem. Mater. 2010, 22, 1739–1748. [Google Scholar] [CrossRef]
- Terreno, E.; Delli Castelli, D.; Viale, A.; Aime, S. Challenges for Molecular Magnetic Resonance Imaging. Chem. Rev. 2010, 110, 3019–3042. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Cunningham, C.H.; Noguchi, K.I.; Chen, I.Y.; Weissman, I.L.; Yeung, A.C.; Robbins, R.C.; Yang, P.C. In Vivo Serial Evaluation of Superparamagnetic Iron-Oxide Labeled Stem Cells by Off-Resonance Positive Contrast. Magn. Reson. Med. 2008, 60, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.X.; Xu, F.J.; Gu, H.C. Experimental study on transverse relaxation rate of protons in water suspensions of magnetite nanoclusters: Dependence of cluster sizes, volume fraction, inter-echo time, and waiting time. J. Magn. Magn. Mater. 2012, 324, 2809–2820. [Google Scholar] [CrossRef]
- Walter, A.; Garofalo, A.; Bonazza, P.; Meyer, F.; Martinez, H.; Fleutot, S.; Billotey, C.; Taleb, J.; Felder-Flesch, D.; Begin-Colin, S. Effect of the Functionalization Process on the Colloidal, Magnetic Resonance Imaging, and Bioelimination Properties of Mono- or Bisphosphonate-Anchored Dendronized Iron Oxide Nanoparticles. Chempluschem 2017, 82, 647–659. [Google Scholar] [CrossRef]
- Williams, J.G. Pertechnetate and the Stomach—Continuing Controversy. J. Nucl. Med. 1983, 24, 633–636. [Google Scholar] [PubMed]
- Hirota, K.; Terada, H. Endocytosis of Particle Formulations by Macrophages and Its Application to Clinical Treatment. In Endocytosis of Particle Formulations by Macrophages and Its Application to Clinical Treatment, Molecular Regulation of Endocytosis; Ceresa, B., Ed.; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Choi, H.S.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Ipe, B.I.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.X.; Zheng, J. Clearance Pathways and Tumor Targeting of Imaging Nanoparticles. ACS Nano 2015, 9, 6655–6674. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, J.V.; Lobovkina, T.; Zare, R.N.; Gambhir, S.S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 2011, 6, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Mandarano, G.L.J.; Lodhia, J.; Eu, P.; Ferris, N.J.; Davidson, R.; Cowell, S.F. Development and use of iron oxide nanoparticles (Part 2): The application of iron oxide contrast agents in MRI. Biomed. Imaging Interv. J. 2010, 6, e13. [Google Scholar] [CrossRef]
- Scharlach, C.; Warmuth, C.; Schellenberger, E. Determination of blood circulation times of superparamagnetic iron oxide nanoparticles by T2*relaxometry using ultrashort echo time (UTE) MRI. Magn. Reson. Imaging 2015, 33, 1173–1177. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.H.; Couvreur, P. Nanotechnology for therapy and imaging of liver diseases. J. Hepatol. 2011, 55, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Nagorniewicz, B.; Storm, G.; Prakash, J. Relaxin-coated superparamagnetic iron-oxide nanoparticles as a novel theranostic approach for the diagnosis and treatment of liver fibrosis. J. Hepatol. 2017, 66, S43. [Google Scholar] [CrossRef]
- Surendran, S.P.; Thomas, R.G.; Moon, M.J.; Jeong, Y.Y. Nanoparticles for the treatment of liver fibrosis. Int. J. Nanomed. 2017, 12, 6997–7006. [Google Scholar] [CrossRef] [PubMed]
- Bartneck, M.; Warzecha, K.T.; Tacke, F. Therapeutic targeting of liver inflammation and fibrosis by nanomedicine. Hepatobiliary Surg. Nutr. 2014, 3, 364–376. [Google Scholar] [PubMed]
