Novel Gold Dendritic Nanoforests Combined with Titanium Nitride for Visible-Light-Enhanced Chemical Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Photodegradation
3. Results and Discussion
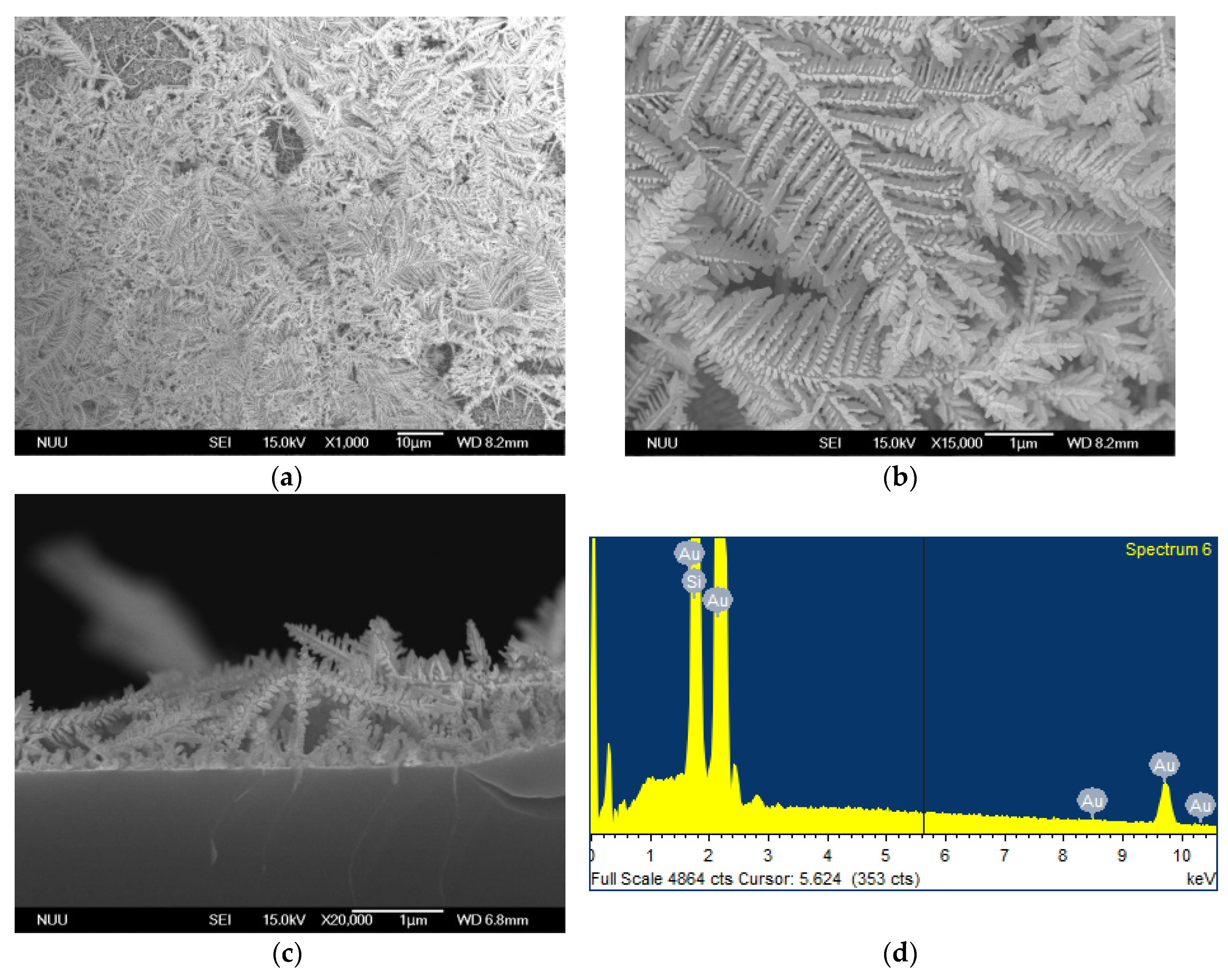
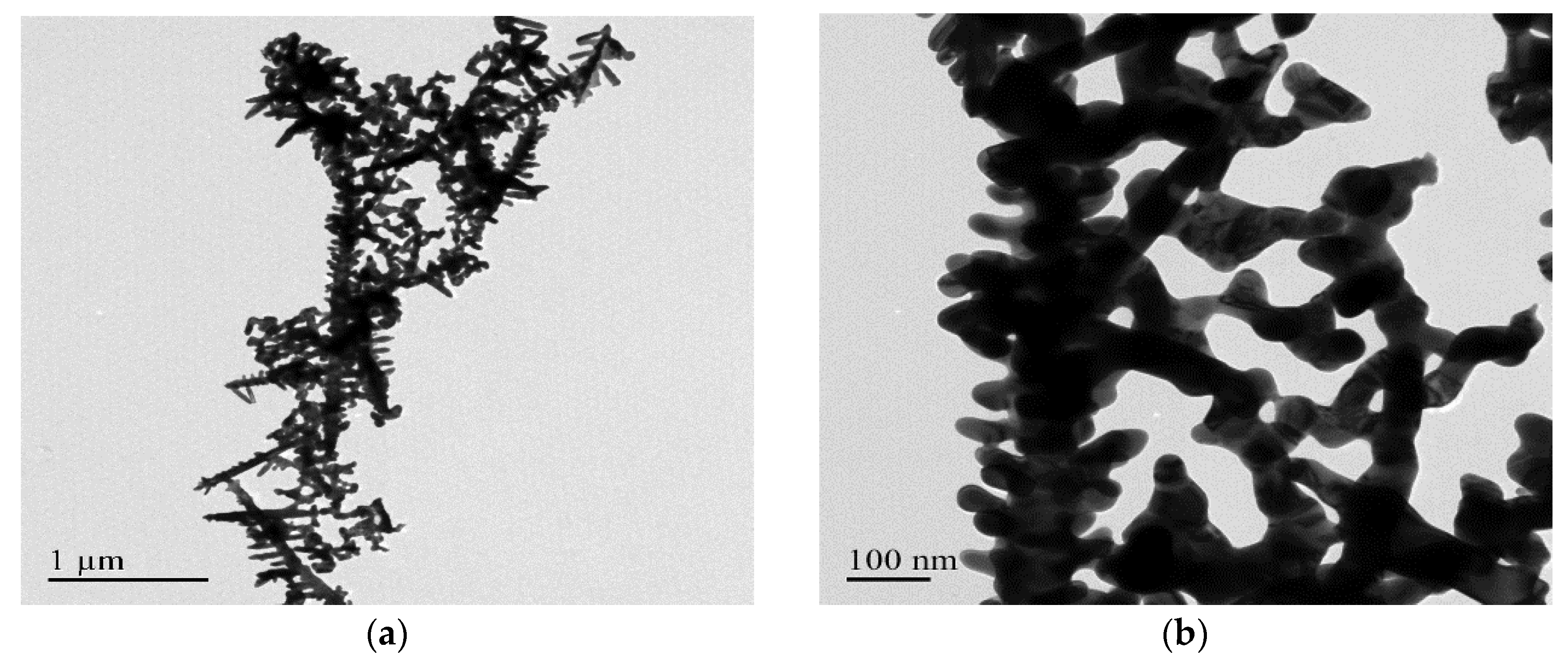
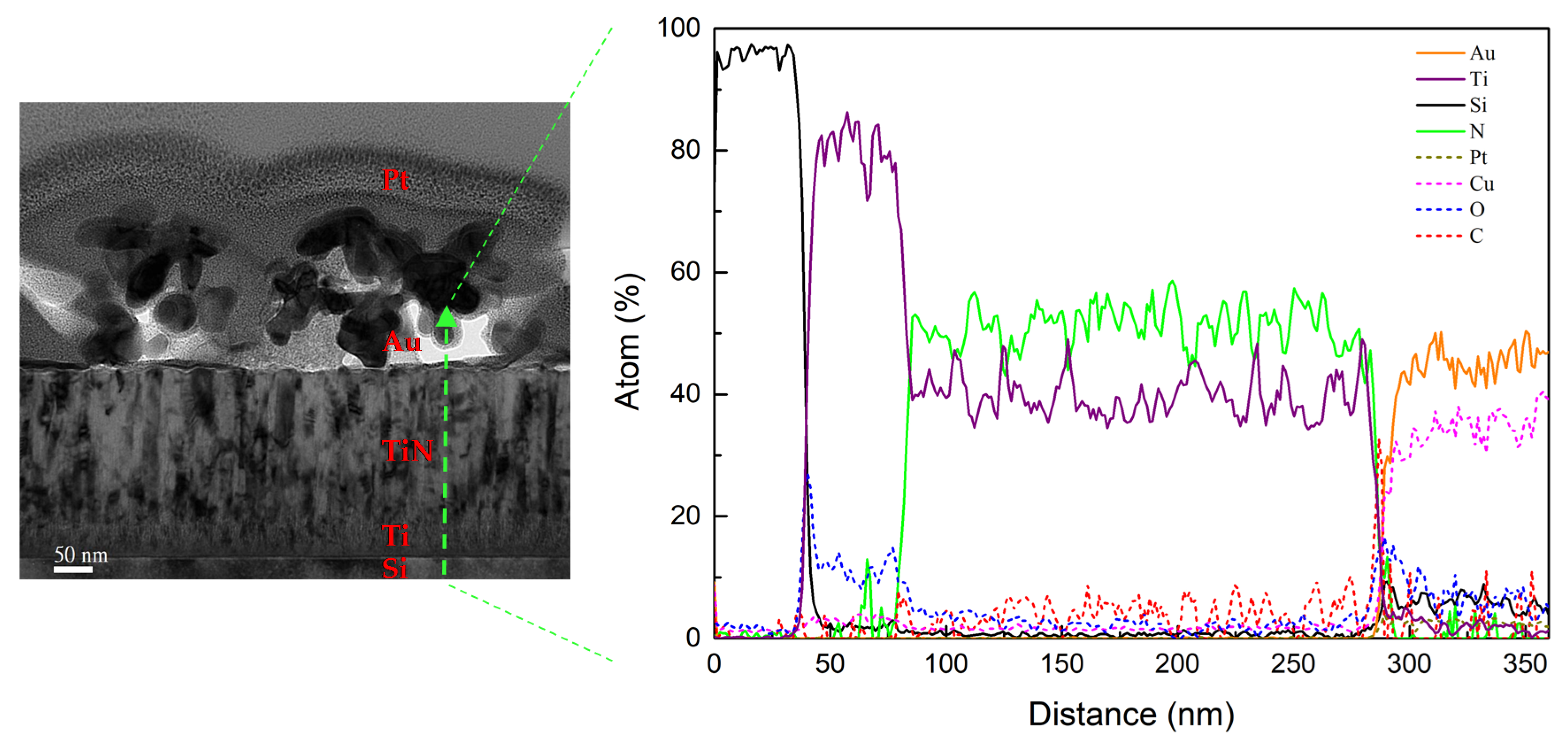
3.1. Morphology and Elemental Analysis
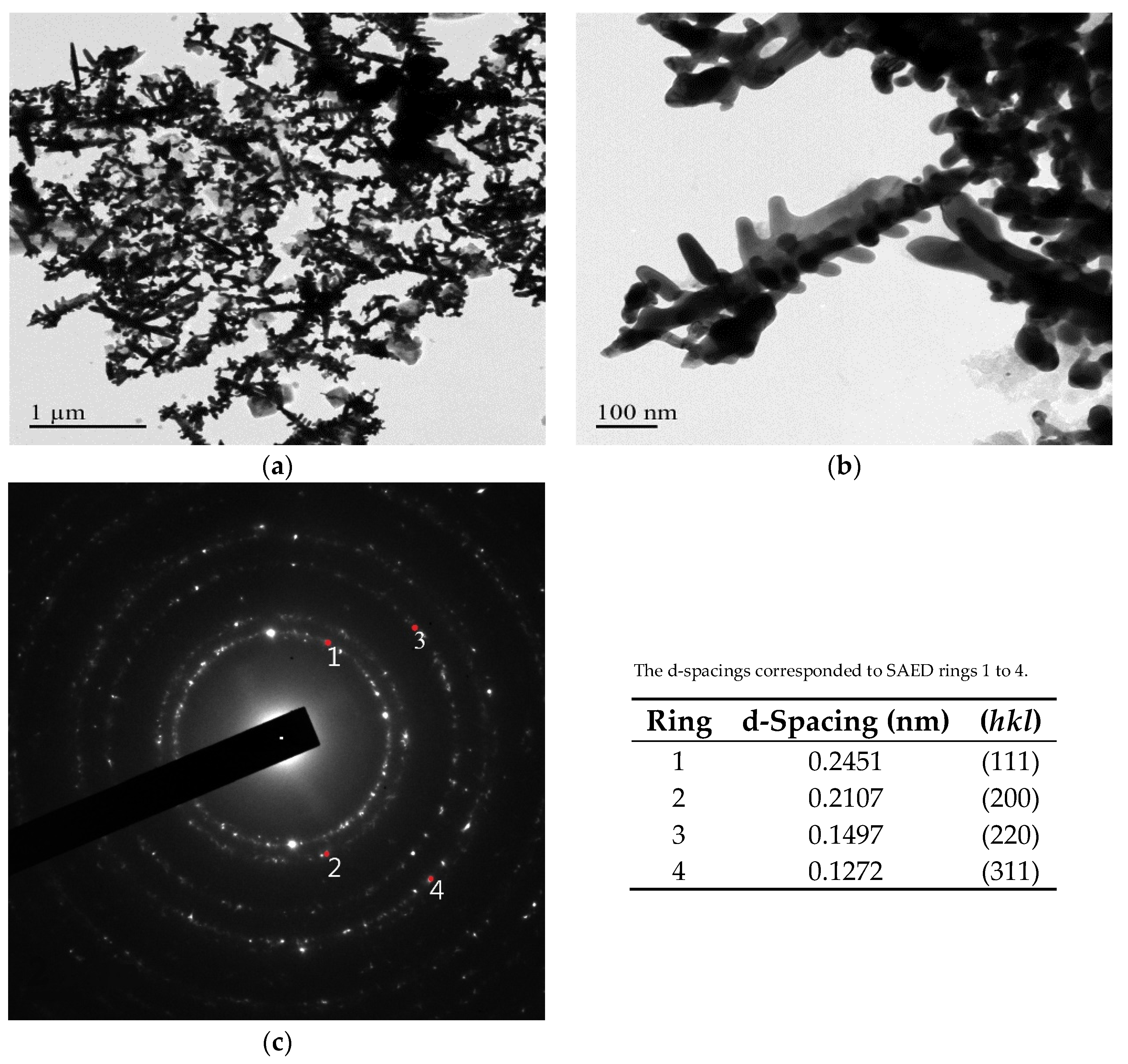
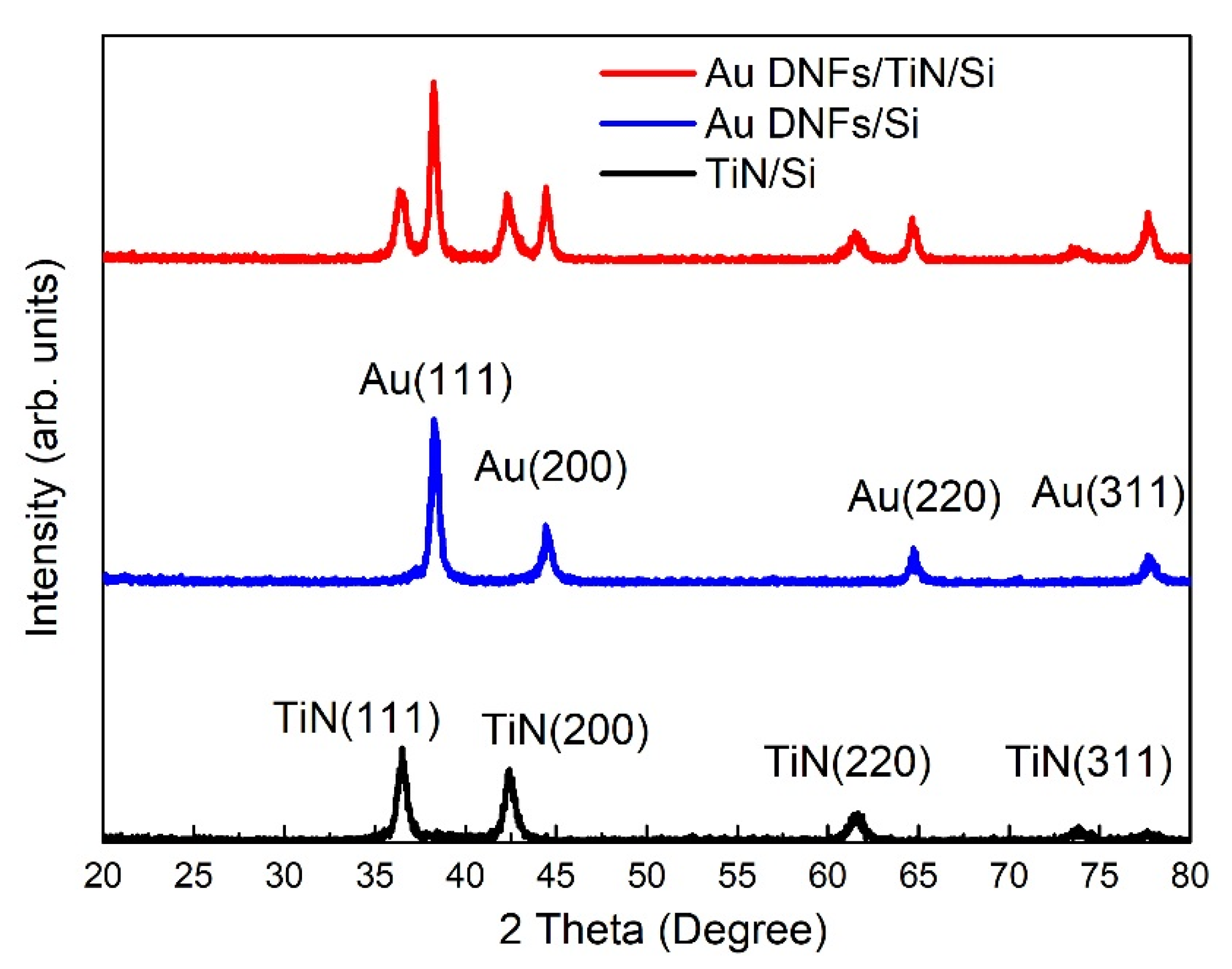
3.2. Crystalline
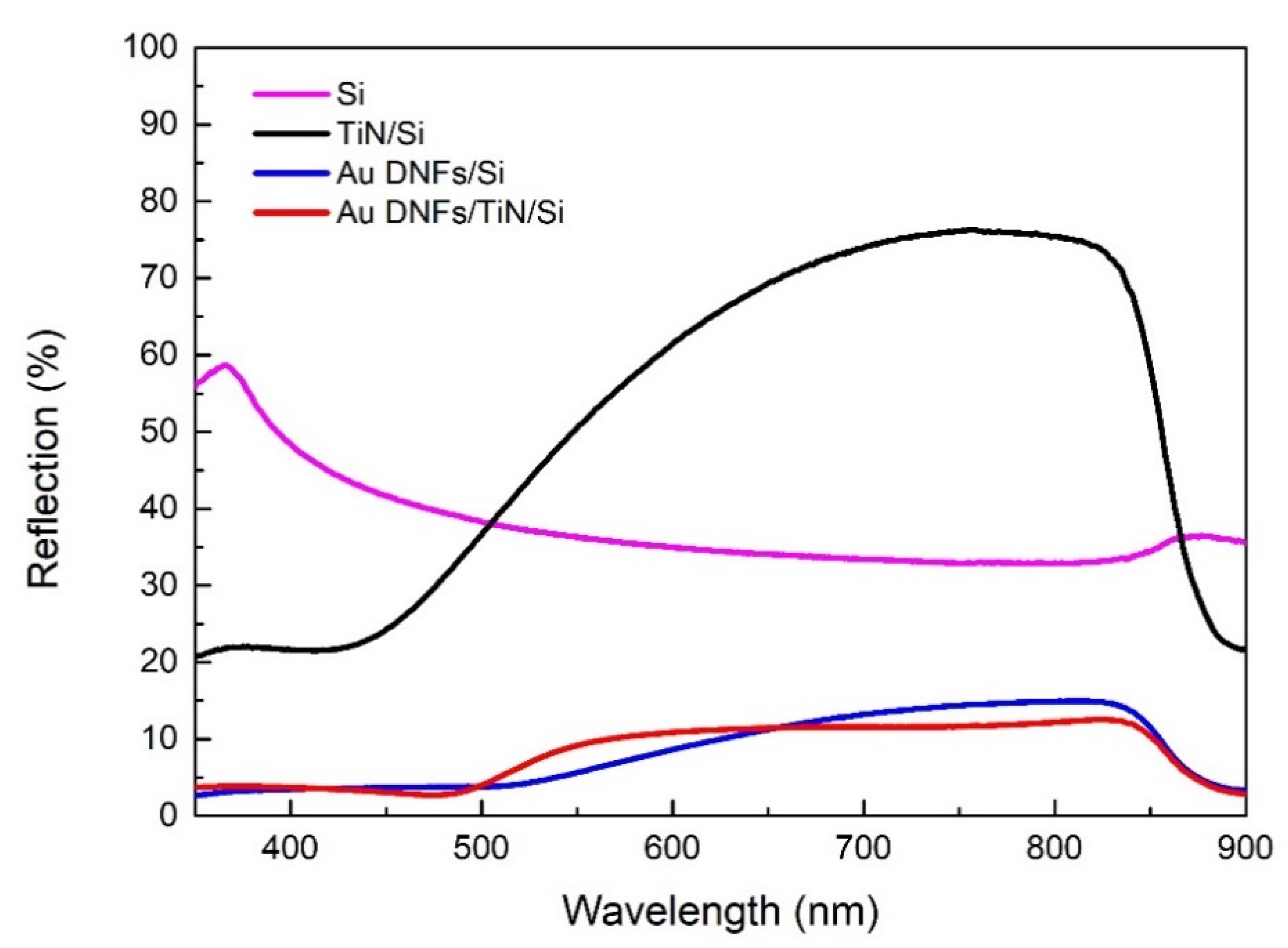
3.3. Reflectance
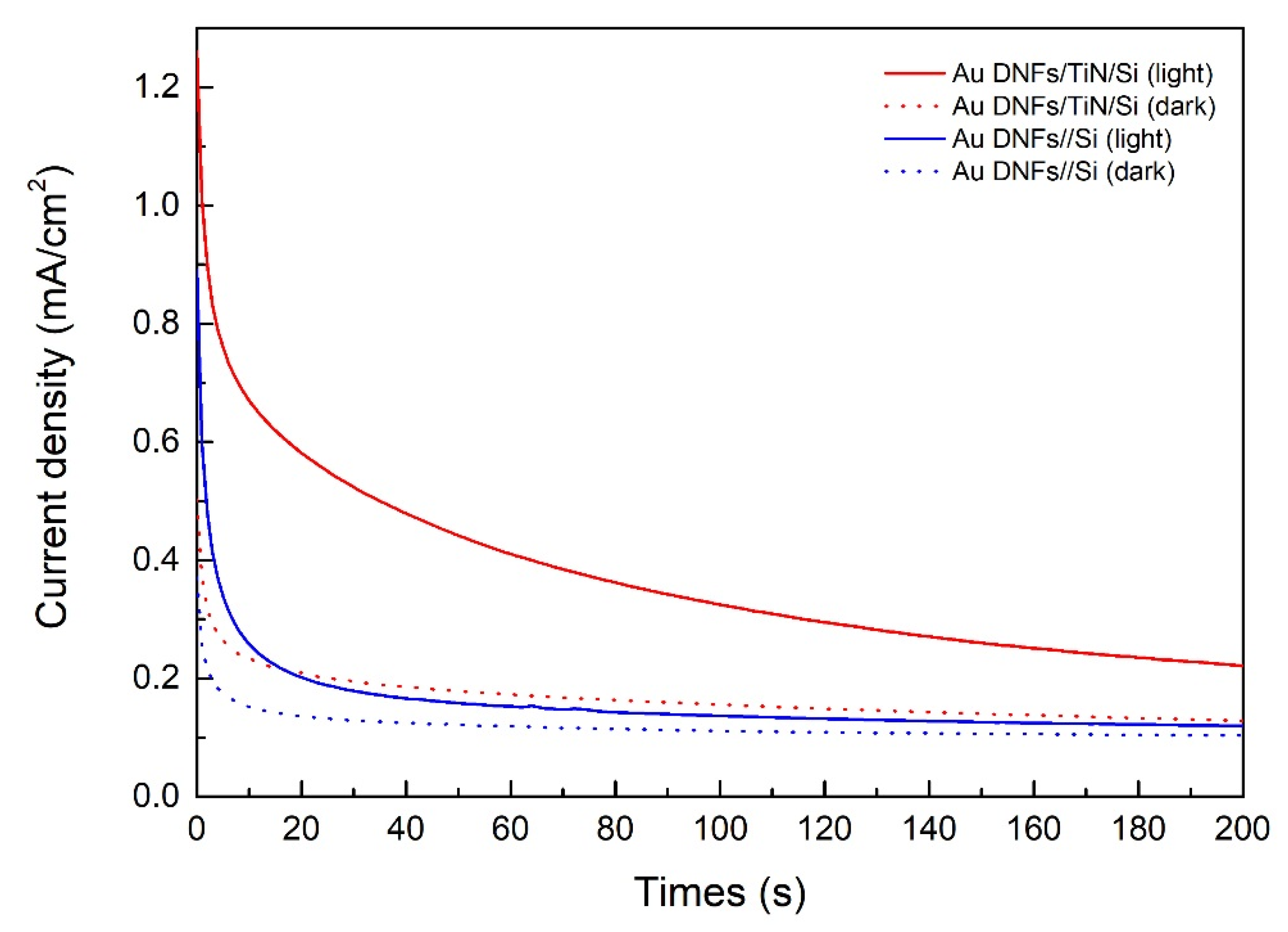
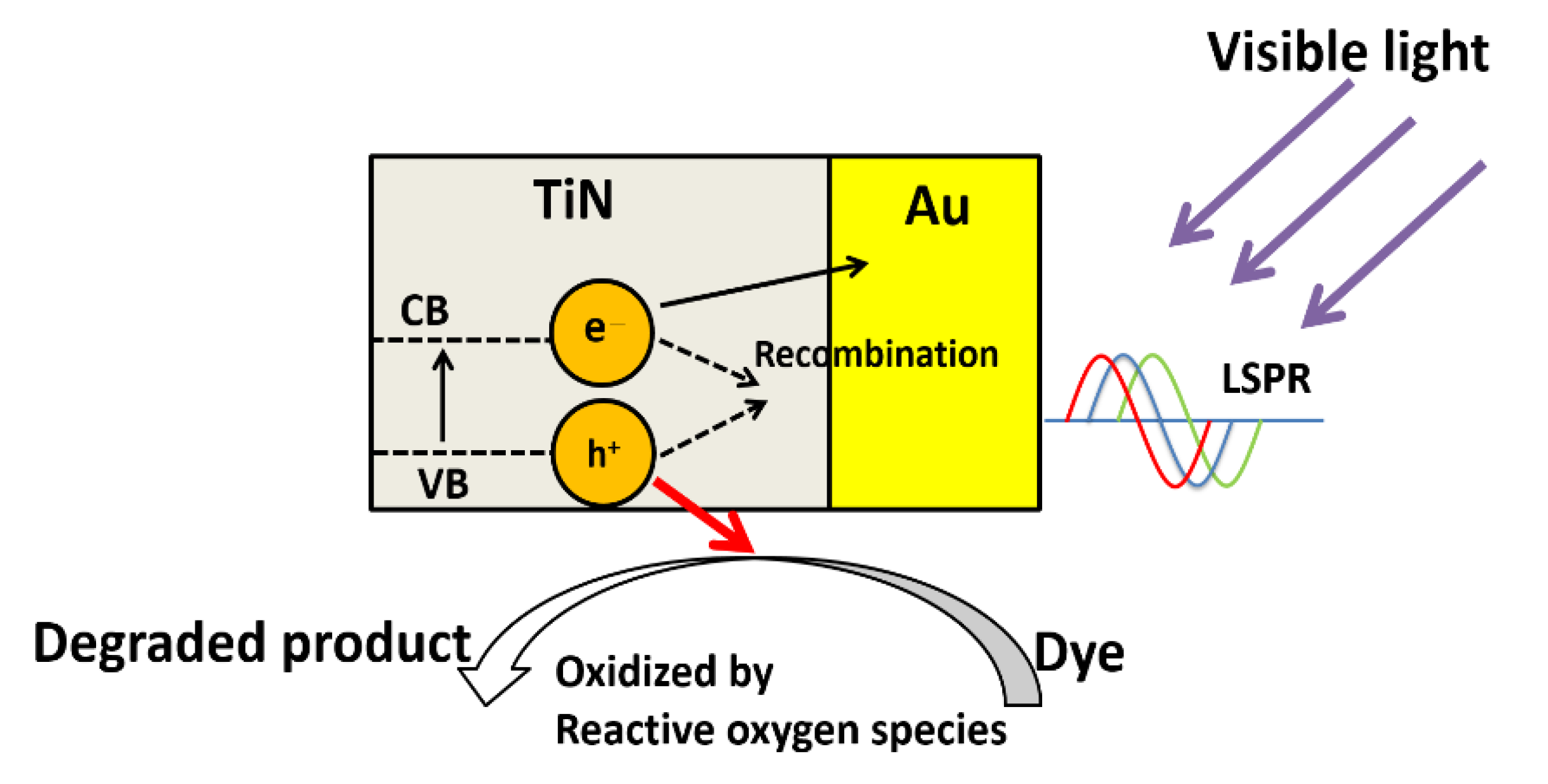
3.4. Photodegradation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turki, A.; Guillard, C.; Dappozze, F.; Berhault, G.; Ksibi, Z.; Kochkar, H. Design of TiO2 nanomaterials for the photodegradation of formic acid—Adsorption isotherms and kinetics study. J. Photochem. Photobiol. A Chem. 2014, 279, 8–16. [Google Scholar] [CrossRef]
- Chatterjee, D.; Mahata, A. Visible light induced photodegradation of organic pollutants on dye adsorbed TiO2 surface. J. Photochem. Photobiol. A Chem. 2002, 153, 199–204. [Google Scholar] [CrossRef]
