Functionalizing Graphene Oxide with Alkylamine by Gamma-ray Irradiation Method
Abstract
:1. Introduction
2. Results and Discussion
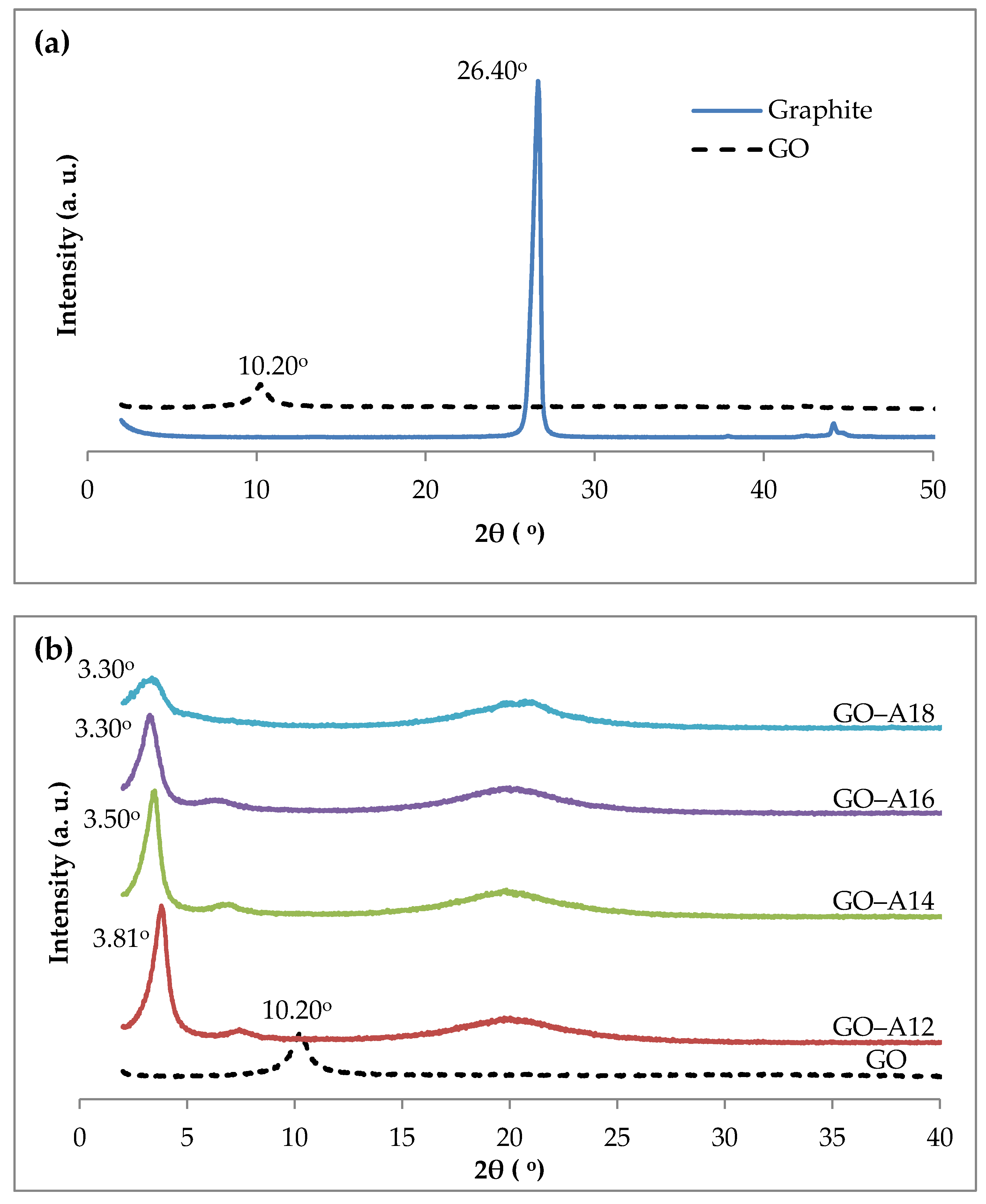
2.1. X-ray Diffraction (XRD)
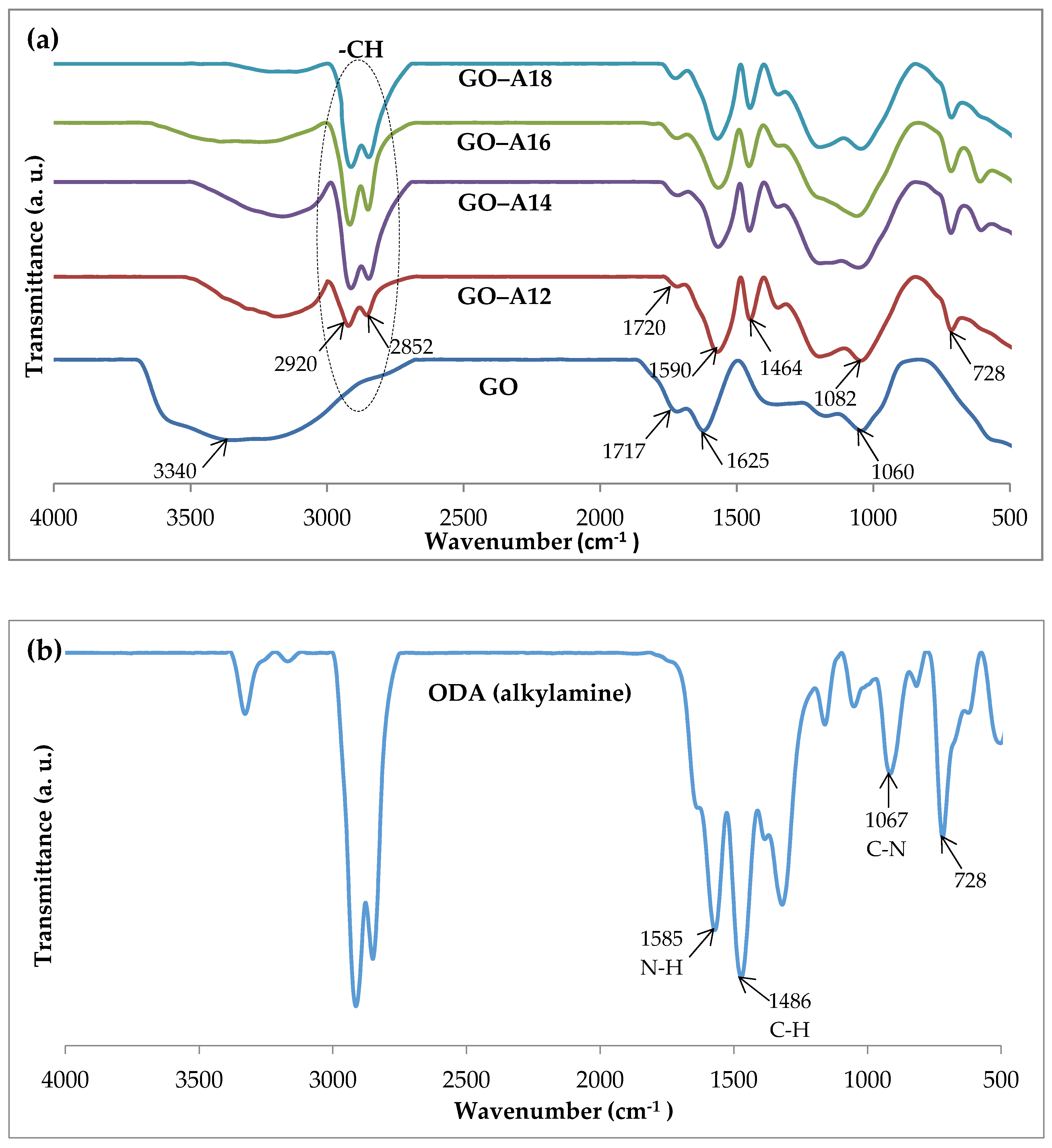
2.2. Fourier Transform Infrared (FTIR)
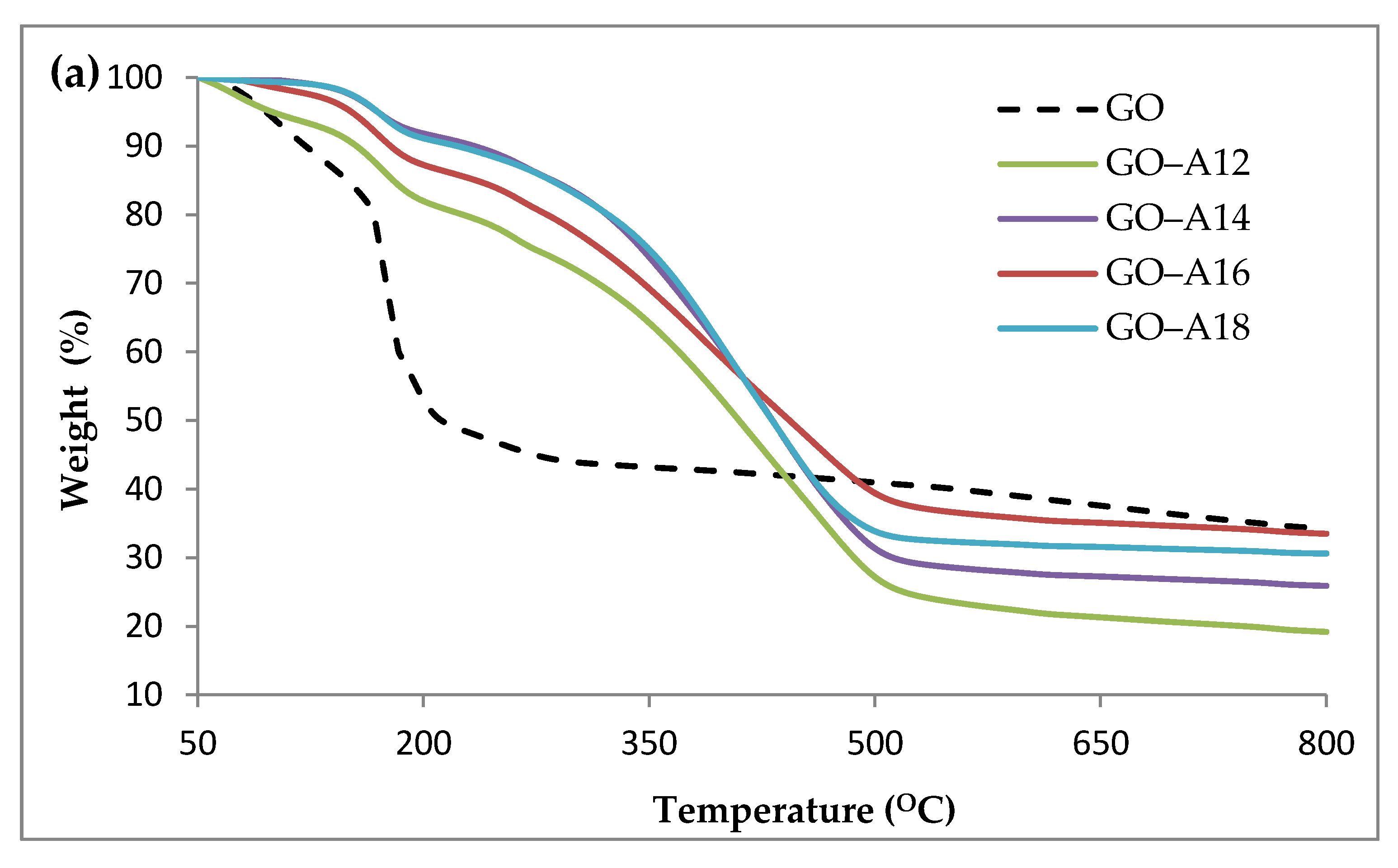
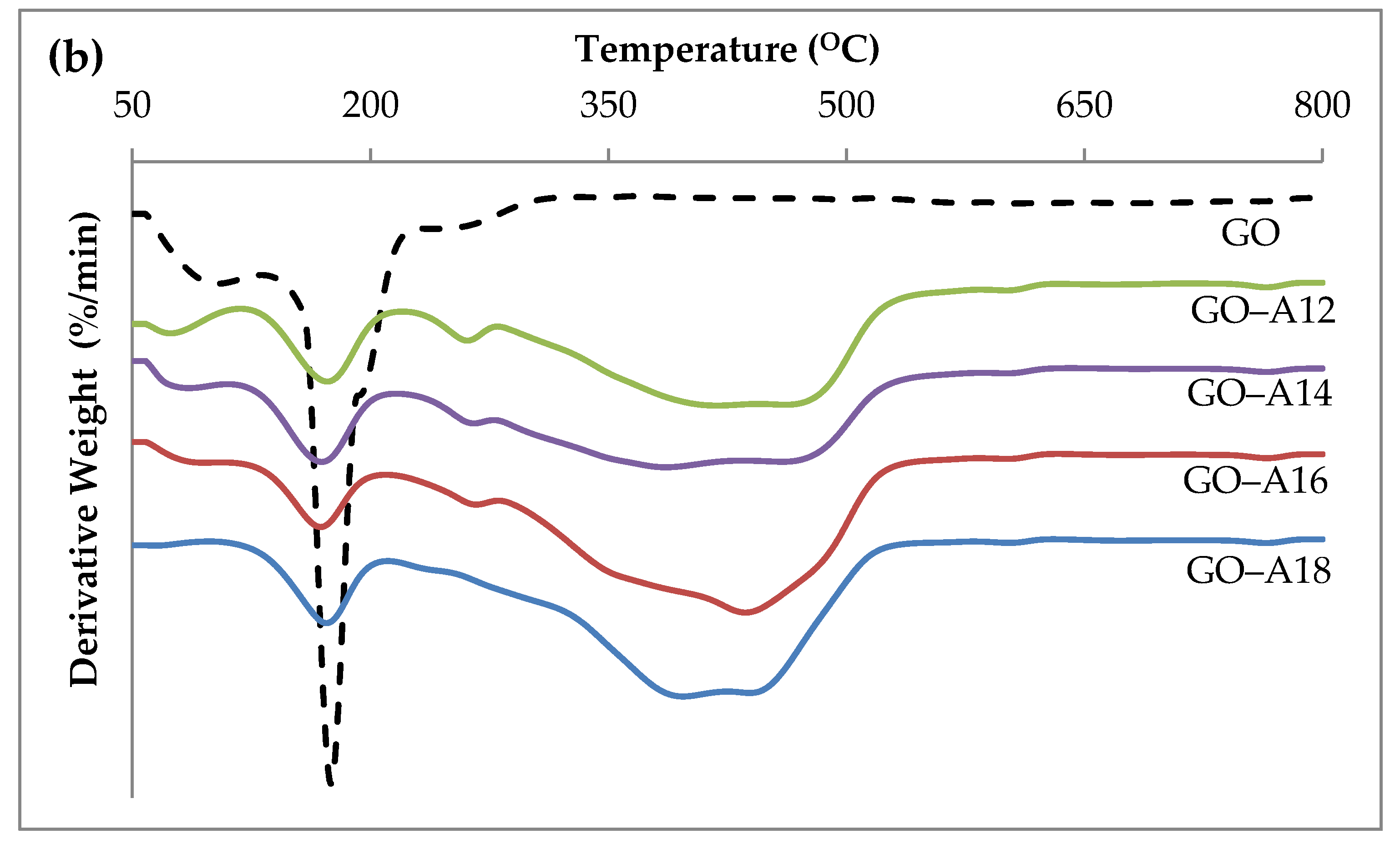
2.3. Thermogravimetric Analysis (TGA)
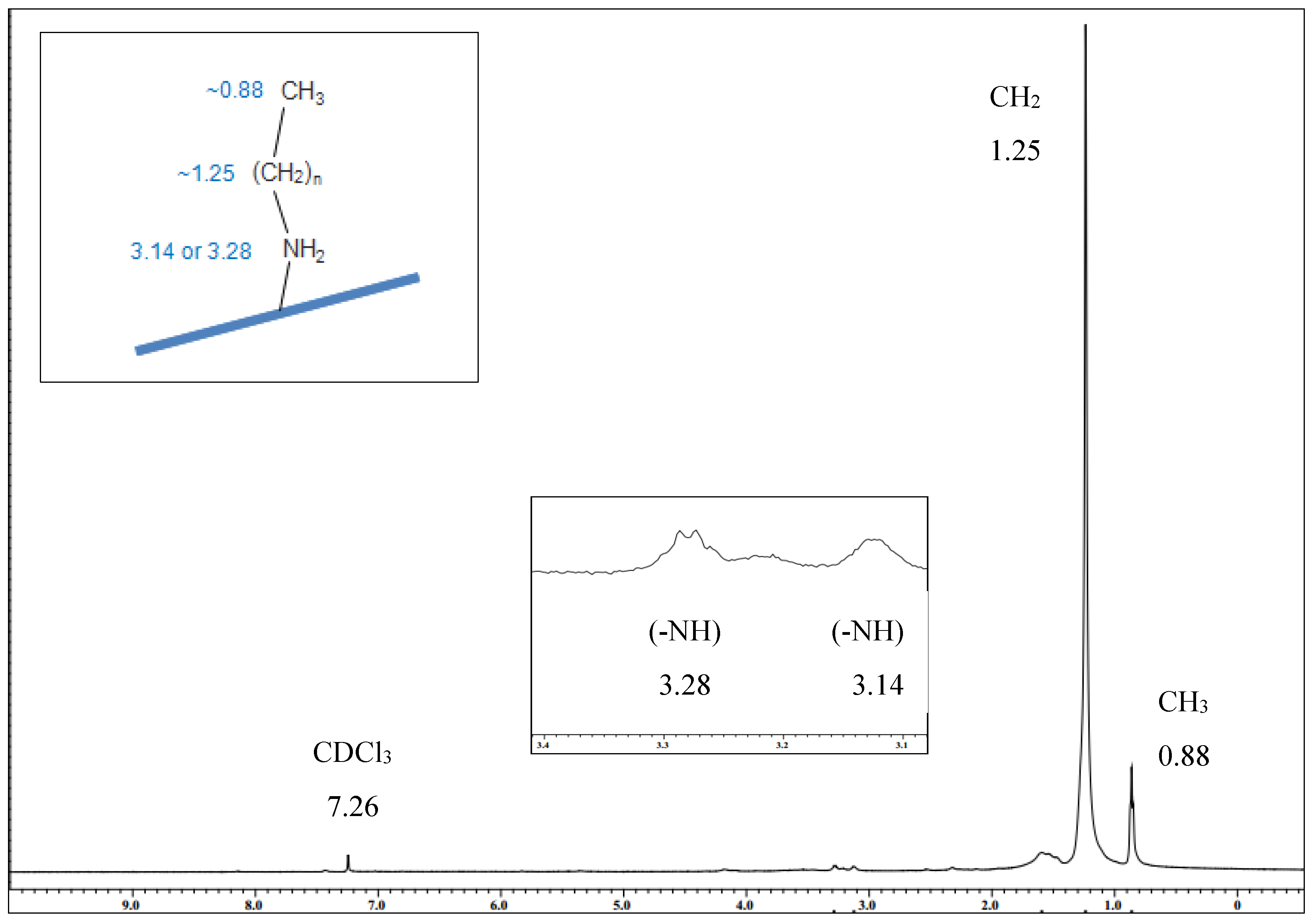
2.4. Nuclear Magnetic Resonance (NMR)
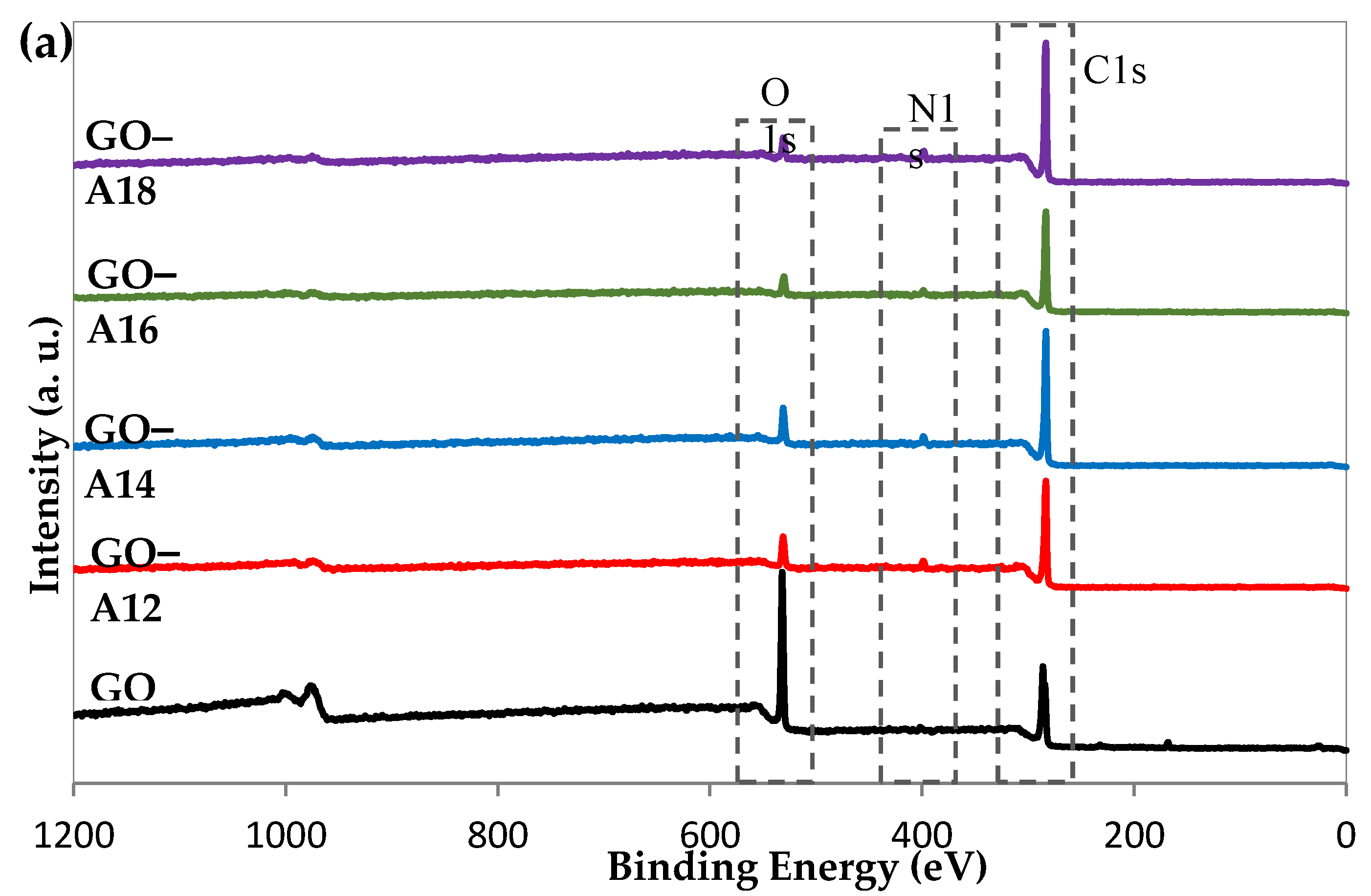
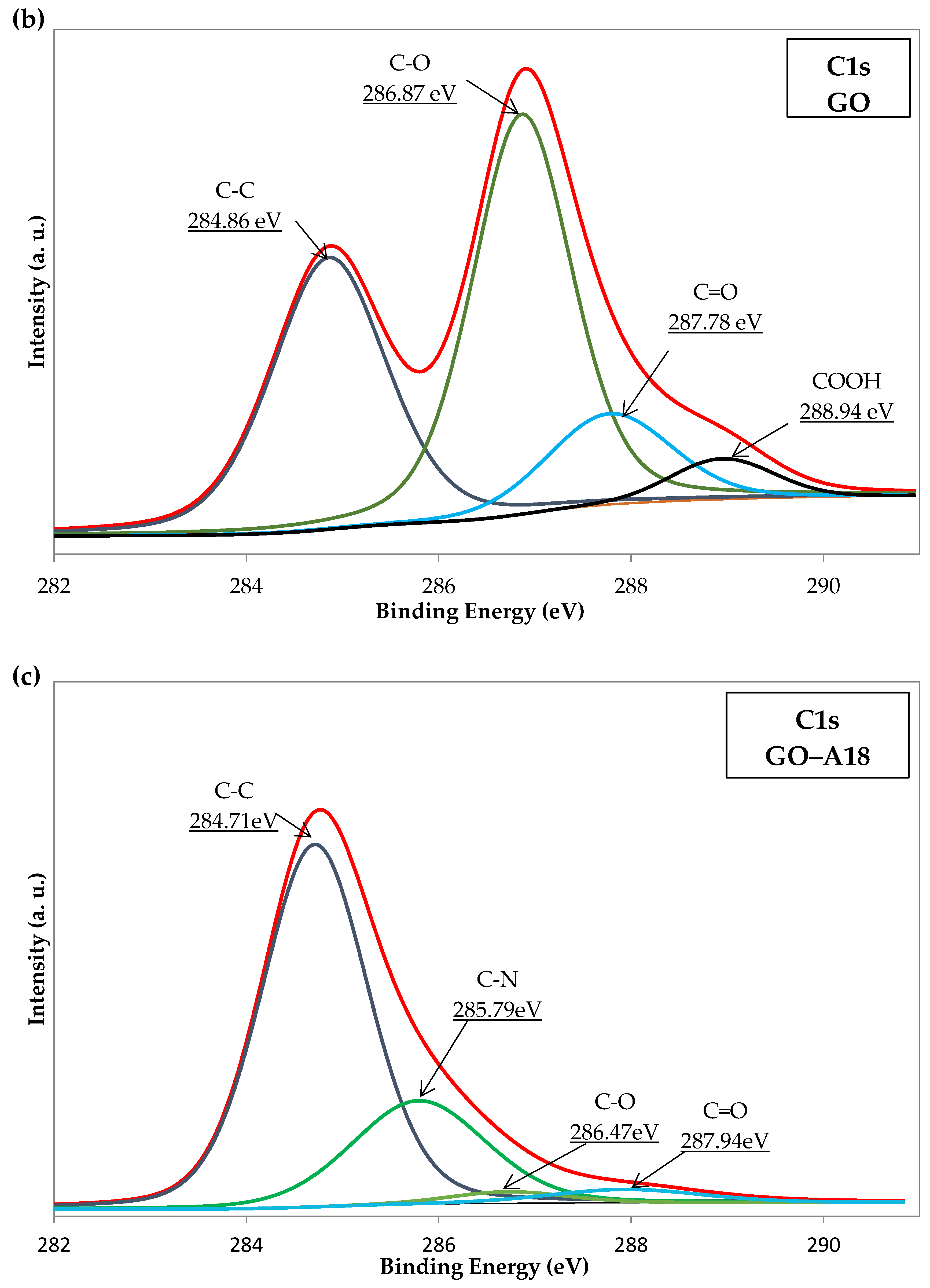
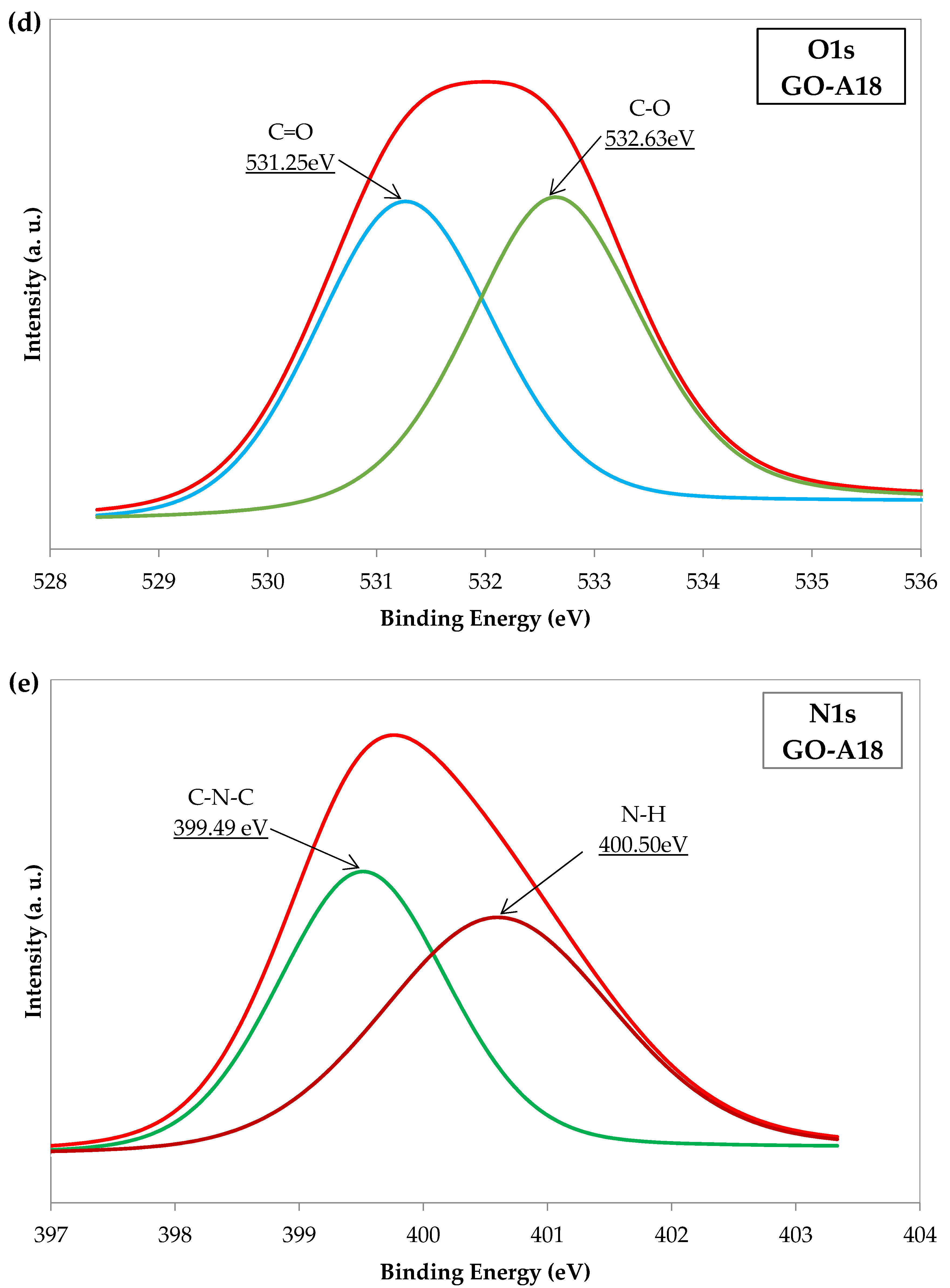
2.5. X-ray Photoelectron Spectroscopy
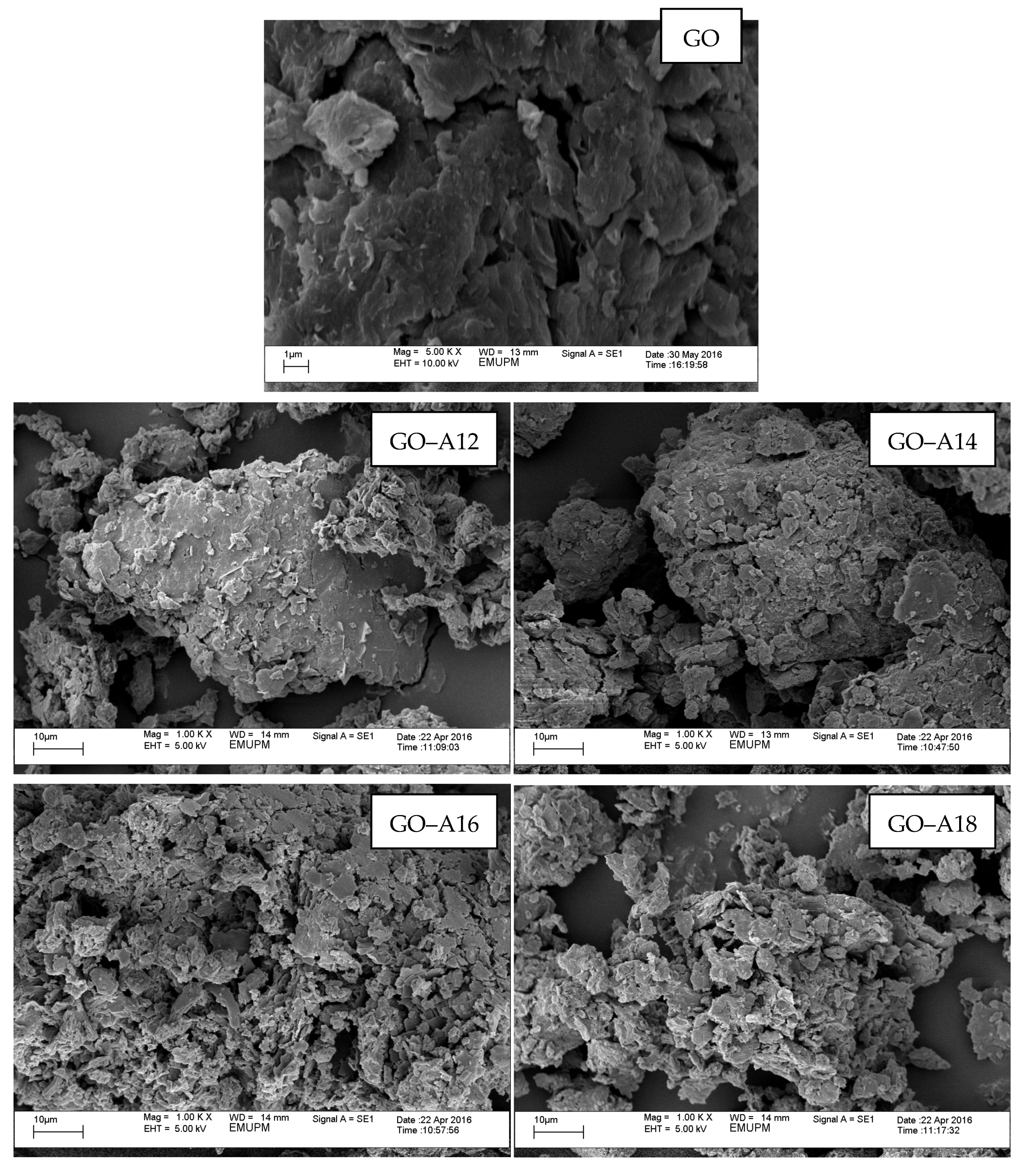
2.6. Scanning Electron Microscopy (SEM)
3. Materials and Methods
3.1. Materials
3.2. Preparation of GO
3.3. Functionalization of GO
3.4. Characterizations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nanotechnol. 2009, 4, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of Graphite Oxide Revisited. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate-treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Li, W.; Tang, X.-Z.; Zhang, H.-B.; Jiang, Z.-G.; Yu, Z.-Z.; Du, X.-S.; Mai, Y.