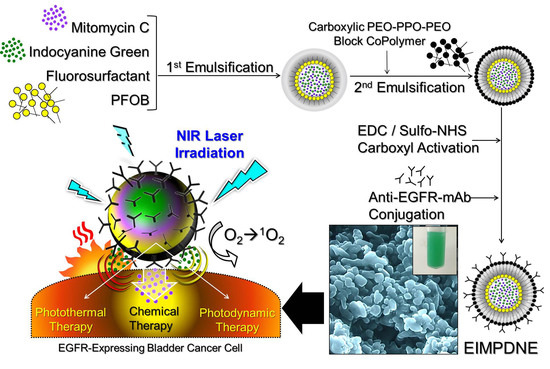
Anti-EGFR Indocyanine Green-Mitomycin C-Loaded Perfluorocarbon Double Nanoemulsion: A Novel Nanostructure for Targeted Photochemotherapy of Bladder Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
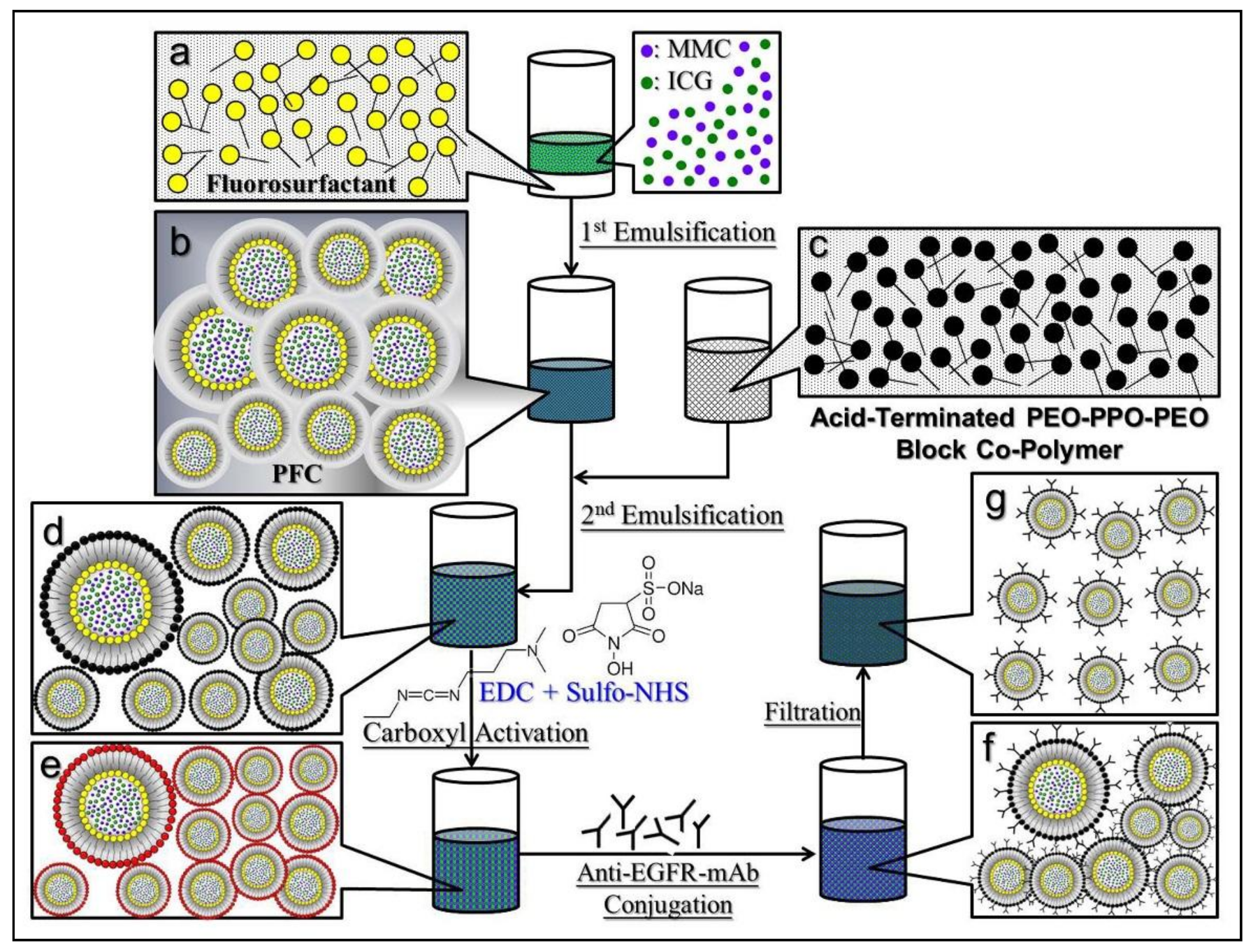
2.1. Fabrication of EIMPDNEs
2.2. Verification of Anti-EGFR-mAb Conjugation
2.3. Assessments of the Physical and Chemical Properties of the EIMPDNEs
2.4. Analysis of EIMPDNE Stability
2.5. Cell Culture
2.6. Verification of EGFR Binding Specificity of the EIMPDNEs
2.7. Assessment of the EIMPDNE-Induced Hyperthermia Effect
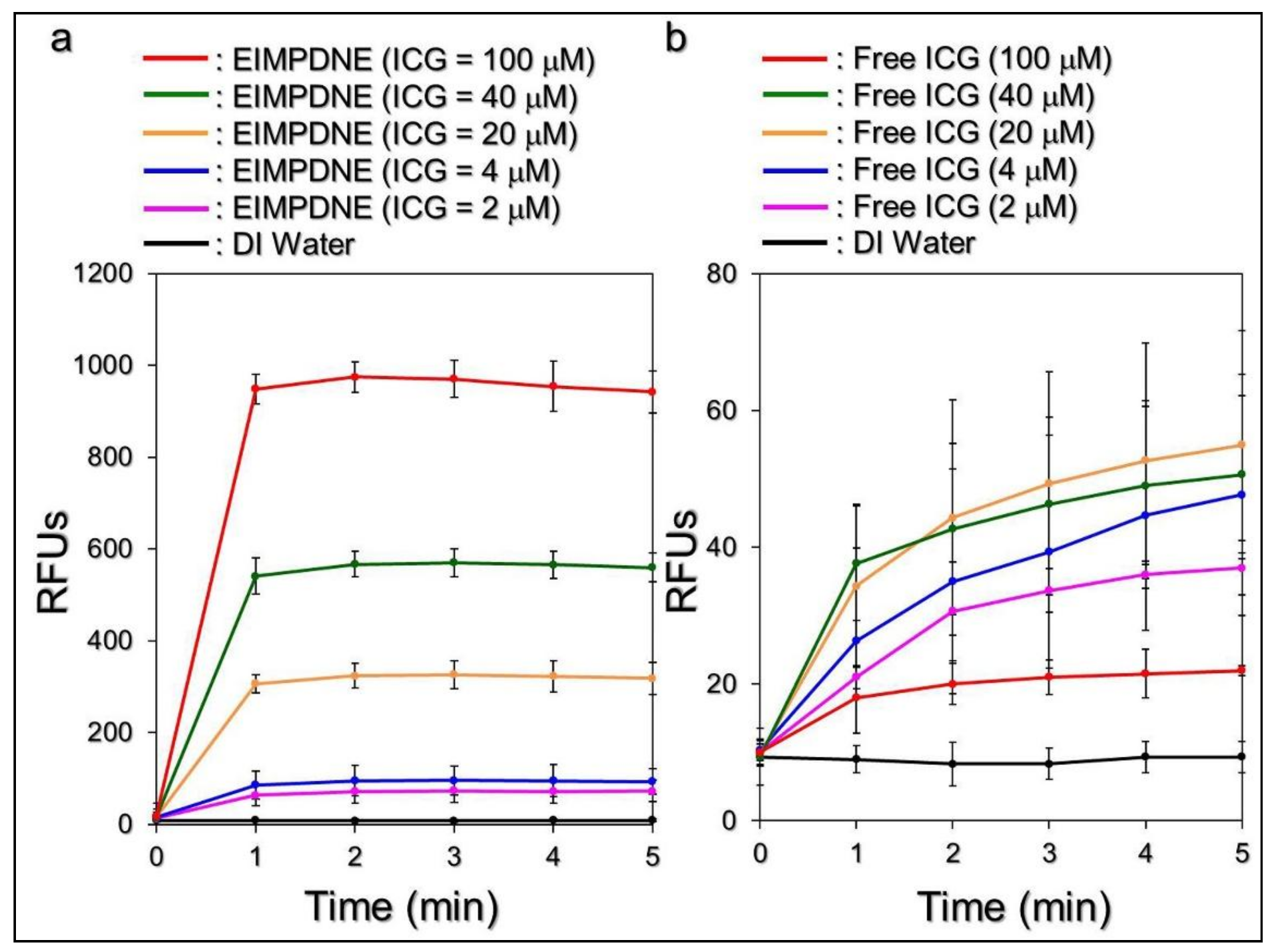
2.8. Assessment of Singlet Oxygen Production Induced by EIMPDNEs
2.9. In Vitro Cytotoxicity Assay
2.10. Statistical Analysis
3. Results and Discussion
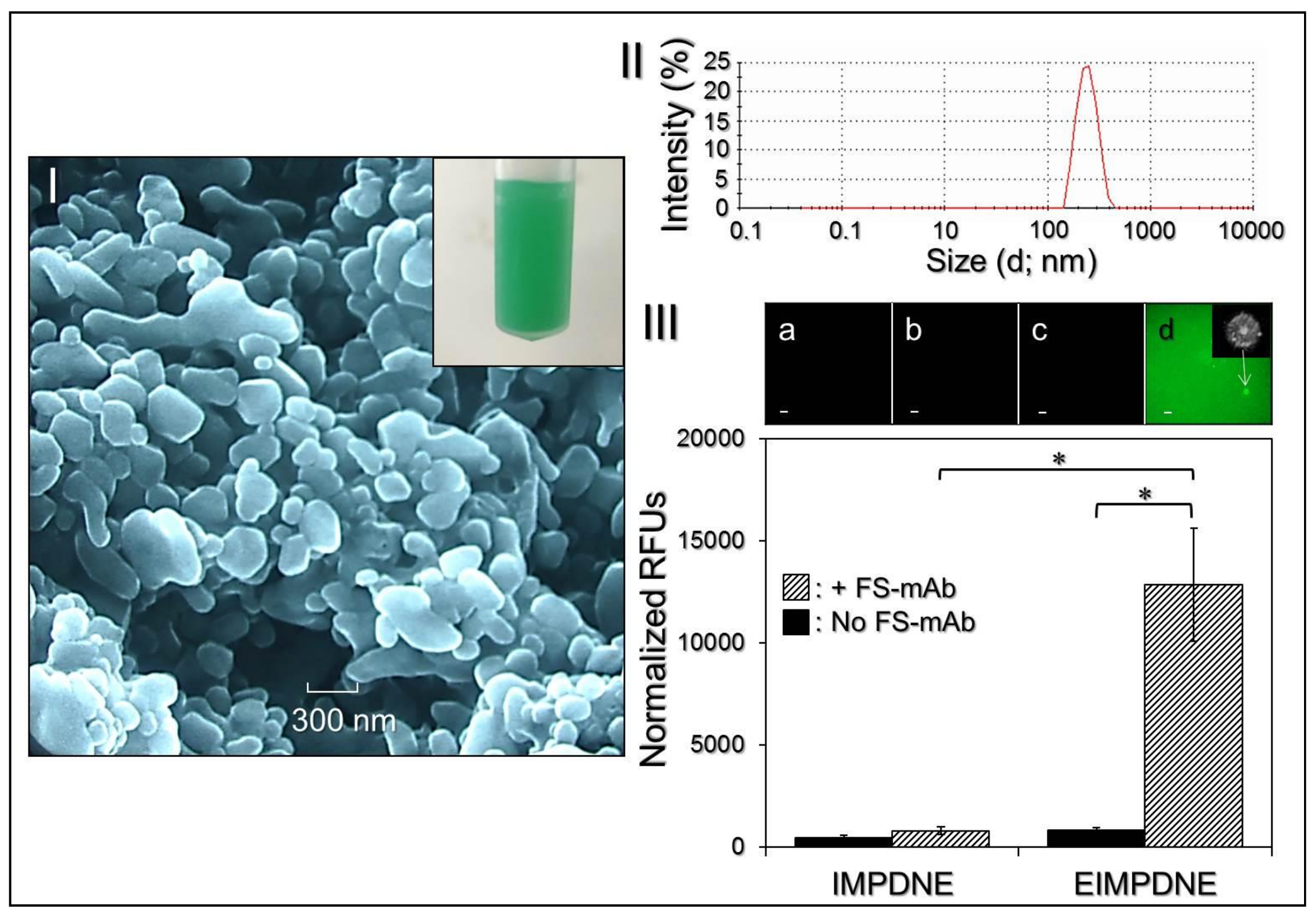
3.1. Characterization of EIMPDNEs
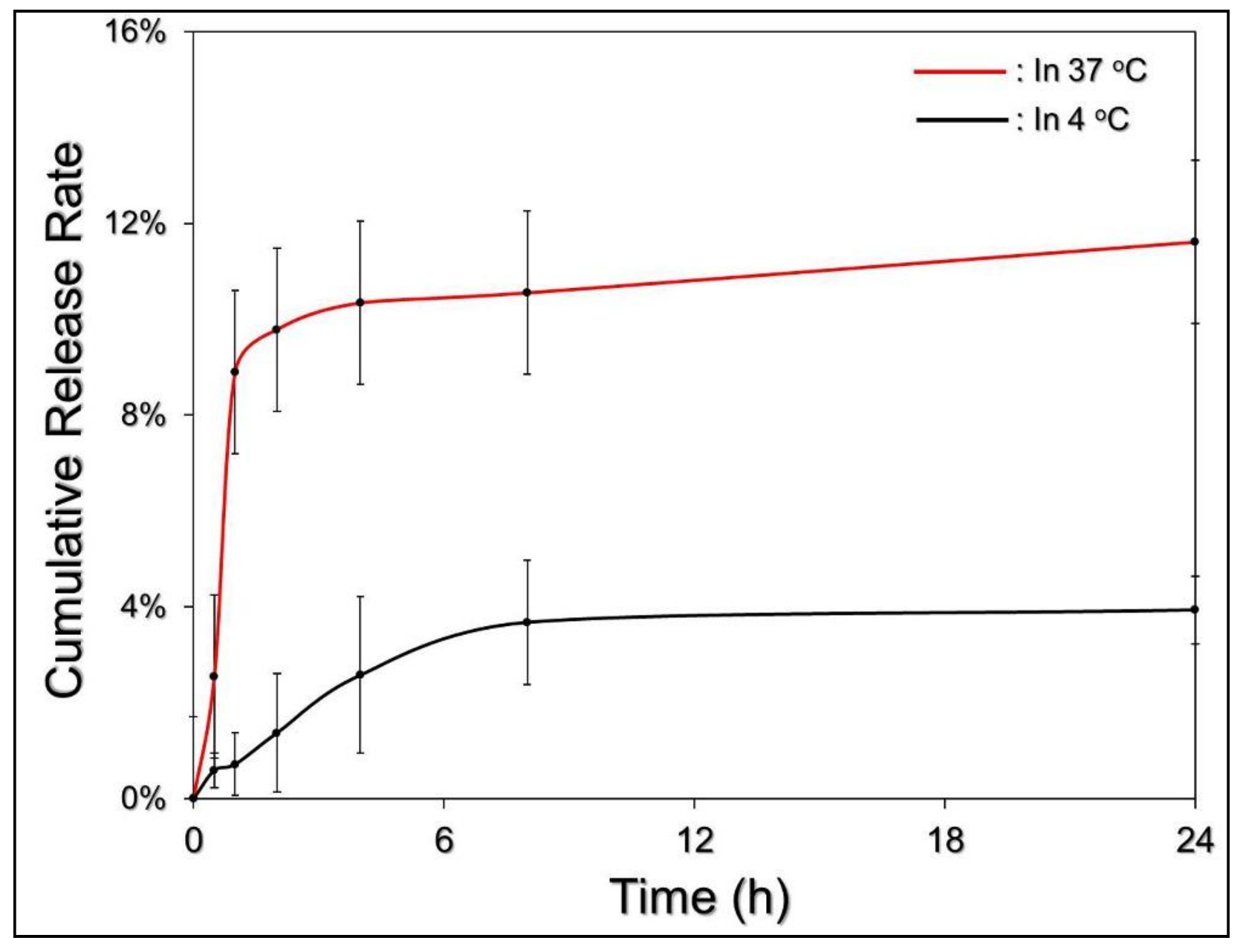
3.2. Thermal Stability of EIMPDNE-Entrapped ICG and Efficiency of MMC Release
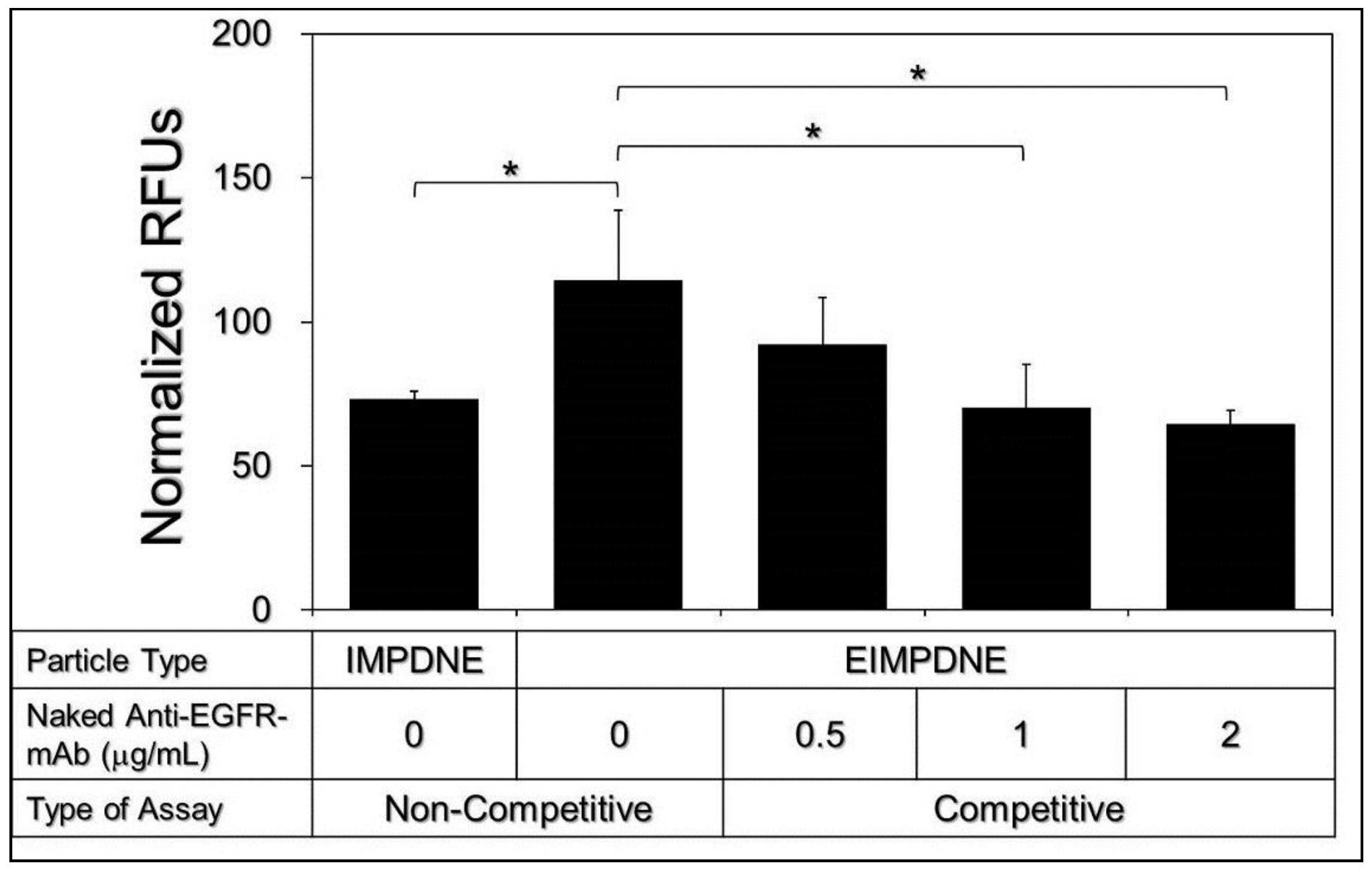
3.3. Binding Specificity of EIMPDNEs
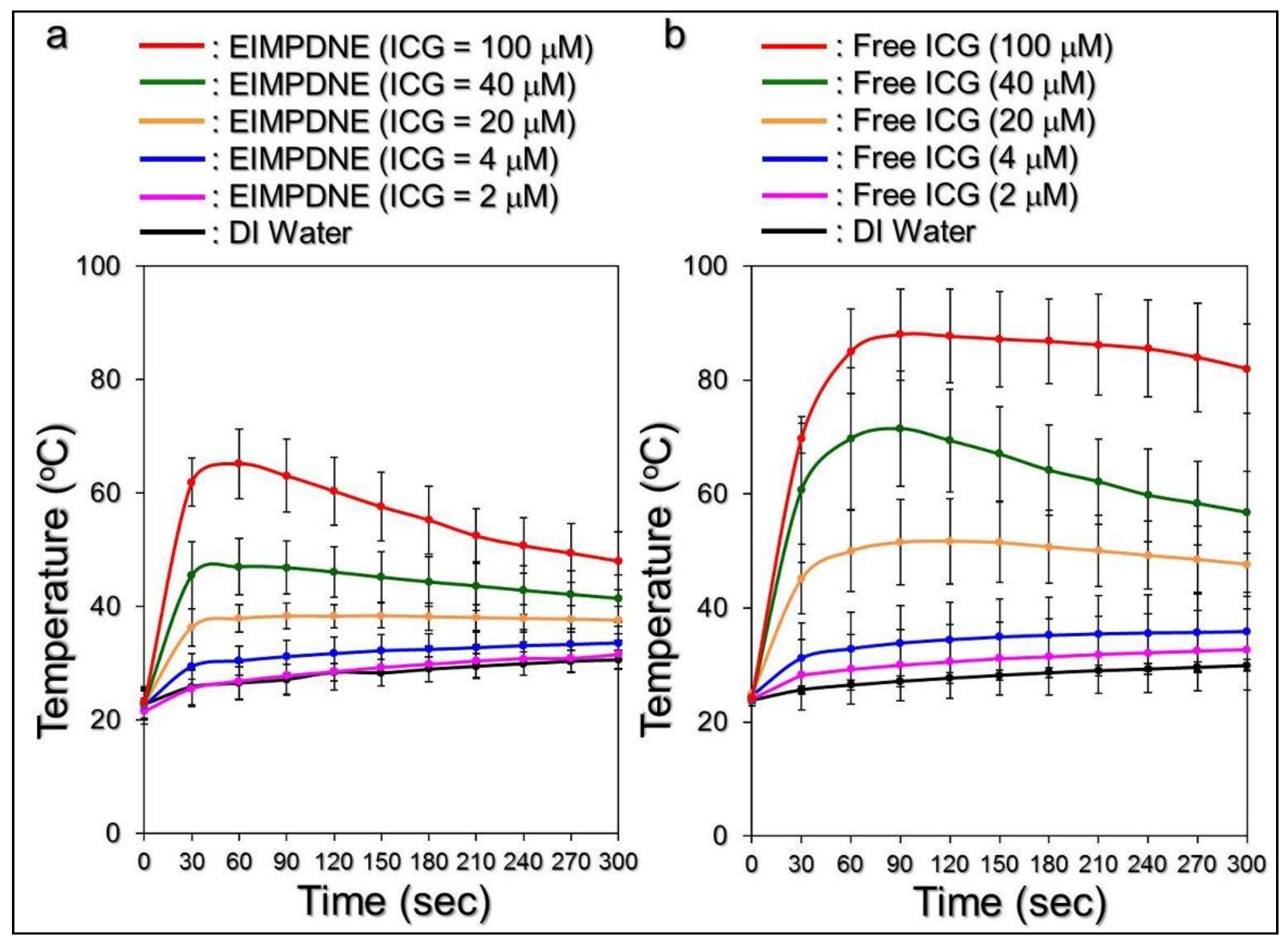
3.4. Effects of Hyperthermia and Singlet Oxygen Generation of EIMPDNEs
3.5. Efficiency of MMC Release under NIR Exposure
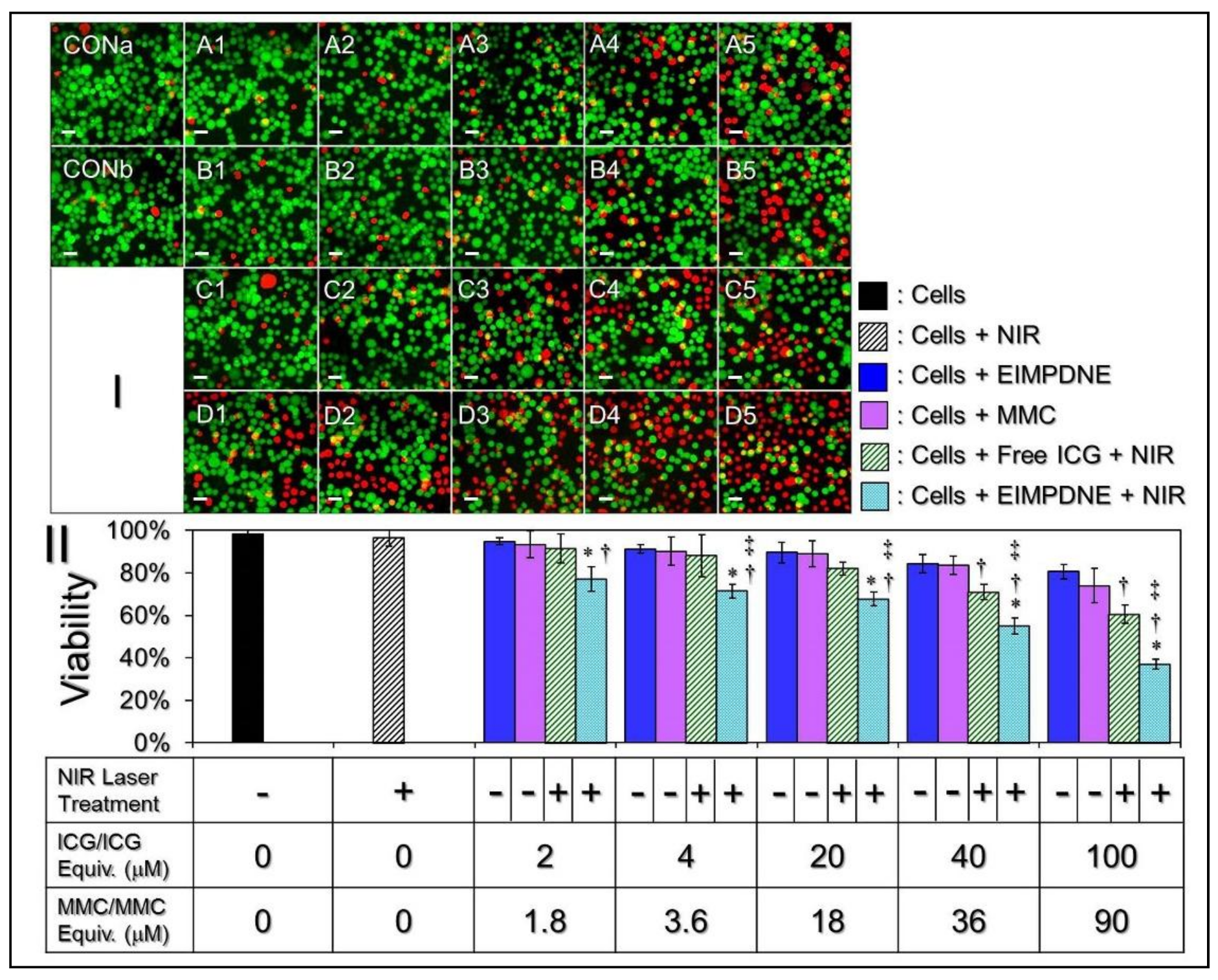
3.6. In Vitro Cytotoxicity of EIMPDNEs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Botteman, M.F.; Pashos, C.L.; Redaelli, A.; Laskin, B.; Hauser, R. The health economics of bladder cancer: A comprehensive review of the published literature. Pharmacoeconomics 2003, 21, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Supit, W.; Mochtar, C.A.; Santoso, R.B.; Umbas, R. Outcomes of radical cystectomy and bladder preservation treatment for muscle-invasive urothelial carcinoma