- Krejčíková, S.; Matějová, L.; Kočí, K.; Obalová, L.; Matěj, Z.; Čapek, L.; Šolcová, O. Preparation and characterization of Ag-doped crystalline titania for photocatalysis applications. Appl. Catal. B Environ. 2012, 111–112, 119–125. [Google Scholar] [CrossRef]
- Thomas, J.; Yoon, M. Facile synthesis of pure TiO2(B) nanofibers doped with gold nanoparticles and solar photocatalytic activities. Appl. Catal. B Environ. 2012, 111–112, 502–508. [Google Scholar] [CrossRef]
- Fu, P.; Zhang, P.; Li, J. Photocatalytic degradation of low concentration formaldehyde and simultaneous elimination of ozone by-product using palladium modified TiO2 films under UV254+185nm irradiation. Appl. Catal. B Environ. 2011, 105, 220–228. [Google Scholar] [CrossRef]
- Vargas Hernández, J.; Coste, S.; Murillo, A.G.; Romo, F.C.; Kassiba, A. Effects of metal doping (Cu, Ag, Eu) on the electronic and optical behavior of nanostructured TiO2. J. Alloys Compd. 2017, 710, 355–363. [Google Scholar] [CrossRef]
- López Ortiz, A.; Zaragoza, M.M.; Gutiérrez, J.S.; Paula, M.M.D.; Collins-Martínez, V. Silver oxidation state effect on the photocatalytic properties of Ag doped TiO2 for hydrogen production under visible light. Int. J. Hydrogen Energy 2015, 40, 17308–17315. [Google Scholar] [CrossRef]
