Electrochemically-Driven Insertion of Biological Nanodiscs into Solid State Membrane Pores as a Basis for “Pore-In-Pore” Membranes
Abstract
:1. Introduction
2. Results and Discussion
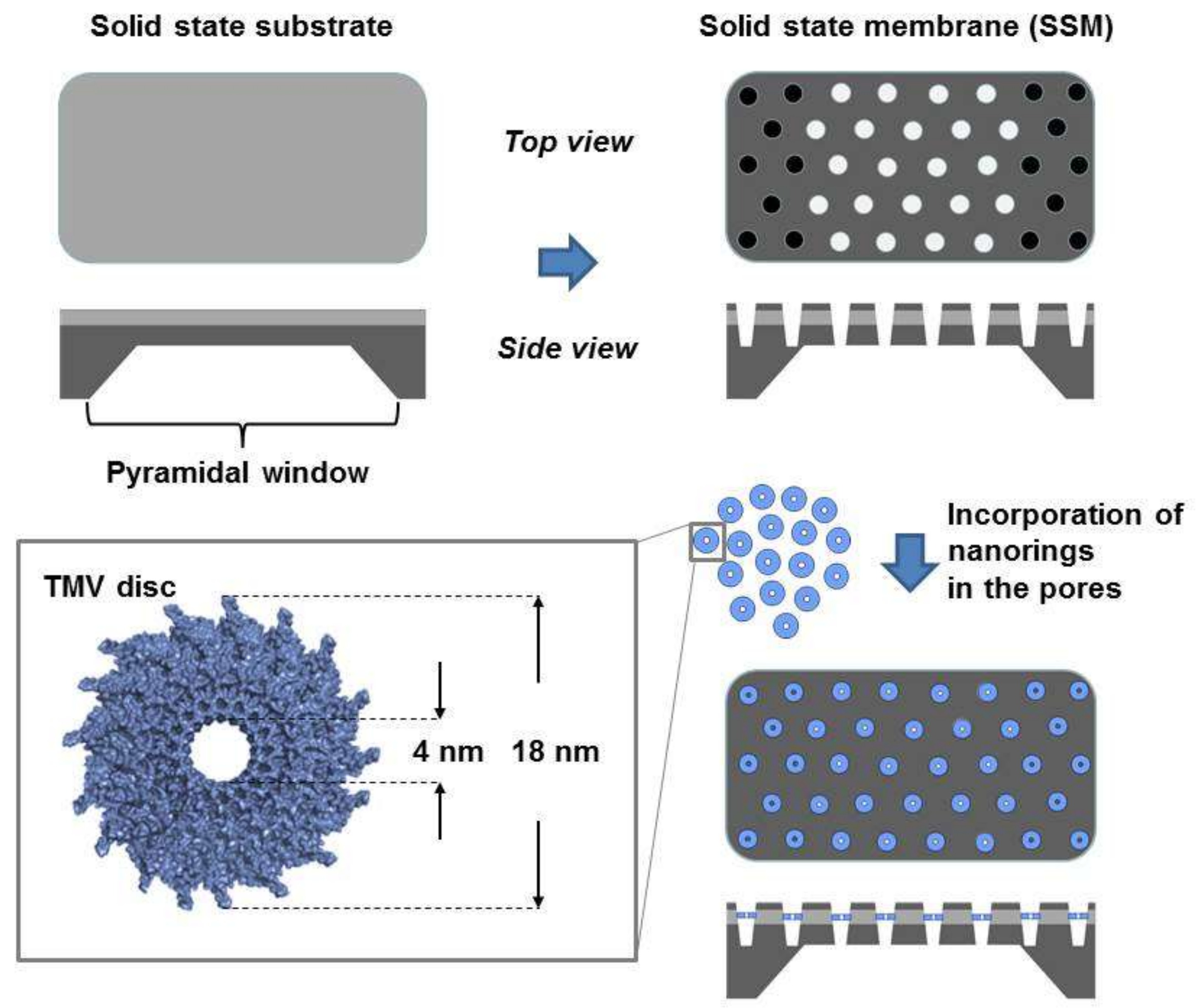
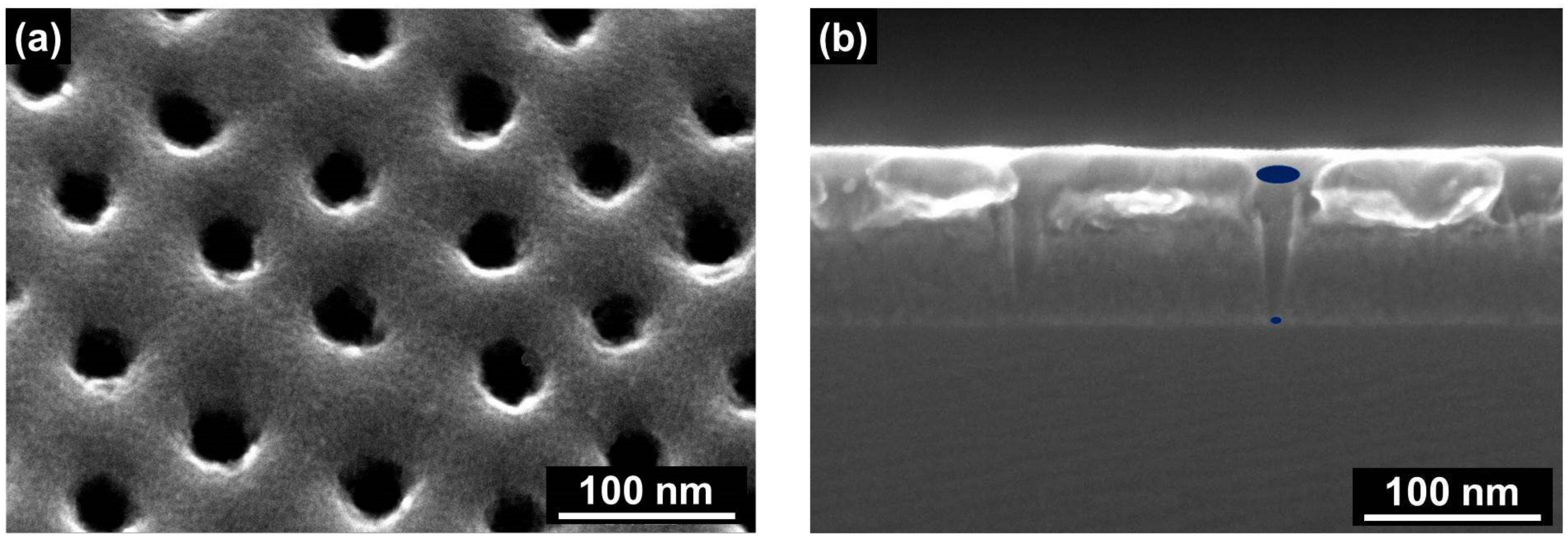
2.1. Characterization of the Solid State Membrane and Viral Nanodiscs
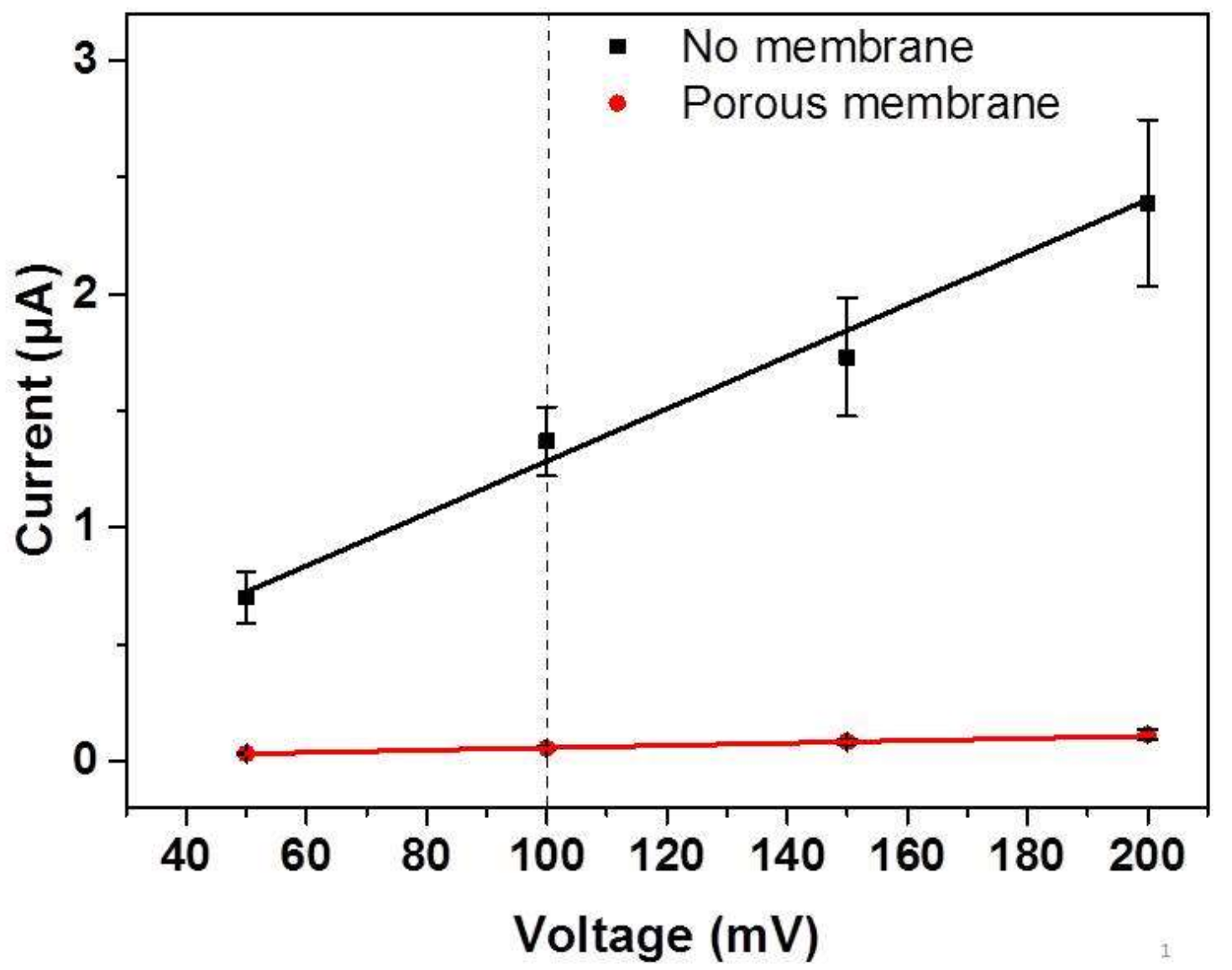
2.2. Electrochemical Characterization of the Membrane
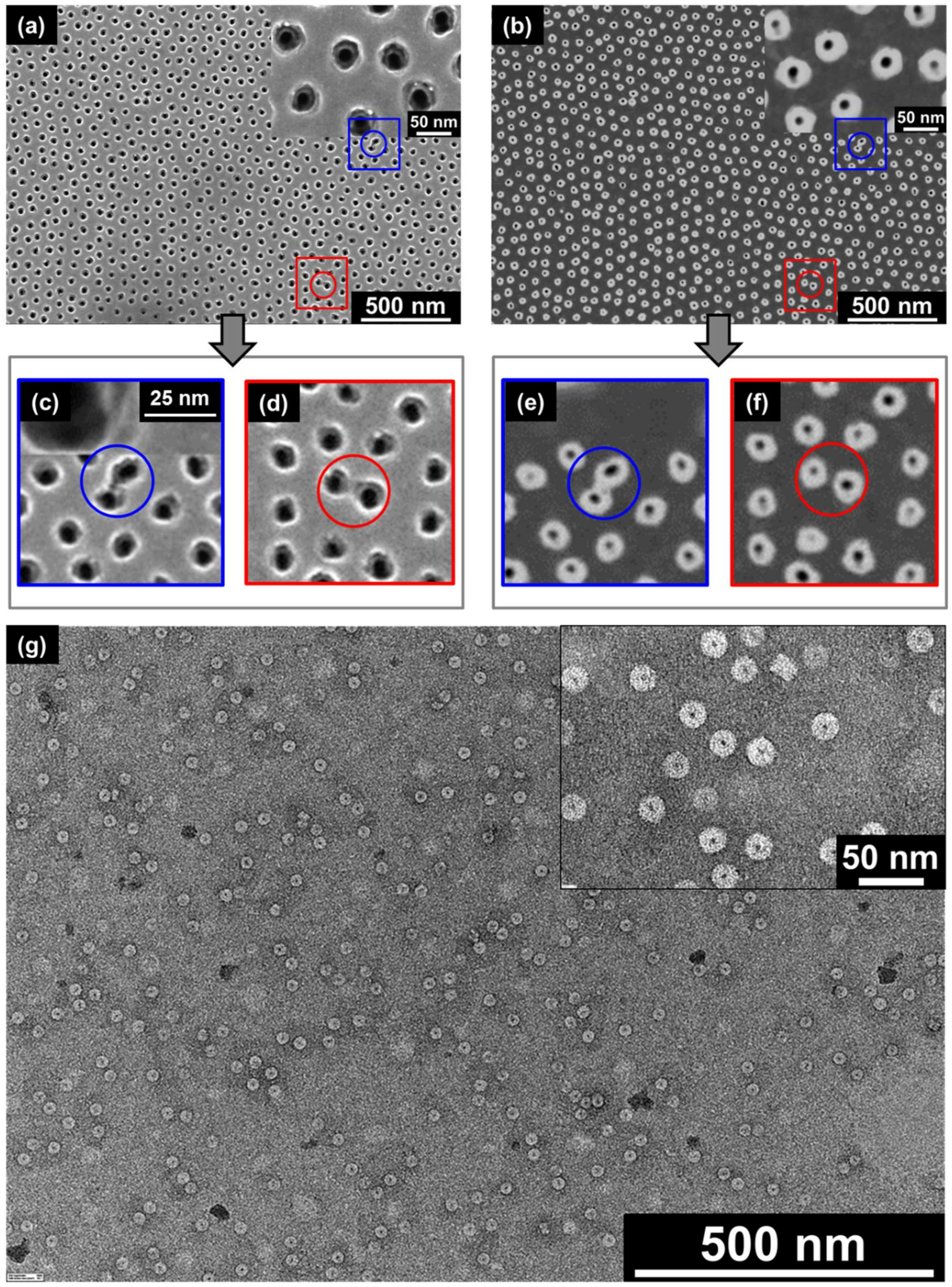
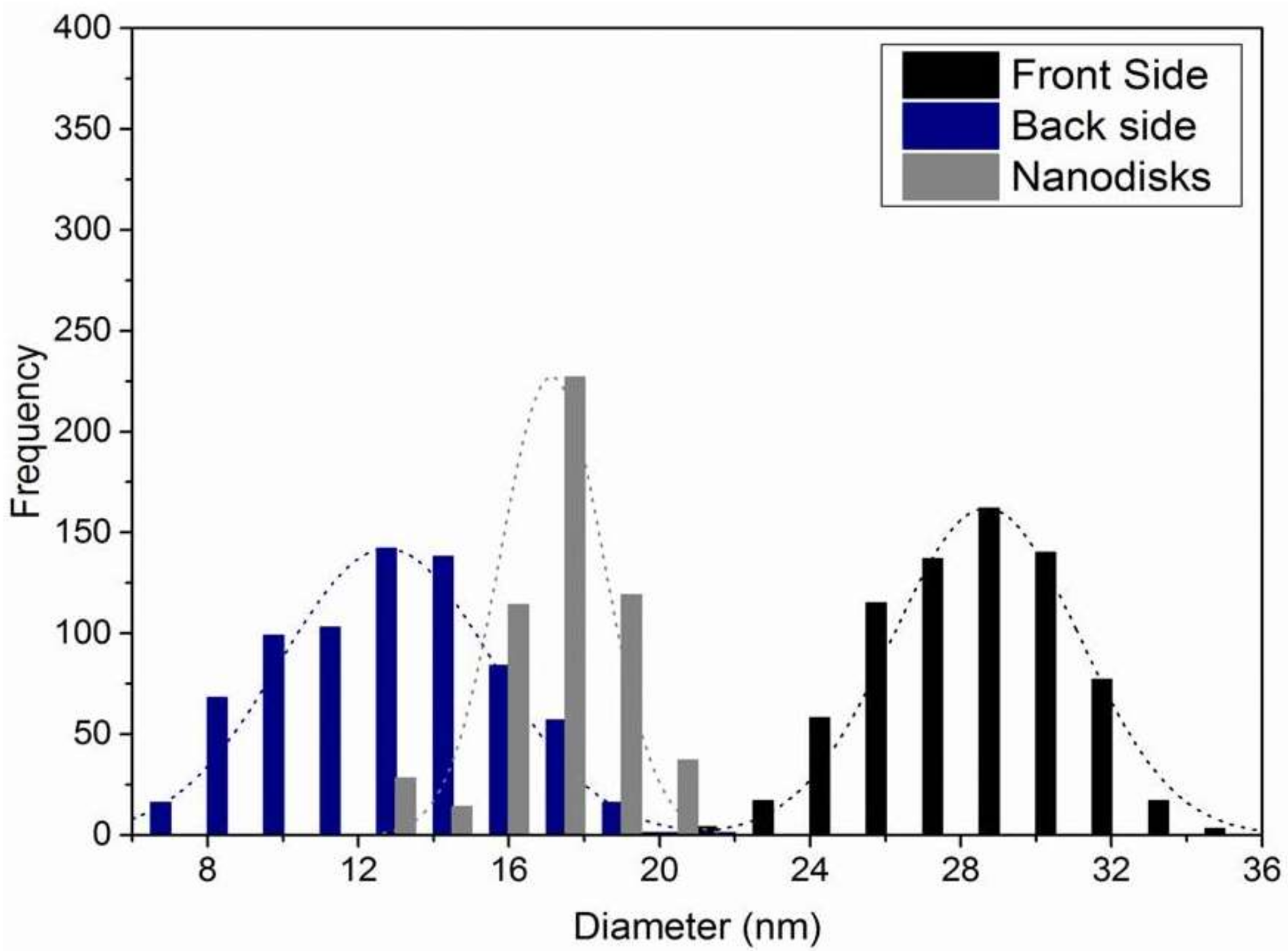
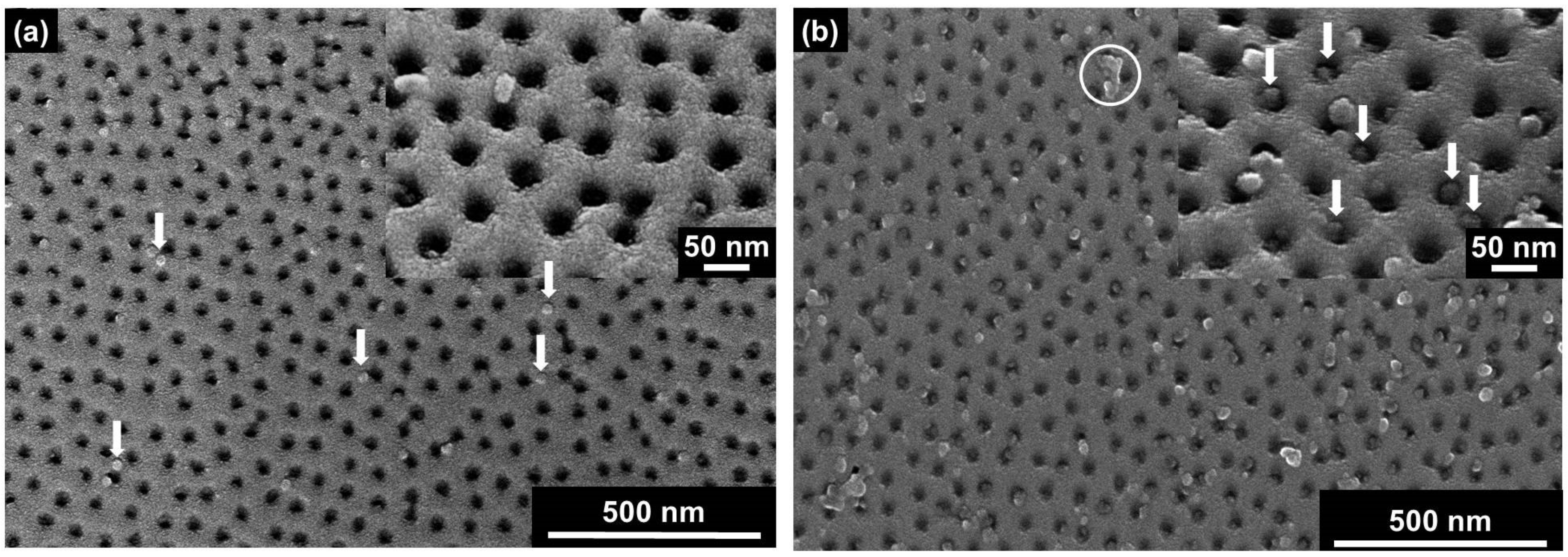
2.3. Direct Observation of TMV Nanodisc Incorporation by SEM
3. Materials and Methods
3.1. Preparation of the Solid State Membrane
3.2. Viral Nanodisc Preparation
3.3. Electrochemical Characterization of the Membrane and Incorporation of the Viral Nanodiscs
3.4. Electron Microscopy
3.5. Evaluation of SEM Images
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deubel, M.; von Freymann, G.; Wegener, M.; Pereira, S.; Busch, K.; Soukoulis, C.M. Direct laser writing of three-dimensional photonic-crystal templates for telecommunications. Nat. Mater. 2004, 3, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Tian, Y.; Jiang, L. Fundamental studies and practical applications of bio-inspired smart solid-state nanopores and nanochannels. Nano Today 2016, 11, 61–81. [Google Scholar] [CrossRef]