of the bladder. Asian J. Surg. 2014, 37, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, A.J.; Mellon, J.K. Epidermal growth factor receptor and bladder cancer. Postgrad. Med. J. 2002, 78, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Wee, P.; Wang, Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers 2017, 9, 52. [Google Scholar]
- Van Cruijsen, H.; Giaccone, G.; Hoekman, K. Epidermal growth factor receptor and angiogenesis: Opportunities for combined anticancer strategies. Int. J. Cancer 2005, 117, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Cheng, J.; Zhang, J.; Zhang, Y.; Chen, X.; Luo, S.; Xie, J. miR-20b reduces 5-FU resistance by suppressing the ADAM9/EGFR signaling pathway in colon cancer. Oncol. Rep. 2017, 37, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Chaux, A.; Cohen, J.S.; Schultz, L.; Albadine, R.; Jadallah, S.; Murphy, K.M.; Sharma, R.; Schoenberg, M.P.; Netto, G.J. High epidermal growth factor receptor immunohistochemical expression in urothelial carcinoma of the bladder is not associated with EGFR mutations in exons 19 and 21: A study using formalin-fixed, paraffin-embedded archival tissues. Hum. Pathol. 2012, 43, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Røtterud, R.; Nesland, J.M.; Berner, A.; Fosså, S.D. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 2005, 95, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Neal, D.E.; Sharples, L.; Smith, K.; Fennelly, J.; Hall, R.R.; Harris, A.L. The epidermal growth factor receptor and the prognosis of bladder cancer. Cancer 1990, 65, 1619–1625. [Google Scholar] [CrossRef]
- Mellon, K.; Wright, C.; Kelly, P.; Horne, C.H.; Neal, D.E. Long-term outcome related to epidermal growth factor receptor status in bladder cancer. J. Urol. 1995, 153, 919–925. [Google Scholar] [CrossRef]