- Wang, H.; Yan, J.; Chang, W.; Zhang, Z. Practical synthesis of aromatic amines by photocatalytic reduction of aromatic nitro compounds on nanoparticles N-doped TiO2. Catal. Commun. 2009, 10, 989–994. [Google Scholar] [CrossRef]
- White, N.; Campbell, A.L.; Grant, J.T.; Pachter, R.; Eyink, K.; Jakubiak, R.; Martinez, G.; Ramana, C.V. Surface/interface analysis and optical properties of RF sputter-deposited nanocrystalline titanium nitride thin films. Appl. Surf. Sci. 2014, 292, 74–85. [Google Scholar] [CrossRef]
- Zhao, J.; Lin, J.; Wei, H.; Li, X.; Zhang, W.; Zhao, G.; Bu, J.; Chen, Y. Surface enhanced Raman scattering substrates based on titanium nitride nanorods. Opt. Mater. 2015, 47, 219–224. [Google Scholar] [CrossRef]
- Zhang, L.A.; Liu, H.N.; Suo, X.X.; Tong, S.; Li, Y.L.; Jiang, Z.T.; Wang, Z. Plasmonic properties of titanium nitride thin films prepared by ion beam assisted deposition. Mater. Lett. 2016, 185, 295–298. [Google Scholar] [CrossRef]
- Lorite, I.; Serrano, A.; Schwartzberg, A.; Bueno, J.; Costa-Krämer, J.L. Surface enhanced Raman spectroscopy by titanium nitride non-continuous thin films. Thin Solid Films 2013, 531, 144–146. [Google Scholar] [CrossRef]