-W. Simultaneous surface functionalization and reduction of graphene oxide with octadecylamine for electrically conductive polystyrene composites. Carbon 2011, 49, 4724–4730. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Wu, P. Alkyl-functionalized graphene nanosheets with improved lipophilicity. Carbon 2010, 48, 1683–1685. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Electrically Conductive “Alkylated” Graphene Paper via Chemical Reduction of Amine-Functionalized Graphene Oxide Paper. Adv. Mater. 2010, 22, 892–896. [Google Scholar] [CrossRef]
- Mei, Q.; Zhang, K.; Guan, G.; Liu, B.; Wang, S.; Zhang, Z. Highly efficient photoluminescent graphene oxide with tunable surface properties. Chem. Commun. 2010, 46, 7319–7321. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Li, Z.; Zhang, H.; Hong, H.; Rebeyev, N.; Ye, Y.; Ma, Y. Amine modified graphene as reversed-dispersive solid phase extraction materials combined with liquid chromatography–tandem mass spectrometry for pesticide multi-residue analysis in oil crops. J. Chromatogr. A 2013, 1286, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Song, H.O. Supramolecular graphene oxide-alkylamine hybrid materials: Variation of dispersibility and improvement of thermal stability. New J. Chem. 2012, 36, 1733–1738. [Google Scholar] [CrossRef]