- Kassouf, W.; Black, P.C.; Tuziak, T.; Bondaruk, J.; Lee, S.; Brown, G.A.; Adam, L.; Wei, C.; Baggerly, K.; Bar-Eli, M.; et al. Distinctive expression pattern of ErbB family receptors signifies an aggressive variant of bladder cancer. J. Urol. 2008, 179, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Messing, E.M. Clinical implications of the expression of epidermal growth factor receptors in human transitional cell carcinoma. Cancer Res. 1990, 50, 2530–2537. [Google Scholar] [PubMed]
- Palom, Y.; Suresh Kumar, G.; Tang, L.Q.; Paz, M.M.; Musser, S.M.; Rockwell, S.; Tomasz, M. Relative toxicities of DNA cross-links and monoadducts: New insights from studies of decarbamoyl mitomycin C and mitomycin C. Chem. Res. Toxicol. 2002, 15, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Tomasz, M. Conjugation of glutathione and other thiols with bioreductively activated mitomycin C. Effect of thiols on the reductive activation rate. Chem. Res. Toxicol. 1994, 7, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Paz, M.M.; Zhang, X.; Lu, J.; Holmgren, A. A new mechanism of action for the anticancer drug mitomycin C: Mechanism-based inhibition of thioredoxin reductase. Chem. Res. Toxicol. 2012, 25, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Filson, C.P.; Montgomery, J.S.; Dailey, S.M.; Crossley, H.S.; Lentz, H.; Tallman, C.T.; He, C.; Weizer, A.Z. Complications associated with single-dose, perioperative mitomycin-C for patients undergoing bladder tumor resection. Urol. Oncol. 2014, 32, 40.e1–40.e8. [Google Scholar] [CrossRef] [PubMed]