- Kim, W.M.; Kim, S.H.; Lee, K.S.; Lee, T.S.; Kim, I.H. Titanium nitride thin film as an adhesion layer for surface plasmon resonance sensor chips. Appl. Surf. Sci. 2012, 261, 749–752. [Google Scholar] [CrossRef]
- Cesiulis, H.; Ziomek-Moroz, M. Electrocrystallization and electrodeposition of silver on titanium nitride. J. Appl. Electrochem. 2000, 30, 1261–1268. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, W.C.; Fong, H.P.; Wan, C.C.; Wang, Y.Y. Displacement reactions between metal ions and nitride barrier layer/silicon substrate. J. Electrochem. Soc. 2002, 149, G309. [Google Scholar] [CrossRef]
- Koo, H.C.; Ahn, E.J.; Kim, J.J. Direct-electroplating of Ag on pretreated TiN surfaces. J. Electrochem. Soc. 2008, 155, D10. [Google Scholar] [CrossRef]
- O’Kelly, J.P.; Mongey, K.F.; Gobil, Y.; Torres, J.; Kelly, P.V.; Crean, G.M. Room temperature electroless plating copper seed layer process for damascene interlevel metal structures. Microelectron. Eng. 2000, 50, 473–479. [Google Scholar] [CrossRef]
- Sakai, N.; Fujiwara, Y.; Arai, M.; Yu, K.; Tatsuma, T. Electrodeposition of gold nanoparticles on ITO: Control of morphology and plasmon resonance-based absorption and scattering. J. Electroanal. Chem. 2009, 628, 7–15. [Google Scholar] [CrossRef]