- Hou, X.; Guo, W.; Jiang, L. Biomimetic smart nanopores and nanochannels. Chem. Soc. Rev. 2011, 40, 2385–2401. [Google Scholar] [CrossRef] [PubMed]
- Carraretto, L.; Teardo, E.; Checchetto, V.; Finazzi, G.; Uozumi, N.; Szabo, I. Ion channels in plant bioenergetic organelles, chloroplasts and mitochondria: From molecular identification to function. Mol. Plant 2016, 9, 371–395. [Google Scholar] [CrossRef] [PubMed]
- Du, H.L.; Li, J.Y.; Zhang, J.; Su, G.; Li, X.Y.; Zhao, Y.L. Separation of hydrogen and nitrogen gases with porous graphene membrane. J. Phys. Chem. C 2011, 115, 23261–23266. [Google Scholar] [CrossRef]
- Yang, S.Y.; Yang, J.A.; Kim, E.S.; Jeon, G.; Oh, E.J.; Choi, K.Y.; Hahn, S.K.; Kim, J.K. Single-file diffusion of protein drugs through cylindrical nanochannels. ACS Nano 2010, 4, 3817–3822. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stein, D.; McMullan, C.; Branton, D.; Aziz, M.J.; Golovchenko, J.A. Ion-beam sculpting at nanometre length scales. Nature 2001, 412, 166–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.; Iqbal, S.M.; Stach, E.A.; King, A.H.; Zaluzec, N.J.; Bashir, R. Fabrication