- Griffin, J.G.; Holzbeierlein, J. Side effects of perioperative intravesical treatment and treatment strategies for these side effects. Urol. Clin. N. Am. 2013, 40, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Wang, C.; Feng, L.; Yang, K.; Liu, Z. Functional nanomaterials for phototherapies of cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Mastropasqua, R.; Di Antonio, L.; Di Staso, S.; Agnifili, L.; Di Gregorio, A.; Ciancaglini, M.; Mastropasqua, L. Optical Coherence Tomography Angiography in Retinal Vascular Diseases and Choroidal Neovascularization. J. Ophthalmol. 2015, 2015, 343515. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, B.E.; Mieog, J.S.; Hutteman, M.; van der Vorst, J.R.; Kuppen, P.J.; Löwik, C.W.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J. Surg. Oncol. 2011, 104, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, C.S.; Moshkelani, D.; Zhang, H. Thermosensitive liposome formulated indocyanine green for near-infrared triggered photodynamic therapy: In vivo evaluation for triple-negative breast cancer. Pharm. Res. 2015, 32, 1604–1614. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.J.; Lowery, A.R.; Thompson, P.A.; Blaney, S.M.; West, J.L. Immunonanoshells for targeted photothermal ablation in medulloblastoma and glioma: An in vitro evaluation using human cell lines. J. Neurooncol. 2008, 86, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mundra, V.; Peng, Y.; Rana, S.; Natarajan, A.; Mahato, R.I. Micellar formulation of indocyanine green for phototherapy of melanoma. J. Control. Release 2015, 220, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Desmettre, T.; Devoisselle, J.M.; Mordon, S. Fluorescence properties and metabolic features of indocyanine green (ICG) as related to angiography. Surv. Ophthalmol. 2000, 45, 15–27. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Nanomedicine: Application of nanobiotechnology in medical practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Lowe, K.C. Perfluorochemical respiratory gas carriers: Benefits to cell culture systems. J. Fluor. Chem. 2002, 118, 19–26. [Google Scholar] [CrossRef]