- Lin, C.T.; Chang, M.N.; Huang, H.J.; Chen, C.H.; Sun, R.J.; Liao, B.H.; Chau, Y.F.C.; Hsiao, C.N.; Shiao, M.H.; Tseng, F.G. Rapid fabrication of three-dimensional gold dendritic nanoforests for visible light-enhanced methanol oxidation. Electrochim. Acta 2016, 192, 15–21. [Google Scholar] [CrossRef]
- Liao, B.H.; Chan, S.H.; Lee, C.C.; Kuo, C.C.; Chen, S.H.; Chiang, D. FTO films deposited in transition and oxide modes by magnetron sputtering using tin metal target. Appl. Opt. 2014, 53, A148–A153. [Google Scholar] [CrossRef] [PubMed]
- Carraroa, C.; Maboudiana, R.; Magagnin, L. Metallization and nanostructuring of semiconductor surfaces by galvanic displacement processes. Surf. Sci. Rep. 2007, 62, 499–525. [Google Scholar] [CrossRef]
- Lahiri, A.; Wen, R.; Kuimalee, S.; Kobayashi, S.; Park, H. One-step growth of needle and dendritic gold nanostructures on silicon for surface enhanced Raman scattering. CrystEngComm 2012, 14, 1241–1246. [Google Scholar] [CrossRef]
- Lahiri, A.; Wen, R.; Kuimalee, S.; Chowdhury, A.; Kobayashi, S.; Zhang, L.; Wang, P.; Fang, Y. Photo-assisted control of gold and silver nanostructures on silicon and its SERRS effect. J. Phys. D Appl. Phys. 2013, 46, 275303. [Google Scholar] [CrossRef]