- Song, X.L.; Luo, X.D.; Zhang, Q.Q.; Zhu, A.P.; Ji, L.J.; Yan, C.F. Preparation and characterization of biofunctionalized chitosan/Fe3O4 magnetic nanoparticles for application in liver magnetic resonance imaging. J. Magn. Magn. Mater. 2015, 388, 116–122. [Google Scholar] [CrossRef]
- Maeng, J.H.; Lee, D.H.; Jung, K.H.; Bae, Y.H.; Park, I.S.; Jeong, S.; Jeon, Y.S.; Shim, C.K.; Kim, W.; Kim, J.; et al. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials 2010, 31, 4995–5006. [Google Scholar] [CrossRef] [PubMed]
- Saraswathy, A.; Nazeer, S.S.; Jeevan, M.; Nimi, N.; Arumugam, S.; Harikrishnan, V.S.; Varma, P.R.H.; Jayasree, R.S. Citrate coated iron oxide nanoparticles with enhanced relaxivity for in vivo magnetic resonance imaging of liver fibrosis. Colloids Surf. B Biointerfaces 2014, 117, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.M.; Fu, C.P.; Li, N.N.; Wang, L.; Xu, X.D.; Yang, D.Y.; Fang, J.Z.; Jiang, X.Q.; Zhang, L.M. Glycosaminoglycan-targeted iron oxide nanoparticles for magnetic resonance imaging of liver carcinoma. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: Considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Hudoklin, S.; Zupancic, D.; Makovec, D.; Kreft, M.E.; Romih, R. Gold nanoparticles as physiological markers of urine internalization into urothelial cells in vivo. Int. J. Nanomed. 2013, 8, 3945–3953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naz, F.; Koul, V.; Srivastava, A.; Gupta, Y.K.; Dinda, A.K. Biokinetics of ultrafine gold nanoparticles (AuNPs) relating to redistribution and urinary excretion: A long-term in vivo study. J. Drug Target. 2016, 24, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Bruns, O.T.; Kaul, M.G.; Hansen, E.C.; Barch, M.; Wisniowska, A.; Chen, O.; Chen, Y.; Li, N.; Okada, S.; et al. Exceedingly small iron oxide nanoparticles as positive MRI contrast agents. Proc. Natl. Acad. Sci. USA 2017, 114, 2325–2330. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Freitas, M.; Porto, G.; Lopez-Quintela, M.A.; Rivas, J.; Freitas, P.; Carvalho, F.; Fernandes, E. Polyacrylic acid-coated and non-coated iron oxide nanoparticles induce cytokine activation in human blood cells through TAK1, p38 MAPK and JNK pro-inflammatory pathways. Arch. Toxicol. 2015, 89, 1759–1769. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostopoulou, A.; Brintakis, K.; Fragogeorgi, E.; Anthousi, A.; Manna, L.; Begin-Colin, S.; Billotey, C.; Ranella, A.; Loudos, G.; Athanassakis, I.; et al. Iron Oxide Colloidal Nanoclusters as Theranostic Vehicles and Their Interactions at the Cellular Level. Nanomaterials 2018, 8, 315. https://doi.org/10.3390/nano8050315
Kostopoulou A, Brintakis K, Fragogeorgi E, Anthousi A, Manna L, Begin-Colin S, Billotey C, Ranella A, Loudos G, Athanassakis I, et al. Iron Oxide Colloidal Nanoclusters as Theranostic Vehicles and Their Interactions at the Cellular Level. Nanomaterials. 2018; 8(5):315. https://doi.org/10.3390/nano8050315
Chicago/Turabian StyleKostopoulou, Athanasia, Konstantinos Brintakis, Eirini Fragogeorgi, Amalia Anthousi, Liberato Manna, Sylvie Begin-Colin, Claire Billotey, Anthi Ranella, George Loudos, Irene Athanassakis, and et al. 2018. "Iron Oxide Colloidal Nanoclusters as Theranostic Vehicles and Their Interactions at the Cellular Level" Nanomaterials 8, no. 5: 315. https://doi.org/10.3390/nano8050315
APA StyleKostopoulou, A., Brintakis, K., Fragogeorgi, E., Anthousi, A., Manna, L., Begin-Colin, S., Billotey, C., Ranella, A., Loudos, G., Athanassakis, I., & Lappas, A. (2018). Iron Oxide Colloidal Nanoclusters as Theranostic Vehicles and Their Interactions at the Cellular Level. Nanomaterials, 8(5), 315. https://doi.org/10.3390/nano8050315