- Björnsson, O.G.; Murphy, R.; Chadwick, V.S.; Björnsson, S. Physiochemical studies on indocyanine green: Molar lineic absorbance, pH tolerance, activation energy and rate of decay in various solvents. J. Clin. Chem. Clin. Biochem. 1983, 21, 453–458. [Google Scholar] [PubMed]
- Sun, C.Z.; Lu, C.T.; Zhao, Y.Z.; Guo, P.; Tian, J.L.; Zhang, L.; Li, X.K.; Lv, H.F.; Dai, D.D.; Li, X. Characterization of the Doxorubicin-Pluronic F68 Conjugate Micelles and Their Effect on Doxorubicin Resistant Human Erythroleukemic Cancer Cells. J. Nanomed. Nanotechnol. 2011, 2, 1000114. [Google Scholar]
- Vivek, R.; Thangam, R.; NipunBabu, V.; Rejeeth, C.; Sivasubramanian, S.; Gunasekaran, P.; Muthuchelian, K.; Kannan, S. Multifunctional HER2-antibody conjugated polymeric nanocarrier-based drug delivery system for multi-drug-resistant breast cancer therapy. ACS Appl. Mater. Interfaces 2014, 6, 6469–6480. [Google Scholar] [CrossRef] [PubMed]
- Chittasupho, C.; Xie, S.X.; Baoum, A.; Yakovleva, T.; Siahaan, T.J.; Berkland, C.J. ICAM-1 targeting of doxorubicin-loaded PLGA nanoparticles to lung epithelial cells. Eur. J. Pharm. Sci. 2009, 37, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P. Emulsions. In Fundamentals of Interface and Colloid Science, 1st ed.; Lyklema, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 4, pp. 8.1–8.94. ISBN 978-0-12-460529-9. [Google Scholar]