- Jung, J.Y.; Guo, Z.; Jee, S.W.; Um, H.D.; Park, K.T.; Lee, J.H. A strong antireflective solar cell prepared by tapering silicon nanowires. Opt. Mater. Express 2010, 18, A286–A292. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Vongehr, S.; Tang, S.; Lu, H.; Shen, J.; Meng, X. Ag dendrite-based Au/Ag bimetallic nanostructures with strongly enhanced catalytic activity. Langmuir 2009, 25, 11890–11896. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, M.; Babu, D.R.; Gengan, R.M.; Chandra, S.; Rao, G.N. Green synthesis of gold nanoparticles using marine algae and evaluation of their catalytic activity. J. Nanostruct. Chem. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Gu, Y.; Jiao, Y.; Zhou, X.; Wu, A.; Buhe, B.; Fu, H. Strongly coupled Ag/TiO2 heterojunctions for effective and stable photothermal catalytic reduction of 4-nitrophenol. Nano Res. 2018, 11, 126–141. [Google Scholar] [CrossRef]
- Hou, W.; Cronin, S.B. A review of surface plasmon resonance-enhanced photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Chau, Y.F.C.; Lee, C.; Huang, H.J.; Lin, C.T.; Chiang, H.P.; Mahadi, A.H.; Voo, N.Y.; Lim, C.M. Plasmonic effects arising from a grooved surface of a gold nanorod. J. Phys. D Appl. Phys. 2017, 50, 125302. [Google Scholar] [CrossRef]
