Trigger-Responsive Gene Transporters for Anticancer Therapy
Abstract
:1. Introduction
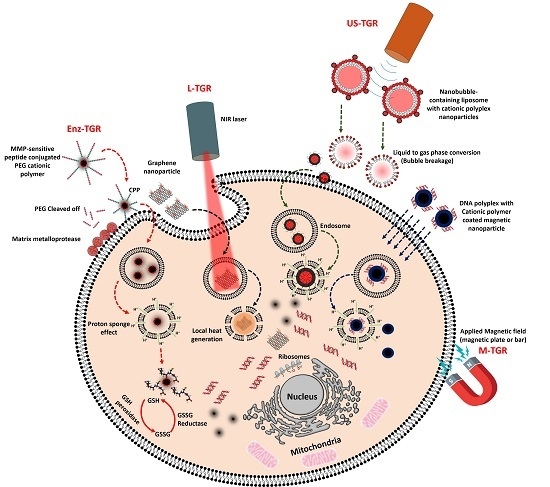
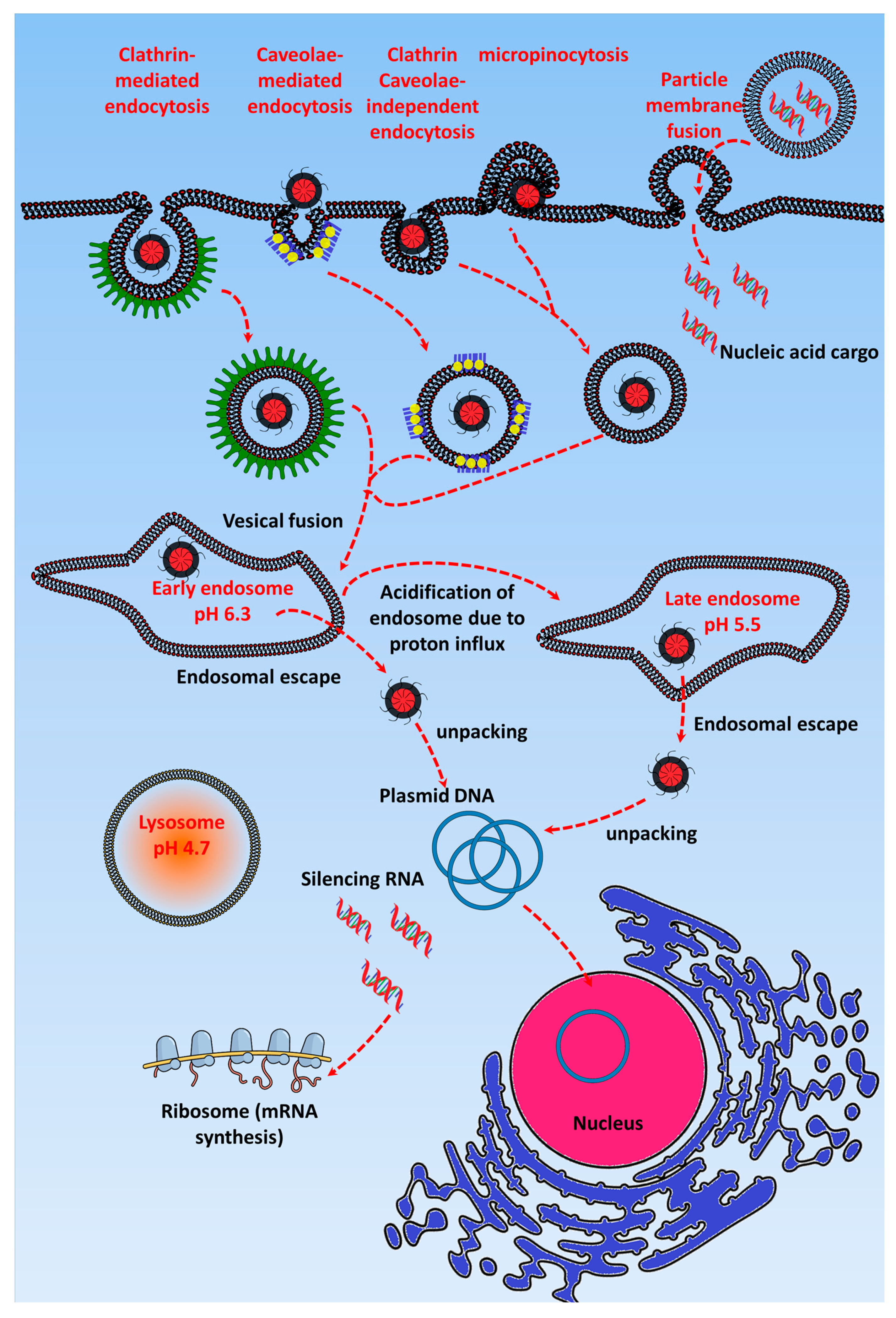
2. Mechanism for Effective Release of Exogenous Nucleic Acids by GT in Cancer Cells
3. Triggers for Gene Release in Cancer Cells Using Gene Transporters
3.1. Enzyme-Triggered Gene Release (Enz-TGR)
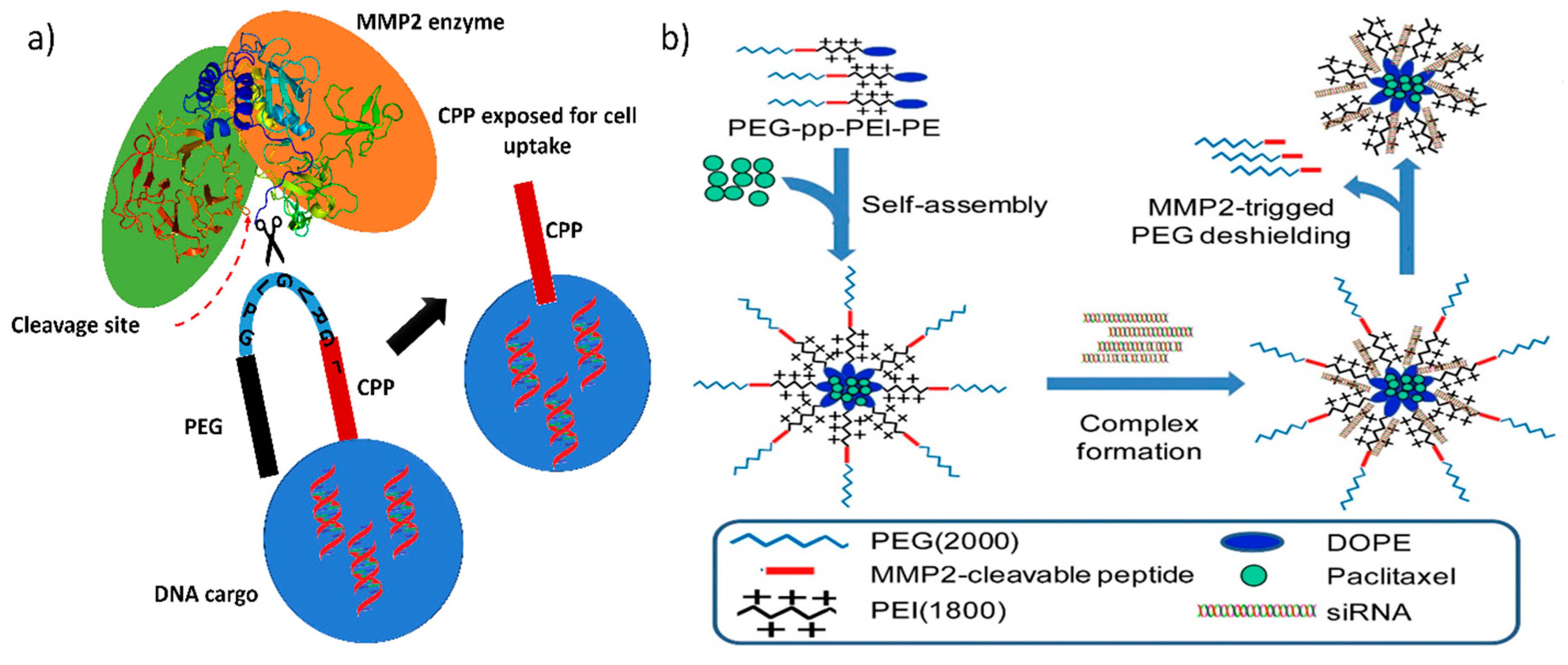
3.1.1. Protease-Triggered Gene Release
3.1.2. Glutathione Enzyme-Triggered Gene Release
3.2. Light-Mediated Gene Release (L-TGR)
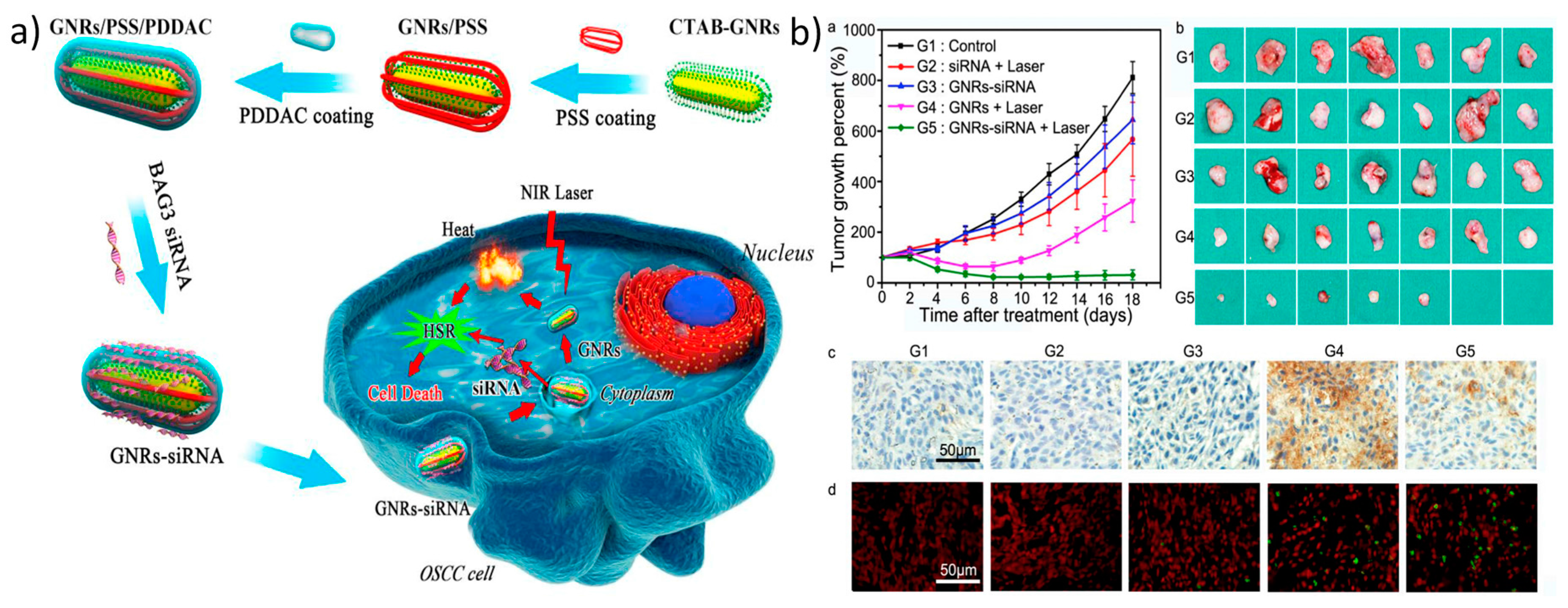
3.2.1. Photothermally Triggered Gene Release
3.2.2. Photochemical Internalization (PCI)-Triggered Gene Release
3.3. Ultrasound-Mediated Gene Release (US-TGR)
3.3.1. US Microbubble-Triggered Gene Release
3.3.2. US Nanobubble-Mediated Gene Transfer
3.4. Magnetic Nanoparticle Mediated Gene Transfer (M-TGR)
4. Challenges Associated with and Future Directions of Trigger-Responsive Gene Transporters
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Alternative magnetic field |
| ATRP | Atom transfer radical polymerization |
| BBB | Blood Brain Barrier |
| CCM | Cancer cell membrane |
| CD | Cytosine deaminase |
| CPP | Cell penetrating peptide |
| CPT | Camptothecin |
| DA | 2,3-Dimethylmaleic anhydride |
| DET | Diethylenetriamine |
| DMAEMA | 2-(Dimethylamino)ethyl methacrylate |
| DMSA | 2,3-Dimercaptosuccinic acid |
| DNA | Deoxyribonucleic acid |
| DOSPA | 2,3-Dioleyloxy-N-[2-spermine carboxamide] ethyl-N,N-dimethyl-1-propanammonium trifluoroacetate |
| DOTAP | 1,2-Dioleoyl-3-trimethylammonium-propane |
| DOTMA | N-[1-(2,3-dioleoyloxy)propel]-N,N,N-trimethylammonium |
| DOX | Doxorubicin |
| DPPC | Dipalmitoylphosphatidylcholine |
| DPTAP | 1,2-dipalmitoyl-3-trimethylammonium-propane |
| DSPE-PEG 2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethylene glycol)-2000] |
| EGFR | Epidermal growth factor receptor |
| Enz-TGR | Enzyme triggered gene release |
| GCS | Glycol chitosan |
| GFP | Green fluorescent protein |
| GNR | Gold Nanorod |
| GSH | Glutathione |
| GSSG | Glutathione disulfide |
| GT(s) | Gene transporter(s) |
| HIV | Human immunodeficiency virus |
| L-TGR | Light triggered gene release |
| MB | Microbubble |
| MDR | Multidrug resistant |
| miRNA | MicroRNA |
| MMP2 | Matrix metalloproteinase-2 |
| MRI | Magnetic Resonance Imaging |
| mRNA | Messenger RNA |
| M-TGR | Magnetic field triggered gene release |
| NET | Neuroepithelial transforming protein 1 |
