Glutathione-Mediated Cu(I)/Cu(II) Complexes: Valence-Dependent Effects on Clearance and In Vivo Imaging Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
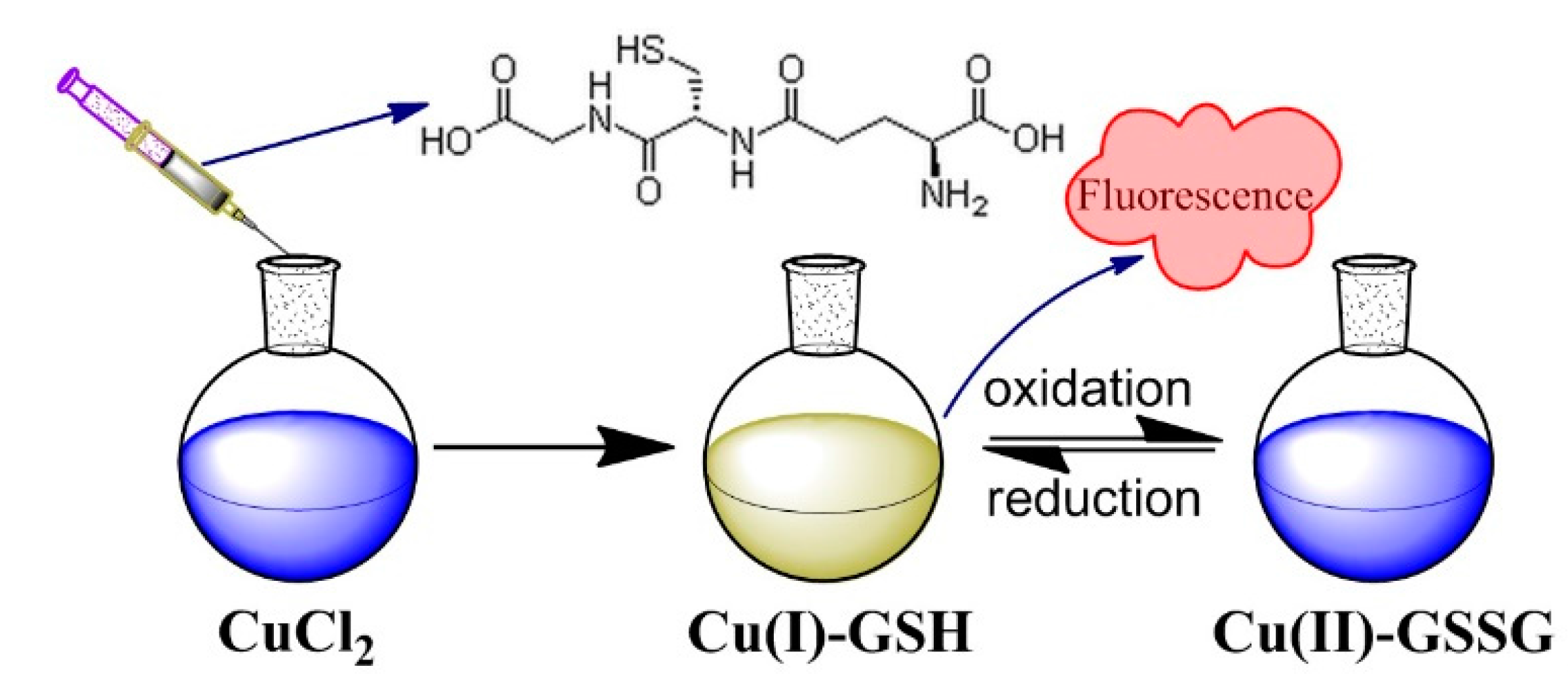
2.2. Synthesis of Cu(I)-GSH and Cu(II)-GSSG Complexes
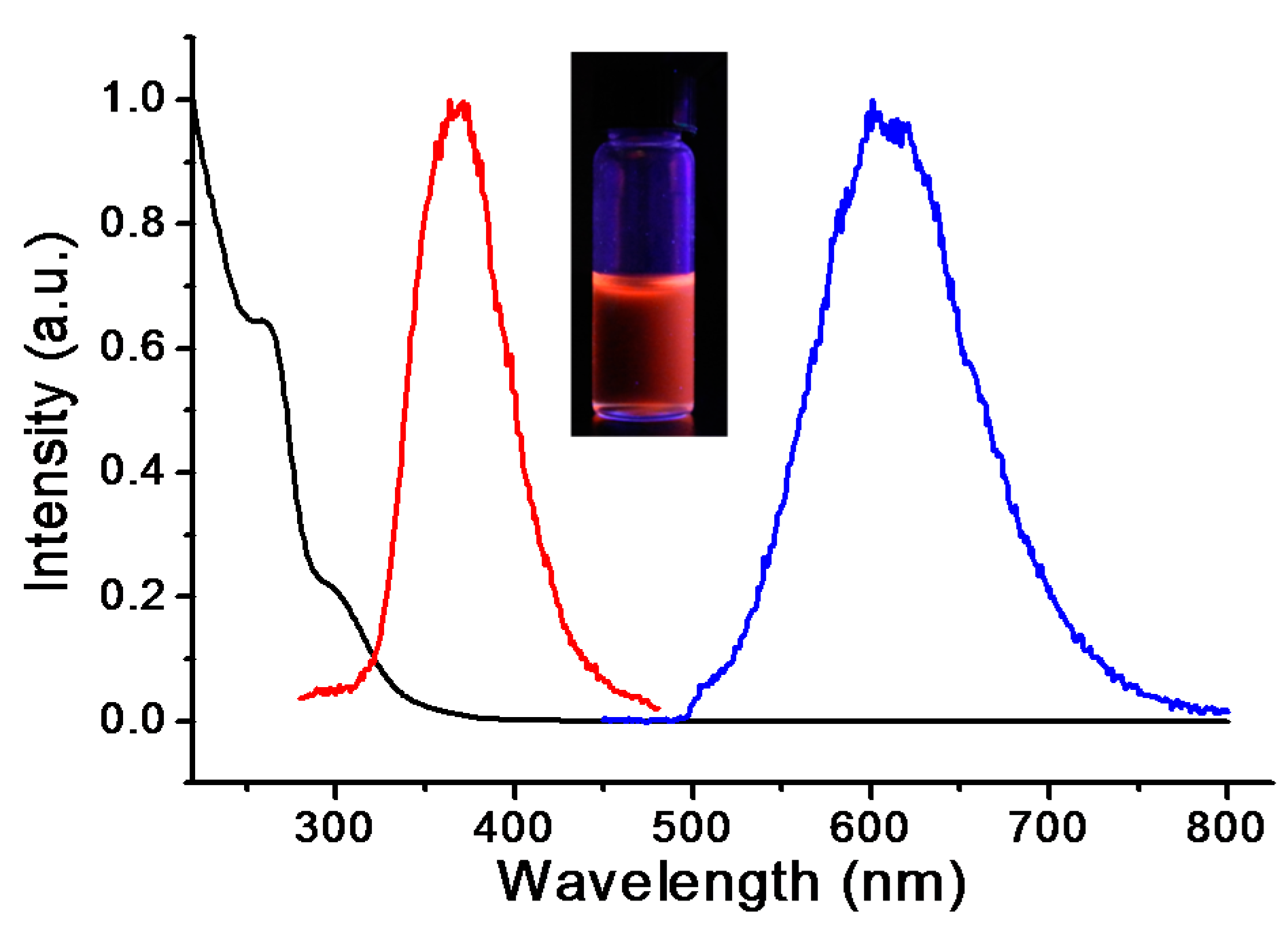
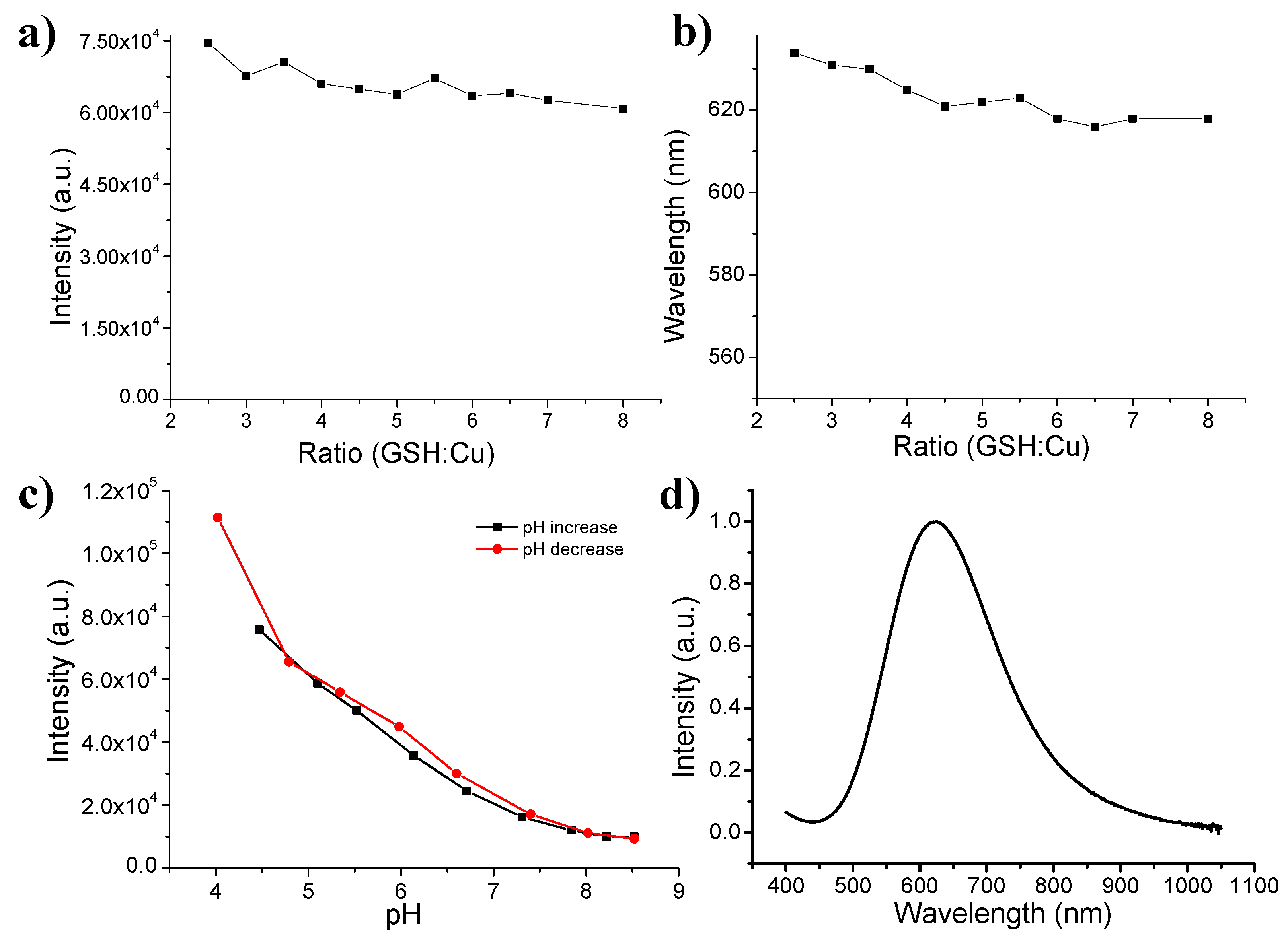
2.3. In Vitro Study of Cu(I)-GSH Complex
2.4. Experimental Animals
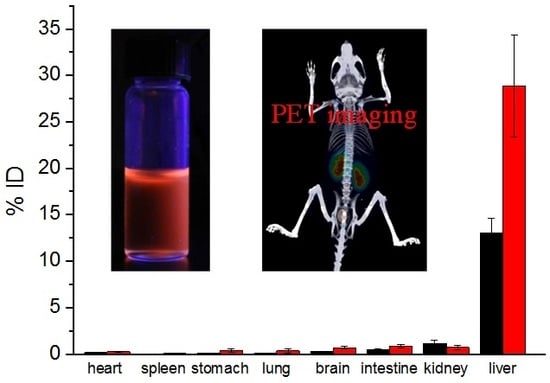
2.5. Biodistribution Studies of Cu(I)-GSH and Cu(II)-GSSG Complex
2.6. Instrumentation
2.7. ICP-MS Measurement of Urine, Blood, and Tissues
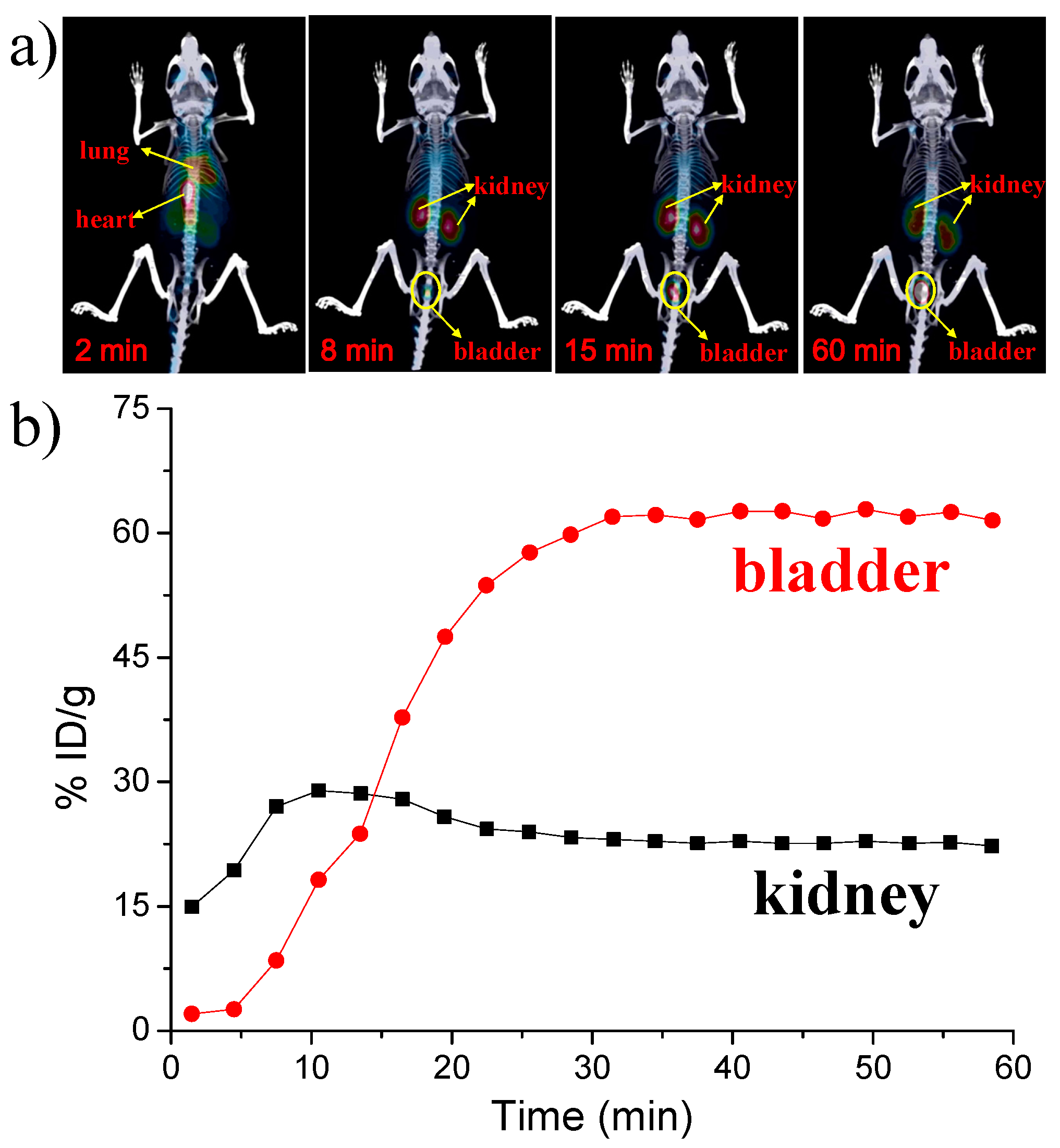
2.8. Micro Positron Emission Tomography (PET)-Computed Tomography (CT) Imaging
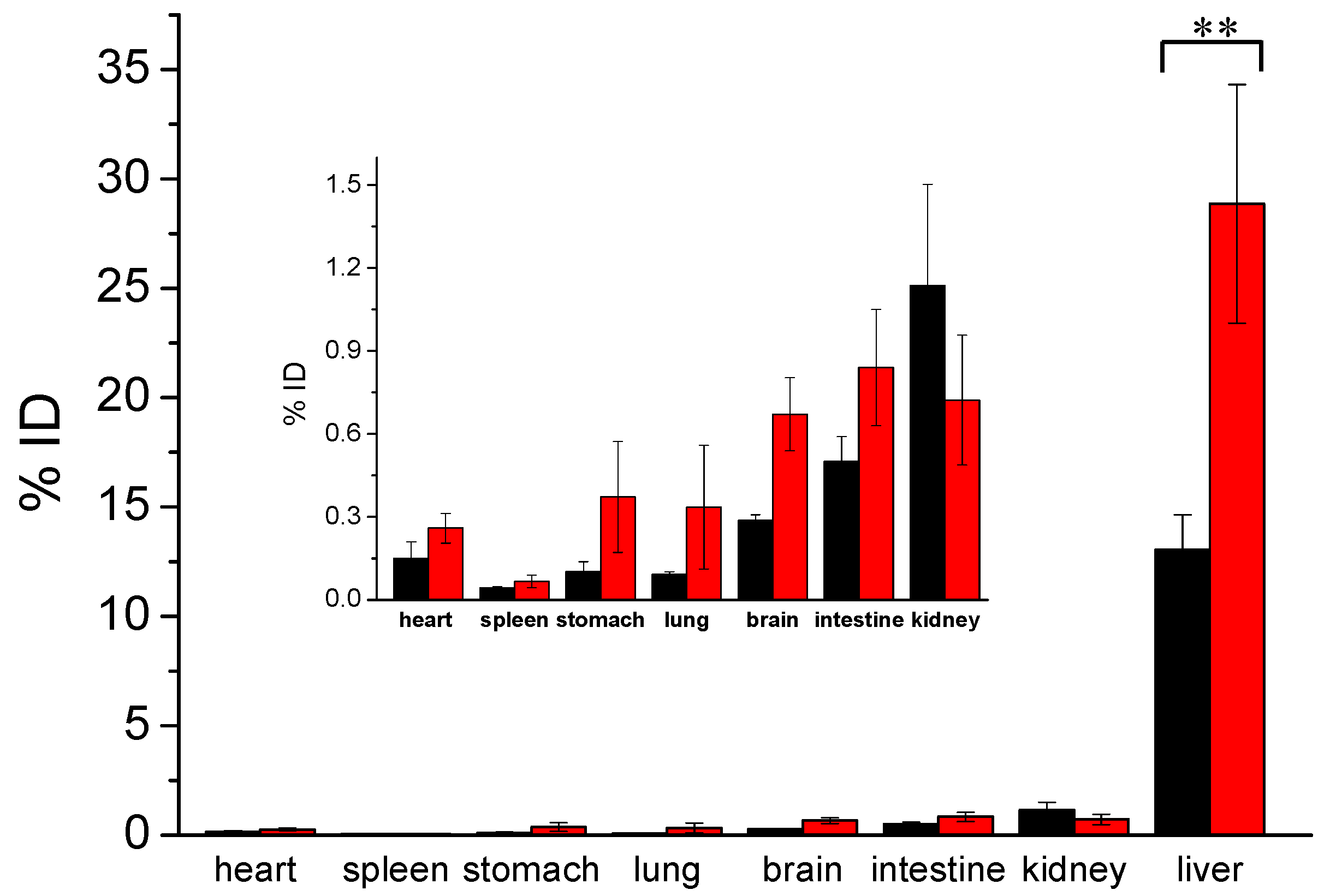
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, Z.L.; Al Zaki, A.; Hui, J.Z.; Muzykantov, V.R.; Tsourkas, A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 2012, 338, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Youns, M.; Hoheisel, J.D.; Efferth, T. Therapeutic and diagnostic applications of nanoparticles. Curr. Drug Targets 2011, 12, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, A.; Chandran, P.; Khan, S.S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.H.; Cui, Y.Y.; Levenson, R.M.; Chung, L.W.K.; Nie, S.M. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat. Biotechnol. 2004, 22, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Gu, L.; Maltzahn von, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 2008, 3, 703–717. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Dickson, R.M.; Yu, J. Developing luminescent silver nanodots for biological applications. Chem. Soc. Rev. 2012, 41, 1867–1891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, S.; Nienhaus, G.U. Ultra-small fluorescent metal nanoclusters: Synthesis and biological applications. Nano Today 2011, 6, 401–418. [Google Scholar]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Meng, H.; Wang, X.; Lin, S.; Ji, Z.; Zhang, H. Nanomaterial toxicity testing in the 21st century: Use of a predictive toxicological approach and high-throughput screening. Acc. Chem. Res. 2013, 46, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ye, D.; Wu, M.; Chen, H.; Zhang, L.; Shi, J.; Wang, L. Break-up of two-dimensional MnO2 nanosheets promotes ultrasensitive pH-triggered theranostics of cancer. Adv. Mater. 2014, 26, 7019–7026. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, C.; Cai, X.J. Glutathione-triggered luminescent silver nanoparticle: A urinary clearable nanoparticle for potential clinical practice. Colloids Surf. B Biointerfaces 2015, 135, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, S.; Zhou, C.; Hao, G.Y.; Liu, J.B.; Ramezani, S.; Yu, M.X.; Sun, X.K.; Zheng, J. Renal clearance and degradation of glutathione-coated copper nanoparticles. Bioconj. Chem. 2015, 26, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.A.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Ntziachristos, V.; Bremer, C.; Weissleder, R. Fluorescence imaging with near-infrared light: New technological advances that enable in vivo molecular imaging. Eur. Radiol. 2003, 13, 195–208. [Google Scholar] [PubMed]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R. A clearer vision for in vivo imaging. Nat. Biotechnol. 2001, 19, 316–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Hu, X.X.; Zhang, X.B. Dye-doped fluorescent silica nanoparticles for live cell and in vivo bioimaging. Nanomaterials 2016, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Bonham, M.; O’Connor, J.M.; Hannigan, B.M.; Strain, J.J. The immune system as a physiological indicator of marginal copper status? Br. J. Nutr. 2002, 87, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Gooneratne, S.R.; Buckley, W.T.; Christensen, D.A. Review of copper deficiency and metabolism in ruminants. Can. J. Anim. Sci. 1989, 69, 819–845. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Meng, H.A.; Xing, G.M.; Chen, C.Y.; Zhao, Y.L.; Jia, G.A.; Wang, T.C.; Yuan, B.C.H.; Wan, M.L.J. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Floriano, P.N.; Noble, C.O.; Schoonmaker, J.M.; Poliakoff, E.D.; McCarley, R.L. Cu(0) Nanoclusters Derived from Poly(propylene imine) Dendrimer Complexes of Cu(II). J. Am. Chem. Soc. 2001, 123, 10545–10553. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Lopez-Alarcon, C.; Olea-Acuna, C.; Aliaga, M.E. Aliaga ME. Role of superoxide anions in the redox changes affecting the physiologically occurring Cu(I)-glutathione complex. Bioinorg. Chem. Appl. 2011, 2011, 674149. [Google Scholar] [CrossRef] [PubMed]
- Pitts, M.; Bayne, K.; Anderson, L.C.; Bernhardt, D.B.; Greene, M.; Klemfuss, H.; Oki, G.S.F.; Rozmiarek, H.; Theran, P.; Van Sluyters, R.C. Institutional Animal Care and Use Committee Guidebook, 2nd ed.; ARENA/OLAW IACUC: Bethesda, MD, USA, 2002. [Google Scholar]
- Cobine, P.A.; George, G.N.; Jones, C.E.; Wickramasinghe, W.A.; Solioz, M.; Dameron, C.T. Copper transfer from the Cu(I) chaperone, CopZ, to the repressor, Zn(II) CopY: Metal coordination environments and protein interactions. Biochemistry 2002, 41, 5822–5829. [Google Scholar] [CrossRef] [PubMed]
- Battin, E.E.; Brumaghim, J.L. Metal specificity in DNA damage prevention by sulfur antioxidants. J. Inorg. Biochem. 2008, 102, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Dameron, C.T.; Winge, D.R.; George, G.N.; Sansone, M.; Hu, S.; Hamer, D. A copper-thiolate polynuclear cluster in the ACE1 transcription factor. Proc. Natl. Acad. Sci. USA 1991, 88, 6127–6131. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhou, C.; Liu, J.; Yu, M.; Zheng, J. One-step interfacial synthesis and assembly of ultrathin luminescent AuNPs/silica membranes. Adv. Mater. 2012, 24, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Awad, H.M.; Boersma, M.G.; Boeren, S.; Bladeren van, P.; Vervoort, J.J.; Rietjens, I.M.C.M. The regioselectivity of glutathione adduct formation with flavonoid quinone/quinone methides is pH-dependent. Chem. Res. Toxicol. 2002, 15, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Casas-Finet, J.R.; Hu, S.; Hamer, D.; Karpel, R.L. Spectroscopic characterization of the copper(I)-thiolate cluster in the DNA-binding domain of yeast ACE1 transcription factor. FEBS Lett. 1991, 281, 205–208. [Google Scholar] [CrossRef]