- Nishi, H.; Tatsuma, T. Mechanistic analysis of plasmon-induced charge separation by the use of chemically synthesized gold nanorods. J. Phys. Chem. C 2018, 122, 2330–2335. [Google Scholar] [CrossRef]
- Bonyár, A.; Csarnovics, I.; Veres, M.; Himics, L.; Csik, A.; Kámán, J.; Balázs, L.; Kökényesi, S. Investigation of the performance of thermally generated gold nanoislands for LSPR and SERS applications. Sens. Actuators B Chem. 2018, 255, 433–439. [Google Scholar] [CrossRef]
- Ayati, A.; Tanhaei, B.; Bamoharram, F.F.; Ahmadpour, A.; Maydannik, P.; Sillanpää, M. Photocatalytic degradation of nitrobenzene by gold nanoparticles decorated polyoxometalate immobilized TiO2 nanotubes. Sep. Purif. Technol. 2016, 171, 62–68. [Google Scholar] [CrossRef]
- Iliev, V.; Tomova, D.; Rakovsky, S. Nanosized N-doped TiO2 and gold modified semiconductors—Photocatalysts for combined UV–visible light destruction of oxalic acid in aqueous solution. Desalination 2010, 260, 101–106. [Google Scholar] [CrossRef]
- Dai, K.; Chen, H.; Peng, T.; Ke, D.; Yi, H. Photocatalytic degradation of methyl orange in aqueous suspension of mesoporous titania nanoparticles. Chemosphere 2007, 69, 1361–1367. [Google Scholar] [CrossRef] [PubMed]