| NIR | Near infrared |
| NKBDO | Nf-KappaB decoy oligonucleotide |
| OMCN | Oxidized mesoporous carbon nanospheres |
| OEI | Oligo ethylenimine |
| PABC | p-Amino benzyloxy carboxyl |
| PAGA | Poly(aminolated glycidyl methacrylate) |
| PAMAM | Polyamidoamine |
| PCI | Photochemical internalization |
| PCL | Poly(ε-caprolactone) |
| PDDAC | Poly(diallyl dimethyl ammonium chloride) |
| PDGFR-α | Platelet-derived growth factor receptor alpha |
| PDPA | Poly(2-(diisopropyl amino) ethyl methacrylate) |
| PE | Phosphoethanolamine |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PF14 | PepFect14 |
| PFC | Perfluorocarbon |
| PhA | Pheophorbide-a |
| PLGA | Poly(lactic- co-glycolic) acid |
| PLL | Polylysine |
| PSS | Poly(sodium 4-styrene sulfonate) |
| PTEN | Phosphatase and tensin homolog |
| PTT | Photothermal therapy |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SAR | Specific absorption rate |
| siRNA | Silencing RNA |
| SIRT2 | Sirtuin 2 |
| SPION | Superparamagnetic iron oxide nanoparticle |
| SWCNT | Single-walled carbon nanotube |
| TAT | HIV transactivator |
| TNF-α | Tumor necrosis factor-alpha |
| TPP | Triphenylphosphonium |
| TRAIL | TNF-related apoptosis-inducing ligand |
| US-TGR | Ultrasound triggered gene release |
| VEGFR-2 | Vascular endothelial growth factor receptor-2 |
| XIAP | X-linked inhibitor of apoptosis protein |
| μOCT | Micro-Optical Coherence Tomography |
References
- Sridharan, K.; Gogtay, N.J. Therapeutic nucleic acids: Current clinical status. Br. J. Clin. Pharmacol. 2016, 82, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Anchordoquy, T. Drug delivery trends in clinical trials and translational medicine: Challenges and opportunities in the delivery of nucleic acid-based therapeutics. J. Pharm. Sci. 2011, 100, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Ziebacz, N.; Wieczorek, S.A.; Kalwarczyk, E.; Sashuk, V.; Kalwarczyk, T.; Kaminski, T.S.; Holyst, R. Formation and structure of pei/DNA complexes: Quantitative analysis. Soft Matter 2011, 7, 6967–6972. [Google Scholar] [CrossRef]
- Zhu, L.; Mahato, R.I. Lipid and polymeric carrier-mediated nucleic acid delivery. Expert Opin. Drug Deliv. 2010, 7, 1209–1226. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.D.; Pofali, P.; Park, T.E.; Singh, B.; Cho, K.; Maharjan, S.; Dandekar, P.; Jain, R.; Choi, Y.J.; Arote, R.; et al. Gene therapy for bone tissue engineering. Tissue Eng. Regen. Med. 2016, 13, 111–125. [Google Scholar] [CrossRef]