- Postal, W.S.; Vogel, E.J.; Young, C.M.; Greenaway, F.T. The binding of copper(II) and zinc(II) to oxidized glutathione. J. Inorg. Biochem. 1985, 25, 25–33. [Google Scholar] [PubMed]
- Aliaga, M.E.; López-Alarcóna, C.; García-Ríob, L.; Martín-Pastorc, M.; Speisky, H. Redox-changes associated with the glutathione-dependent ability of the Cu(II)–GSSG complex to generate superoxide Bioorgan. Med. Chem. 2012, 20, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Shtyrlin, V.G.; Zyavkina, Y.I.; Ilakin, V.S.; Garipov, R.R.; Zakharov, A.V. Structure, stability, and ligand exchange of copper(II) complexes with oxidized glutathione. J. Inorg. Biochem. 2005, 99, 1335–1346. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.X.; Zhou, C.; Liu, J.B.; Hankins, J.D.; Zheng, J. Luminescent gold nanoparticles with pH-dependent membrane adsorption. J. Am. Chem. Soc. 2011, 133, 11014–11017. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Ipe, B.I.; Misra, P.; Lee, J.H.; Bawendi, M.G.; Frangioni, J.V. Tissue-and organ-selective biodistribution of NIR fluorescent quantum dots. Nano Lett. 2009, 9, 2354–2359. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Anderson, C.J. Chelators for copper radionuclides in positron emission tomography radiopharmaceuticals. J. Label Compd. Radiopharm. 2014, 57, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Jones-Wilson, T.M.; Deal, K.A.; Anderson, C.J.; McCarthy, D.W.; Kovacs, Z.; Motekaitis, R.J.; Sherry, A.D.; Martell, A.E.; Welch, M.J. The in vivo behavior of per-64-labeled azamacrocyclic complexes. Nucl. Med. Biol. 1998, 25, 523–530. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.-N.; Liu, Y.; Zhou, C.; Yang, S. Glutathione-Mediated Cu(I)/Cu(II) Complexes: Valence-Dependent Effects on Clearance and In Vivo Imaging Application. Nanomaterials 2017, 7, 132. https://doi.org/10.3390/nano7060132
Yin S-N, Liu Y, Zhou C, Yang S. Glutathione-Mediated Cu(I)/Cu(II) Complexes: Valence-Dependent Effects on Clearance and In Vivo Imaging Application. Nanomaterials. 2017; 7(6):132. https://doi.org/10.3390/nano7060132
Chicago/Turabian StyleYin, Su-Na, Yuanyuan Liu, Chen Zhou, and Shengyang Yang. 2017. "Glutathione-Mediated Cu(I)/Cu(II) Complexes: Valence-Dependent Effects on Clearance and In Vivo Imaging Application" Nanomaterials 7, no. 6: 132. https://doi.org/10.3390/nano7060132