- Shanmugharaj, A.M.; Yoon, J.H.; Yang, W.J.; Ryu, S.H. Synthesis, characterization, and surface wettability properties of amine functionalized graphene oxide films with varying amine chain lengths. J. Colloid Interface Sci. 2013, 401, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Kumar, N.; Panda, K.; Melvin, A.A.; Joshi, S.; Dash, S.; Tyagi, A.K. Effective Noncovalent Functionalization of Poly(ethylene glycol) to Reduced Graphene Oxide Nanosheets through γ-Radiolysis for Enhanced Lubrication. J. Phys. Chem. C 2016, 120, 2139–2148. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Li, L.; Ma, H.; Yu, M.; Li, J. γ-ray irradiation effects on graphene oxide in an ethylenediamine aqueous solution. Radiat. Phys. Chem. 2014, 94, 80–83. [Google Scholar] [CrossRef]
- Dumée, L.F.; Feng, C.; He, L.; Yi, Z.; She, F.; Peng, Z.; Gao, W.; Banos, C.; Davies, J.B.; Huynh, C.; et al. Single step preparation of meso-porous and reduced graphene oxide by gamma-ray irradiation in gaseous phase. Carbon 2014, 70, 313–318. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Wang, Z.; Xie, S.; Zhang, Y.; Shen, Y.; Yu, M.; Deng, B.; Huang, Q.; Fan, C.; et al. Radiation induced reduction: An effective and clean route to synthesize functionalized graphene. J. Mater. Chem. 2012, 22, 7775–7781. [Google Scholar] [CrossRef]
- Safibonab, B.; Reyhani, A.; Nozad Golikand, A.; Mortazavi, S.Z.; Mirershadi, S.; Ghoranneviss, M. Improving the surface properties of multi-walled carbon nanotubes after irradiation with gamma rays. Appl. Surf. Sci. 2011, 258, 766–773. [Google Scholar] [CrossRef]
- Miao, M.; Hawkins, S.C.; Cai, J.Y.; Gengenbach, T.R.; Knott, R.; Huynh, C.P. Effect of gamma-irradiation on the mechanical properties of carbon nanotube yarns. Carbon 2011, 49, 4940–4947. [Google Scholar] [CrossRef]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hofmann, U.; König, E. Untersuchungen über Graphitoxyd. Z. Anorg. Allg. Chem. 1937, 234, 311–336. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-Layer Assembly of Ultrathin Composite Films from Micron-Sized Graphite Oxide Sheets and Polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.; Li, C.; Shi, G. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 2015, 81, 826–834. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Z.; Wang, S.; Zhang, Y. Preparation and properties of octadecylamine modified graphene oxide/styrene-butadiene rubber composites through an improved melt compounding method. J. Appl. Polym. Sci. 2016, 133, 42907. [Google Scholar] [CrossRef]
- Gao, W. The Chemistry of Graphene Oxide. In Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications; Gao, W., Ed.; Springer: Cham, Switzerland, 2015; pp. 61–95. [Google Scholar]
- Li, Z.; Zhang, W.; Luo, Y.; Yang, J.; Hou, J.G. How Graphene Is Cut upon Oxidation? J. Am. Chem. Soc. 2009, 131, 6320–6321. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tao, J.; Ezzeddine, A.; Mahfouz, R.; Al-Shahrani, A.; Alabedi, G.; Khashab, N. Superior Performance Nanocomposites from Uniformly Dispersed Octadecylamine Functionalized Multi-Walled Carbon Nanotubes. C J. Carbon Res. 2015, 1, 58–76. [Google Scholar] [CrossRef]
- Yan, J.-L.; Chen, G.-J.; Cao, J.; Yang, W.; Xie, B.-H.; Yang, M.-B. Functionalized graphene oxide with ethylenediamine and 1,6-hexanediamine. New Carbon Mater. 2012, 27, 370–376. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Dumée, L.F.; Feng, C.; He, L.; Allioux, F.-M.; Yi, Z.; Gao, W.; Banos, C.; Davies, J.B.; Kong, L. Tuning the grade of graphene: Gamma ray irradiation of free-standing graphene oxide films in gaseous phase. Appl. Surf. Sci. 2014, 322, 126–135. [Google Scholar] [CrossRef]