- Leblond, J.; Mignet, N.; Largeau, C.; Spanedda, M.V.; Seguin, J.; Scherman, D.; Herscovici, J. Lipopolythioureas: A new non-cationic system for gene transfer. Bioconj. Chem. 2007, 18, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, Y.; Jiang, Q.; Sun, Y.; Deng, L.; Liang, Z.; Du, Q.; Xing, J.; Zhao, Y.; Wang, P.C.; et al. Enhanced gene delivery and siRNA silencing by gold nanoparticles coated with charge-reversal polyelectrolyte. ACS Nano 2010, 4, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, C.; Lee, I.; Cowan, K.R.; Suh, J. Coating barium titanate nanoparticles with polyethylenimine improves cellular uptake and allows for coupled imaging and gene delivery. Colloids Surf. B 2013, 112, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Matsushita-Ishiodori, Y.; Ohtsuki, T. Photoinduced rna interference. Acc. Chem. Res. 2012, 45, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lian, G.; Liao, C.; Wang, W.; Zeng, L.; Qian, C.; Huang, K.; Shuai, X. Characterization of polyethylene glycol-grafted polyethylenimine and superparamagnetic iron oxide nanoparticles (PEG-g-PEI-SPION) as an mri-visible vector for siRNA delivery in gastric cancer in vitro and in vivo. J. Gastroenterol. 2013, 48, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Branco, M.C.; Schneider, J.P. Self-assembling materials for therapeutic delivery. Acta Biomater. 2009, 5, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Llizo, A.; Wang, C.; Xu, G.; Yang, Y. Nanostructure-induced DNA condensation. Nanoscale 2013, 5, 8288–8306. [Google Scholar] [CrossRef] [PubMed]
- Ainalem, M.L.; Bartles, A.; Muck, J.; Dias, R.S.; Carnerup, A.M.; Zink, D.; Nylander, T. DNA compaction induced by a cationic polymer or surfactant impact gene expression and DNA degradation. PLoS ONE 2014, 9, e92692. [Google Scholar] [CrossRef] [PubMed]
- Maurstad, G.; Stokke, B.T.; Varum, K.M.; Strand, S.P. Pegylated chitosan complexes DNA while improving polyplex colloidal stability and gene transfection efficiency. Carbohydr. Polym. 2013, 94, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Fant, K.; Esbjorner, E.K.; Jenkins, A.; Grossel, M.C.; Lincoln, P.; Norden, B. Effects of pegylation and acetylation of pamam dendrimers on DNA binding, cytotoxicity and in vitro transfection efficiency. Mol. Pharm. 2010, 7, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Eloy, J.O.; Petrilli, R.; Lopez, R.F.; Lee, R.J. Stimuli-responsive nanoparticles for siRNA delivery. Curr. Pharm. Des. 2015, 21, 4131–4144. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.L.; Piccirillo, C.A.; Tabrizian, M. Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur. J. Pharm. 2008, 68, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Von Gersdorff, K.; Sanders, N.N.; Vandenbroucke, R.; De Smedt, S.C.; Wagner, E.; Ogris, M. The internalization route resulting in successful gene expression depends on both cell line and polyethylenimine polyplex type. Mol. Ther. 2006, 14, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Grzesik, B.A.; Vohwinkel, C.U.; Morty, R.E.; Mayer, K.; Herold, S.; Seeger, W.; Vadasz, I. Efficient gene delivery to primary alveolar epithelial cells by nucleofection. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L786–L794. [Google Scholar] [CrossRef] [PubMed]
- Potter, H.; Heller, R. Transfection by electroporation. Curr. Protoc. Immunol. 2017, 117, 10.15.1–10.15.9. [Google Scholar] [CrossRef] [PubMed]
- Andreason, G.L.; Evans, G.A. Introduction and expression of DNA molecules in eukaryotic cells by electroporation. Biotechniques 1988, 6, 650–660. [Google Scholar] [PubMed]
- Almofti, M.R.; Harashima, H.; Shinohara, Y.; Almofti, A.; Baba, Y.; Kiwada, H. Cationic liposome-mediated gene delivery: Biophysical study and mechanism of internalization. Arch. Biochem. Biophys. 2003, 410, 246–253. [Google Scholar] [CrossRef]
- Tashima, T. Intelligent substance delivery into cells using cell-penetrating peptides. Bioorg. Med. Chem. Lett. 2017, 27, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Nakase, I.; Akita, H.; Kogure, K.; Graslund, A.; Langel, U.; Harashima, H.; Futaki, S. Efficient intracellular delivery of nucleic acid pharmaceuticals using cell-penetrating peptides. Acc. Chem. Res. 2012, 45, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Lai, G.H.; Schmidt, N.W.; Sun, V.Z.; Rodriguez, A.R.; Tong, R.; Tang, L.; Cheng, J.; Deming, T.J.; Kamei, D.T.; et al. Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc. Natl. Acad. Sci. USA 2011, 108, 16883–16888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Becton, M.; Wang, X. Designing nanoparticle translocation through cell membranes by varying amphiphilic polymer coatings. J. Phys. Chem. B 2015, 119, 3786–3794. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Li, Z.; Gao, H. Surface-structure-regulated penetration of nanoparticles across a cell membrane. Nanoscale 2012, 4, 3768–3775. [Google Scholar] [CrossRef] [PubMed]