- Fang, Y.P.; Hu, P.Y.; Huang, Y.B. Diminishing the side effect of mitomycin C by using pH-sensitive liposomes: In vitro characterization and in vivo pharmacokinetics. Drug Des. Dev. Ther. 2018, 12, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, J.; Wu, H.; Chang, Y.; Yuan, C.; Liu, C.; Wang, S.; Hou, Z.; Dai, L. Orthogonally functionalized nanoscale micelles for active targeted codelivery of methotrexate and mitomycin C with synergistic anticancer effect. Mol. Pharm. 2015, 12, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lin, J.; Wu, H.; Jia, M.; Yuan, C.; Chang, Y.; Hou, Z.; Dai, L. Novel methotrexate prodrug-targeted drug delivery system based on PEG–lipid–PLA hybrid nanoparticles for enhanced anticancer efficacy and reduced toxicity of mitomycin C. J. Mater. Chem. B 2014, 2, 6534–6548. [Google Scholar] [CrossRef]
- Harush-Frenkel, O.; Debotton, N.; Benita, S.; Altschuler, Y. Targeting of nanoparticles to the clathrin-mediated endocytic pathway. Biochem. Biophys. Res. Commun. 2007, 353, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ogris, M.; Steinlein, P.; Carotta, S.; Brunner, S.; Wagner, E. DNA/polyethylenimine transfection particles: Influence of ligands, polymer size, and PEGylation on internalization and gene expression. AAPS PharmSci 2001, 3, 43. [Google Scholar] [CrossRef]
- Ferreira, B.M.S.; Ramalho, J.B.V.S.; Lucas, E.F. Demulsification of eater-in-crude oil emulsions by microwave radiation: Effect of aging, demulsifier addition, and selective heating. Energy Fuels 2013, 27, 615–621. [Google Scholar] [CrossRef]
- Ruhi, M.K.; Can, A.A.; Gülsoy, M. Dose-dependent Photochemical/Photothermal Toxicity of Indocyanine Green-Based Therapy on Three Different Cancer Cell Lines. Photodiagnosis Photodyn. Ther. 2018, 21, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, S.N.; Gazelle, G.S.; Halpern, E.F.; Rittman, W.J.; Mueller, P.R.; Rosenthal, D.I. Radiofrequency tissue ablation: Importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad. Radiol. 1996, 3, 212–218. [Google Scholar] [CrossRef]