- Ansón-Casaos, A.; Puértolas, J.A.; Pascual, F.J.; Hernández-Ferrer, J.; Castell, P.; Benito, A.M.; Maser, W.K.; Martínez, M.T. The effect of gamma-irradiation on few-layered graphene materials. Appl. Surf. Sci. 2014, 301, 264–272. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing Sugar: New Functional Molecules for the Green Synthesis of Graphene Nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Mungse, H.P.; Khatri, O.P. Dispersion of alkylated graphene in organic solvents and its potential for lubrication applications. J. Mater. Chem. 2012, 22, 21032–21039. [Google Scholar] [CrossRef]
- Sobon, G.; Sotor, J.; Jagiello, J.; Kozinski, R.; Zdrojek, M.; Holdynski, M.; Paletko, P.; Boguslawski, J.; Lipinska, L.; Abramski, K.M. Graphene Oxide vs. Reduced Graphene Oxide as saturable absorbers for Er-doped passively mode-locked fiber laser. Opt. Express 2012, 20, 19463–19473. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.H.; Shanmugharaj, A.M. Influence of long-chain alkylamine-modified graphene oxide on the crystallization, mechanical and electrical properties of isotactic polypropylene nanocomposites. Chem. Eng. J. 2014, 244, 552–560. [Google Scholar] [CrossRef]