- Gkeka, P.; Angelikopoulos, P.; Sarkisov, L.; Cournia, Z. Membrane partitioning of anionic, ligand-coated nanoparticles is accompanied by ligand snorkeling, local disordering, and cholesterol depletion. PLoS Comput. Biol. 2014, 10, e1003917. [Google Scholar] [CrossRef] [PubMed]
- Van Lehn, R.C.; Alexander-Katz, A. Free energy change for insertion of charged, monolayer-protected nanoparticles into lipid bilayers. Soft Matter 2014, 10, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, T.; Sheng, R.; Sun, J.; Wang, Z.; Cao, A. Achieving high gene delivery performance with caveolae-mediated endocytosis pathway by (l)-arginine/(l)-histidine co-modified cationic gene carriers. Colloids Surf. B 2016, 148, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Conese, M.; Hoekstra, D. Gene transfer by means of lipo- and polyplexes: Role of clathrin and caveolae-mediated endocytosis. J. Liposome Res. 2006, 16, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.A.; Choi, C.H.; Zhang, C.; Hao, L.; Mirkin, C.A. Intracellular fate of spherical nucleic acid nanoparticle conjugates. J. Am. Chem. Soc. 2014, 136, 7726–7733. [Google Scholar] [CrossRef] [PubMed]
- Selby, L.I.; Cortez-Jugo, C.M.; Such, G.K.; Johnston, A.P. Nanoescapology: Progress toward understanding the endosomal escape of polymeric nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Thomas, M.; Klibanov, A.M.; Langer, R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J. Gene Med. 2005, 7, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Kichler, A.; Leborgne, C.; Coeytaux, E.; Danos, O. Polyethylenimine-mediated gene delivery: A mechanistic study. J. Gene Med. 2001, 3, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Benjaminsen, R.V.; Mattebjerg, M.A.; Henriksen, J.R.; Moghimi, S.M.; Andresen, T.L. The possible “proton sponge” effect of polyethylenimine (PEI) does not include change in lysosomal pH. Mol. Ther. 2013, 21, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Barry, M.A.; Saggau, P.; Wu, K.K.; Mikos, A.G. Poly(ethylenimine)-mediated transfection: A new paradigm for gene delivery. J. Biomed. Mater. Res. 2000, 51, 321–328. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Park, T.E.; Jiang, T.; Kang, S.K.; Choi, Y.J.; Cho, C.S. Tuning the buffering capacity of polyethylenimine with glycerol molecules for efficient gene delivery: Staying in or out of the endosomes. Macromol. Biosci. 2015, 15, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; LaRochelle, J.R.; Jiang, B.; Lian, W.; Hard, R.L.; Selner, N.G.; Luechapanichkul, R.; Barrios, A.M.; Pei, D. Early endosomal escape of a cyclic cell-penetrating peptide allows effective cytosolic cargo delivery. Biochemistry 2014, 53, 4034–4046. [Google Scholar] [CrossRef] [PubMed]