- Haen, S.P.; Pereira, P.L.; Salih, H.R.; Rammensee, H.G.; Gouttefangeas, C. More than just tumor destruction: Immunomodulation by thermal ablation of cancer. Clin. Dev. Immunol. 2011, 2011, 160250. [Google Scholar] [CrossRef] [PubMed]
- Coffey, D.S.; Getzenberg, R.H.; DeWeese, T.L. Hyperthermic biology and cancer therapies: A hypothesis for the “Lance Armstrong effect”. JAMA 2006, 296, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Badawi, A.A.; El-Nabarawi, M.A.; El-Setouhy, D.A.; Alsammit, S.A. Characterization and stability testing of itraconazole solid dispersions containing crystalllization inhibitors. Am. J. Drug Discov. Dev. 2011, 1, 144–159. [Google Scholar]
- Krege, S.; Giani, G.; Meyer, R.; Otto, T.; Rübben, H. A randomized multicenter trial of adjuvant therapy in superficial bladder cancer: Transurethral resection only versus transurethral resection plus mitomycin C versus transurethral resection plus bacillus Calmette-Guerin. Participating Clinics. J. Urol. 1996, 156, 962–966. [Google Scholar] [CrossRef]
- Friedrich, M.G.; Pichlmeier, U.; Schwaibold, H.; Conrad, S.; Huland, H. Long-term intravesical adjuvant chemotherapy further reduces recurrence rate compared with short-term intravesical chemotherapy and short-term therapy with Bacillus Calmette-Guérin (BCG) in patients with non-muscle-invasive bladder carcinoma. Eur. Urol. 2007, 52, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Svatek, R.S.; Zhao, X.R.; Morales, E.E.; Jha, M.K.; Tseng, T.Y.; Hugen, C.M.; Hurez, V.; Hernandez, J.; Curiel, T.J. Sequential intravesical mitomycin plus bacillus calmette-guérin for non-muscle-invasive