- Ma, W.-S.; Li, J.; Deng, B.-J.; Zhao, X.-S. Preparation and characterization of long-chain alkyl silane-functionalized graphene film. J. Mater. Sci. 2013, 48, 156–161. [Google Scholar] [CrossRef]
- Bao, H.; Pan, Y.; Ping, Y.; Sahoo, N.G.; Wu, T.; Li, L.; Li, J.; Gan, L.H. Chitosan-Functionalized Graphene Oxide as a Nanocarrier for Drug and Gene Delivery. Small 2011, 7, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Carreira, L.; Mendes, L.; Ribeiro, M.; Sebastião, P. Organically-Expanded Graphite/Octadecylamine: Structural, Thermal and Relaxation Evaluation. Mater. Sci. Appl. 2013, 4, 281–286. [Google Scholar] [CrossRef]
| Method | Year | Oxidants | Solvent | Reference |
|---|---|---|---|---|
| Brodie | 1859 | KClO3 | HNO3 | [19] |
| Staudenmaier | 1898 | KClO3 | HNO3 (fuming), H2SO4 | [20] |
| Hofmann | 1937 | KClO3 | HNO3, H2SO4 | [21] |
| Hummers | 1958 | NaNO3, KMnO4 | H2SO4 | [22] |
| Modified Hummers | 1999 | K2S2O8, P2O5, KMnO4 | H2SO4 | [23] |
| 2010 | KMnO4 | H2SO4, H3PO4 | [24] |
| Sample | O/C Ratio | N/O Ratio |