- Pichon, C.; Billiet, L.; Midoux, P. Chemical vectors for gene delivery: Uptake and intracellular trafficking. Curr. Opin. Biotechnol. 2010, 21, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Collins, E.; Birchall, J.C.; Williams, J.L.; Gumbleton, M. Nuclear localisation and pdna condensation in non-viral gene delivery. J. Gene Med. 2007, 9, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, H.; Sun, Y.; Qiu, L.; Zhang, J.; Guan, G.; Zhao, X.; Qiao, M.; Cheng, L.; Chen, D. A pH-sensitive gene delivery system based on folic acid-PEG-chitosan-PAMAM-plasmid DNA complexes for cancer cell targeting. Biomaterials 2013, 34, 10120–10132. [Google Scholar] [CrossRef] [PubMed]
- Kongkatigumjorn, N.; Cortez-Jugo, C.; Czuba, E.; Wong, A.S.; Hodgetts, R.Y.; Johnston, A.P.; Such, G.K. Probing endosomal escape using phlexi nanoparticles. Macromol. Biosci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Zhang, H.; Dai, S.; Du, X.; Bi, J.; Qiao, S.Z. Intracellular microenvironment responsive polymers: A multiple-stage transport platform for high-performance gene delivery. Small 2014, 10, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Ashley, C.E.; Carnes, E.C.; Phillips, G.K.; Padilla, D.; Durfee, P.N.; Brown, P.A.; Hanna, T.N.; Liu, J.; Phillips, B.; Carter, M.B.; et al. The targeted delivery of multicomponent cargos to cancer cells by nanoporous particle-supported lipid bilayers. Nat. Mater. 2011, 10, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, C.; Shew, C.Y.; Ito, T.; Koyama, Y. Loosening of DNA/polycation complexes by synthetic polyampholyte to improve the transcription efficiency: Effect of charge balance in the polyampholyte. Biophys. J. 2010, 98, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Veiman, K.L.; Kunnapuu, K.; Lehto, T.; Kiisholts, K.; Parn, K.; Langel, U.; Kurrikoff, K. PEG shielded MMP sensitive CPPs for efficient and tumor specific gene delivery in vivo. J. Control. Release 2015, 209, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, W.J. Photothermally controlled gene delivery by reduced graphene oxide-polyethylenimine nanocomposite. Small 2014, 10, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, P.; Li, J.; Zheng, R.; Zheng, B.; Cheng, D.; Li, R.; Lai, J.; Shuai, X. Ultrasound-sensitive siRNA-loaded nanobubbles formed by hetero-assembly of polymeric micelles and liposomes and their therapeutic effect in gliomas. Biomaterials 2013, 34, 4532–4543. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.L.; Chou, H.L.; Liao, Z.X.; Huang, S.J.; Ke, J.H.; Liu, Y.S.; Chiu, C.C.; Wang, L.F. Chondroitin sulfate-polyethylenimine copolymer-coated superparamagnetic iron oxide nanoparticles as an efficient magneto-gene carrier for microrna-encoding plasmid DNA delivery. Nanoscale 2015, 7, 8554–8565. [Google Scholar] [CrossRef] [PubMed]
- Taranejoo, S.; Chandrasekaran, R.; Cheng, W.; Hourigan, K. Bioreducible pei-functionalized glycol chitosan: A novel gene vector with reduced cytotoxicity and improved transfection efficiency. Carbohydr. Polym. 2016, 153, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, Y.; Chen, Z.; Xie, S.; Luo, X.; Li, X. Synergistic antitumor efficacy of redox and ph dually responsive micelleplexes for co-delivery of camptothecin and genes. Acta Biomater. 2017, 49, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, K.; Hu, Q.; Ding, L.; Yu, F.; Zhou, Z.; Zhou, Y.; Li, J.; Sun, M.; Oupicky, D. Combining fluorination and bioreducibility for improved siRNA polyplex delivery. ACS Appl. Mater. Interfaces 2017, 9, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Rong, L.; Lei, Q.; Cao, P.X.; Qin, S.Y.; Zheng, D.W.; Jia, H.Z.; Zhu, J.Y.; Cheng, S.X.; Zhuo, R.X.; et al. A surface charge-switchable and folate modified system for co-delivery of proapoptosis peptide and p53 plasmid in cancer therapy. Biomaterials 2016, 77, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Perche, F.; Wang, T.; Torchilin, V.P. Matrix metalloproteinase 2-sensitive multifunctional polymeric micelles for tumor-specific co-delivery of siRNA and hydrophobic drugs. Biomaterials 2014, 35, 4213–4222. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.X.; Yang, X.Z.; Sun, C.Y.; Mao, C.Q.; Zhu, Y.H.; Wang, J. Matrix metalloproteinase 2-responsive micelle for siRNA delivery. Biomaterials 2014, 35, 7622–7634. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhou, Z.; Fan, M.; Gong, T.; Zhang, Z.; Sun, X. PEGylated cationic vectors containing a protease-sensitive peptide as a mirRNA delivery system for treating breast cancer. Mol. Pharm. 2017, 14, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Yingyuad, P.; Mevel, M.; Prata, C.; Kontogiorgis, C.; Thanou, M.; Miller, A.D. Enzyme-triggered PEGylated siRNA-nanoparticles for controlled release of siRNA. J. RNAi Gene Silenc. 2014, 10, 490–499. [Google Scholar]