and characterization of solid-state nanopores using a field emission scanning electron microscope. Appl. Phys. Lett. 2006, 88, 103109. [Google Scholar] [CrossRef]
- Storm, A.J.; Chen, J.H.; Ling, X.S.; Zandbergen, H.W.; Dekker, C. Fabrication of solid-state nanopores with single-nanometre precision. Nat. Mater. 2003, 2, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Krapf, D.; Zandbergen, M.; Zandbergen, H.; Batson, P.E. Formation of nanopores in a SiN∕SiO2 membrane with an electron beam. Appl. Phys. Lett. 2005, 87, 113106. [Google Scholar] [CrossRef]
- Garaj, S.; Hubbard, W.; Reina, A.; Kong, J.; Branton, D.; Golovchenko, J.A. Graphene as a subnanometre trans-electrode membrane. Nature 2010, 467, 190–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchant, C.A.; Healy, K.; Wanunu, M.; Ray, V.; Peterman, N.; Bartel, J.; Fischbein, M.D.; Venta, K.; Luo, Z.; Johnson, A.T.C.; et al. DNA Translocation through Graphene Nanopores. Nano Lett. 2010, 10, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Park, S.R.; Ling, X.S. Lithography-Free Formation of Nanopores in Plastic Membranes Using Laser Heating. Nano Lett. 2006, 6, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, W.; Geng, H.; Hou, X.; Shuai, Z.; Jiang, L. Layer-by-layer removal of insulating few-layer mica flakes for asymmetric ultra-thin nanopore fabrication. Nano Res. 2012, 5, 99–108. [Google Scholar] [CrossRef]
- James, T.; Kalinin, Y.V.; Chan, C.-C.; Randhawa, J.S.; Gaevski, M.; Gracias, D.H. Voltage-Gated Ion Transport through Semiconducting Conical Nanopores Formed by Metal Nanoparticle-Assisted Plasma Etching. Nano Lett. 2012, 12, 3437–3442. [Google Scholar] [CrossRef] [PubMed]
- Apel, P.Y.; Korchev, Y.E.; Siwy, Z.; Spohr, R.; Yoshida, M. Diode-like single-ion track membrane prepared by electro-stopping. Nucl. Instrum. Methods Phys. Res. Sect. B 2001, 184, 337–346. [Google Scholar] [CrossRef]
- Kovalev, Y.S.; Levkovich, N.V.; Kuklin, A.I.; Apel’, P.Y. Surfactant aggregation in solutions applied for track etching and its possible effect on the pore shape in track membranes. Colloid J. 2009, 71, 634. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization and applications. Thin Solid Films 1997, 297, 192–201. [Google Scholar] [CrossRef]
- Poinern, G.E.J.; Ali, N.; Fawcett, D. Progress in Nano-Engineered Anodic Aluminum Oxide Membrane Development. Materials 2011, 4, 487–526. [Google Scholar] [CrossRef] [PubMed]