|---|---|---|
| GO | 2.34 | 0.08 |
| GO–A12 | 0.51 | 0.91 |
| GO–A14 | 0.48 | 0.74 |
| GO–A16 | 0.36 | 0.74 |
| GO–A18 | 0.30 | 1.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad Daud, N.; Chieng, B.W.; Ibrahim, N.A.; Talib, Z.A.; Muhamad, E.N.; Abidin, Z.Z. Functionalizing Graphene Oxide with Alkylamine by Gamma-ray Irradiation Method. Nanomaterials 2017, 7, 135. https://doi.org/10.3390/nano7060135
Ahmad Daud N, Chieng BW, Ibrahim NA, Talib ZA, Muhamad EN, Abidin ZZ. Functionalizing Graphene Oxide with Alkylamine by Gamma-ray Irradiation Method. Nanomaterials. 2017; 7(6):135. https://doi.org/10.3390/nano7060135
Chicago/Turabian StyleAhmad Daud, Noraniza, Buong Woei Chieng, Nor Azowa Ibrahim, Zainal Abidin Talib, Ernee Noryana Muhamad, and Zurina Zainal Abidin. 2017. "Functionalizing Graphene Oxide with Alkylamine by Gamma-ray Irradiation Method" Nanomaterials 7, no. 6: 135. https://doi.org/10.3390/nano7060135
APA StyleAhmad Daud, N., Chieng, B. W., Ibrahim, N. A., Talib, Z. A., Muhamad, E. N., & Abidin, Z. Z. (2017). Functionalizing Graphene Oxide with Alkylamine by Gamma-ray Irradiation Method. Nanomaterials, 7(6), 135. https://doi.org/10.3390/nano7060135