| Layer | DC Power (W) | Impulse Period (µs) | Ar Flow Rate (sccm) | N2 Flow Rate (sccm) |
|---|---|---|---|---|
| Ti | 250 | 90 | 20 | - |
| TiN | 300 | 1000 | 30 | 1.5 |
| Sample | Reaction Rate Constant (×10−3, 1/min) |
|---|---|
| Au DNFs/Si (dark) | 7.0 |
| Au DNFs/TiN/Si (dark) | 8.0 |
| Au DNFs/Si (light) | 9.7 |
| Au DNFs/TiN/Si (light) | 12.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiao, M.-H.; Lin, C.-T.; Zeng, J.-J.; Lin, Y.-S. Novel Gold Dendritic Nanoforests Combined with Titanium Nitride for Visible-Light-Enhanced Chemical Degradation. Nanomaterials 2018, 8, 282. https://doi.org/10.3390/nano8050282
Shiao M-H, Lin C-T, Zeng J-J, Lin Y-S. Novel Gold Dendritic Nanoforests Combined with Titanium Nitride for Visible-Light-Enhanced Chemical Degradation. Nanomaterials. 2018; 8(5):282. https://doi.org/10.3390/nano8050282
Chicago/Turabian StyleShiao, Ming-Hua, Chun-Ting Lin, Jian-Jia Zeng, and Yung-Sheng Lin. 2018. "Novel Gold Dendritic Nanoforests Combined with Titanium Nitride for Visible-Light-Enhanced Chemical Degradation" Nanomaterials 8, no. 5: 282. https://doi.org/10.3390/nano8050282