- Krismastuti, F.S.H.; Bayat, H.; Voelcker, N.H.; Schönherr, H. Real Time Monitoring of Layer-by-Layer Polyelectrolyte Deposition and Bacterial Enzyme Detection in Nanoporous Anodized Aluminum Oxide. Anal. Chem. 2015, 87, 3856–3863. [Google Scholar] [CrossRef] [PubMed]
- Butler, P.J.G. Self-assembly of tobacco mosaic virus: The role of an intermediate aggregate in generating both specificity and speed. Philos. Trans. R. Soc. Lond. Ser. B 1999, 354, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Shekhah, O.; Wang, H.; Kowarik, S.; Schreiber, F.; Paulus, M.; Tolan, M.; Sternemann, C.; Evers, F.; Zacher, D.; Fischer, R.A.; et al. Step-by-step route for the synthesis of metal-organic frameworks. J. Amer. Chem. Soc. 2007, 129, 15118–15119. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; Li, G.M.; Li, H.L. Selective binding and removal of guests in a microporous metal-organic framework. Nature 1995, 378, 703–706. [Google Scholar] [CrossRef]
- Azucena, C.; Eber, F.J.; Trouillet, V.; Hirtz, M.; Heissler, S.; Franzreb, M.; Fuchs, H.; Wege, C.; Gliemann, H. New Approaches for Bottom-Up Assembly of Tobacco Mosaic Virus-Derived Nucleoprotein Tubes on Defined Patterns on Silica- and Polymer-Based Substrates. Langmuir 2012, 28, 14867–14877. [Google Scholar] [CrossRef] [PubMed]