urothelial bladder carcinoma: Translational and phase I clinical trial. Clin. Cancer Res. 2015, 21, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Kabanov, A.V.; Batrakova, E.V.; Alakhov, V.Y. Pluronic block copolymers for overcoming drug resistance in cancer. Adv. Drug Deliv. Rev. 2002, 54, 759–779. [Google Scholar] [CrossRef]
- Morgillo, F.; Bareschino, M.A.; Bianco, R.; Tortora, G.; Ciardiello, F. Primary and acquired resistance to anti-EGFR targeted drugs in cancer therapy. Differentiation 2007, 75, 788–799. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef] [PubMed]
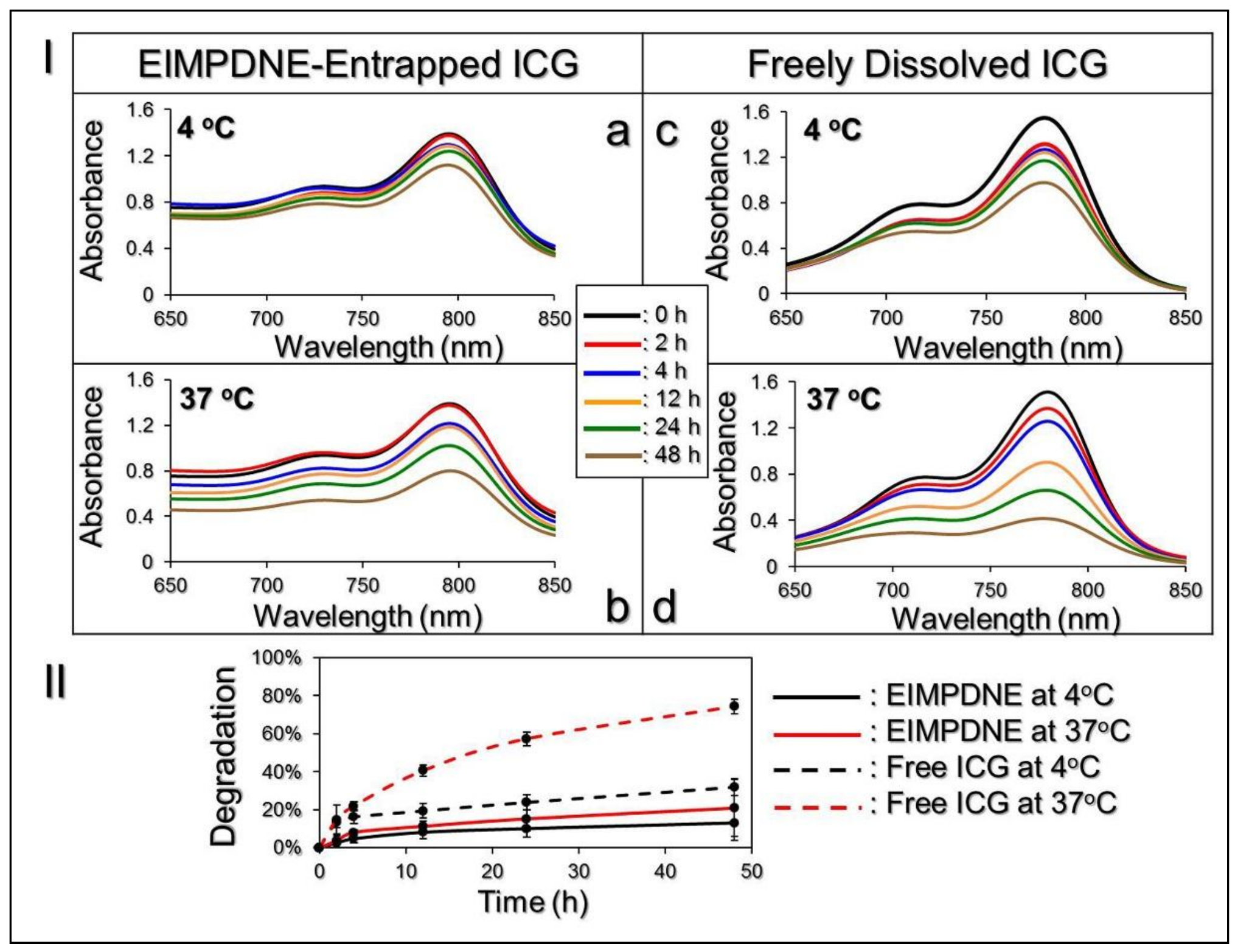

| Group/Temperature Setting | Residual Rate of ICG (Ct */C0) | kd (h−1) |
|---|---|---|
| EIMPDNE-entrapped ICG | ||
| 4 °C in the dark | 86.90% | 0.0029 † |
| 37 °C in the dark | 79.08% | 0.0049 † |
| Free ICG in DI Water | ||
| 4 °C in the dark | 68.07% | 0.0081 |
| 37 °C in the dark | 25.62% | 0.0284 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Lin, Y.-C. Anti-EGFR Indocyanine Green-Mitomycin C-Loaded Perfluorocarbon Double Nanoemulsion: A Novel Nanostructure for Targeted Photochemotherapy of Bladder Cancer Cells. Nanomaterials 2018, 8, 283. https://doi.org/10.3390/nano8050283
Lee Y-H, Lin Y-C. Anti-EGFR Indocyanine Green-Mitomycin C-Loaded Perfluorocarbon Double Nanoemulsion: A Novel Nanostructure for Targeted Photochemotherapy of Bladder Cancer Cells. Nanomaterials. 2018; 8(5):283. https://doi.org/10.3390/nano8050283
Chicago/Turabian StyleLee, Yu-Hsiang, and Yu-Chun Lin. 2018. "Anti-EGFR Indocyanine Green-Mitomycin C-Loaded Perfluorocarbon Double Nanoemulsion: A Novel Nanostructure for Targeted Photochemotherapy of Bladder Cancer Cells" Nanomaterials 8, no. 5: 283. https://doi.org/10.3390/nano8050283