- Ladnorg, T.; Welle, A.; Heisser, S.; Woell, C.; Gliemann, H. Site-selective growth of surface-anchored metal-organic frameworks on self-assembled monolayer patterns prepared by AFM nanografting. Beilstein J. Nanotechnol. 2013, 4, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Munuera, C.; Shekhah, O.; Wang, H.; Woll, C.; Ocal, C. The controlled growth of oriented metal-organic frameworks on functionalized surfaces as followed by scanning force microscopy. Phys. Chem. Chem. Phys. 2008, 10, 7257–7261. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-M.; Akin, D.; Xuan, Y.; Peide, D.Y.; Guo, P.; Bashir, R. Capture and alignment of phi29 viral particles in sub-40 nanometer porous alumina membranes. Biomed. Microdevices 2009, 11, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.R.; Scott, A.; Rotem, D.; Mehta, K.K.; Bayley, H.; Dekker, C. Hybrid pore formation by directed insertion of α-haemolysin into solid-state nanopores. Nat. Nanotechnol. 2010, 5, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ainsa, S.; Misiunas, K.; Thacker, V.V.; Hemmig, E.A.; Keyser, U.F. Voltage-dependent properties of DNA origami nanopores. Nano Lett. 2014, 14, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Seidenstücker, A. Physikaliche Voraussetzungen für die Realisierung einer Bio-Anorganischen Hybridmembran Mit Uniformen Nanoporen. Ph.D. Thesis, University of Ulm, Ulm, Germany, 2015. [Google Scholar]
- Altintoprak, K.; Seidenstücker, A.; Krolla-Sidenstein, P.; Plettl, A.; Jeske, H.; Gliemann, H.; Wege, C. RNA-stabilized protein nanorings: High-precision adapters for biohybrid design. Bioinspired Biomim. Nanobiomater. 2017, 6, 208–223. [Google Scholar] [CrossRef]
- Seidenstücker, A.; Beirle, S.; Enderle, F.; Ziemann, P.; Marti, O.; Plettl, A. Nanoporous silicon nitride based membranes of controlled pore size, shape and areal density as well as their electrophoretic and molecular filtering characterization. Beilstein J. Nanotechnol. 2018. accepted for publication. [Google Scholar]
- Protein Interfaces, Surfaces and Assemblies Service PISA at the European Bioinformatics Institute. Available online: http://www.ebi.ac.uk/pdbe/prot_int/pistart.html (accessed on 12 February 2018).
- Krissinel, E.; Henrick, K. Inference of Macromolecular Assemblies from Crystalline State. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 12 April 2018).
- Lan, W.-J.; Holden, D.A.; Zhang, B.; White, H.S. Nanoparticle transport in conical-shaped nanopores. Anal. Chem. 2011, 83, 3840–3847. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, H.; Wu, L.; Xie, X.; Kong, J.; Ye, X.; Liu, L. Voltage-driven translocation of DNA through a high throughput conical solid-state nanopore. PLoS ONE 2012, 7, e46014. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chen, Y.; Zhou, Q.; Wang, R.; Xia, B.; Ma, D.; Luo, K.; Liu, Q. Translocation of rigid rod-shaped virus through various solid-state nanopores. Anal. Chem. 2016, 88, 2502–2510. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, L.; Ni, Z.; Chen, Y. Detecting a single molecule using a micropore-nanopore hybrid chip. Nanoscale Res. Lett. 2013, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Spatz, J.P.; Mössmer, S.; Hartmann, C.; Möller, M.; Herzog, T.; Krieger, M.; Boyen, H.-G.; Ziemann, P.; Kabius, B. Ordered deposition of inorganic clusters from micellar block copolymer films. Langmuir 2000, 16, 407–415. [Google Scholar] [CrossRef]
- Härtling, T.; Seidenstücker, A.; Olk, P.; Plettl, A.; Ziemann, P.; Eng, L.M. Controlled photochemical particle growth in two-dimensional ordered metal nanoparticle arrays. Nanotechnology 2010, 21, 145309. [Google Scholar] [CrossRef] [PubMed]
- Brieger, S.; Dubbers, O.; Fricker, S.; Manzke, A.; Pfahler, C.; Plettl, A.; Ziemann, P. An approach for the fabrication of hexagonally ordered arrays of cylindrical nanoholes in crystalline and amorphous silicon based on the self-organization of polymer micelles. Nanotechnology 2006, 17, 4991. [Google Scholar] [CrossRef]
- Goelet, P.; Lomonossoff, G.P.; Butler, P.J.; Akam, M.E.; Gait, M.J.; Karn, J. Nucleotide sequence of tobacco mosaic virus RNA. Proc. Natl. Acad. Sci. USA 1982, 79, 5818–5822. [Google Scholar] [CrossRef] [PubMed]
- Kadri, A.; Maiss, E.; Amsharov, N.; Bittner, A.M.; Balci, S.; Kern, K.; Jeske, H.; Wege, C. Engineered Tobacco mosaic virus mutants with distinct physical characteristics in planta and enhanced metallization properties. Virus Res. 2011, 157, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F.C.; Eber, F.J.; Eiben, S.; Müller, A.; Jeske, H.; Spatz, J.P.; Wege, C. TMV nanorods with programmed longitudinal domains of differently addressable coat proteins. Nanoscale 2013, 5, 3808–3816. [Google Scholar] [CrossRef] [PubMed]
- Gooding, G.V.; Hebert, T.T. A simple technique for purification of tobacco mosaic virus in large quantities. Phytopathology 1967, 57, 1285. [Google Scholar] [PubMed]
- Fraenkel-Conrat, H. Degradation of tobacco mosaic virus with acetic acid. Virology 1957, 4, 1–4. [Google Scholar] [CrossRef]
- Eiben, S.; Stitz, N.; Eber, F.; Wagner, J.; Atanasova, P.; Bill, J.; Wege, C.; Jeske, H. Tailoring the surface properties of tobacco mosaic virions by the integration of bacterially expressed mutant coat protein. Virus Res. 2014, 180, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Durham, A.C.; Klug, A. Polymerization of tobacco mosaic virus protein and its control. Nat. New Biol. 1971, 229, 42–46. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farajollahi, F.; Seidenstücker, A.; Altintoprak, K.; Walther, P.; Ziemann, P.; Plettl, A.; Marti, O.; Wege, C.; Gliemann, H. Electrochemically-Driven Insertion of Biological Nanodiscs into Solid State Membrane Pores as a Basis for “Pore-In-Pore” Membranes. Nanomaterials 2018, 8, 237. https://doi.org/10.3390/nano8040237
Farajollahi F, Seidenstücker A, Altintoprak K, Walther P, Ziemann P, Plettl A, Marti O, Wege C, Gliemann H. Electrochemically-Driven Insertion of Biological Nanodiscs into Solid State Membrane Pores as a Basis for “Pore-In-Pore” Membranes. Nanomaterials. 2018; 8(4):237. https://doi.org/10.3390/nano8040237
Chicago/Turabian StyleFarajollahi, Farid, Axel Seidenstücker, Klara Altintoprak, Paul Walther, Paul Ziemann, Alfred Plettl, Othmar Marti, Christina Wege, and Hartmut Gliemann. 2018. "Electrochemically-Driven Insertion of Biological Nanodiscs into Solid State Membrane Pores as a Basis for “Pore-In-Pore” Membranes" Nanomaterials 8, no. 4: 237. https://doi.org/10.3390/nano8040237