- Ni, Q.; Teng, Z.; Dang, M.; Tian, Y.; Zhang, Y.; Huang, P.; Su, X.; Lu, N.; Yang, Z.; Tian, W.; et al. Gold nanorod embedded large-pore mesoporous organosilica nanospheres for gene and photothermal cooperative therapy of triple negative breast cancer. Nanoscale 2017, 9, 1466–1474. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Li, X.; Yang, Y.; Jing, L.; Yue, X.; Chen, X.; Dai, Z. Chitosan functionalized cus nanoparticles boots gene transfection via photothermal effect. Curr. Drug Deliv. 2016, 14, 334–341. [Google Scholar] [CrossRef]
- Kong, F.; Liu, F.; Li, W.; Guo, X.; Wang, Z.; Zhang, H.; Li, Q.; Luo, L.; Du, Y.; Jin, Y.; et al. Smart carbon nanotubes with laser-controlled behavior in gene delivery and therapy through a non-digestive trafficking pathway. Small 2016, 12, 6753–6766. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shi, J.; Zhang, H.; Li, H.; Gao, Y.; Wang, Z.; Wang, H.; Li, L.; Zhang, C.; Chen, C.; et al. Synergistic anticancer effect of RNAi and photothermal therapy mediated by functionalized single-walled carbon nanotubes. Biomaterials 2013, 34, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Matsushita-Ishiodori, Y.; Kuwabara, R.; Sakakoshi, H.; Endoh, T.; Ohtsuki, T. Photosensitizing carrier proteins for photoinducible rna interference. Bioconj. Chem. 2011, 22, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, T.; Fukushima, S.; Kumagai, M.; Machitani, K.; Arnida; Matsumoto, Y.; Oba, M.; Miyata, K.; Osada, K.; Nishiyama, N.; et al. Three-layered polyplex micelle as a multifunctional nanocarrier platform for light-induced systemic gene transfer. Nat. Commun. 2014, 5, 3545. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Park, W.; Na, K. Photo-activatable ternary complex based on a multifunctional shielding material for targeted shRNA delivery in cancer treatment. Biomaterials 2013, 34, 8991–8999. [Google Scholar] [CrossRef] [PubMed]
- Mullick Chowdhury, S.; Wang, T.Y.; Bachawal, S.; Dev Yuan, Y.; Zhang, C.J.; Liu, B. A photoactivatable AIE polymer for light-controlled gene delivery: Concurrent endo/lysosomal escape and DNA unpacking. Angew. Chem. Int. Ed. Engl. 2015, 54, 11419–11423. [Google Scholar]
- De Cock, I.; Lajoinie, G.; Versluis, M.; De Smedt, S.C.; Lentacker, I. Sonoprinting and the importance of microbubble loading for the ultrasound mediated cellular delivery of nanoparticles. Biomaterials 2016, 83, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Choe, J.W.; Pu, K.; Devulapally, R.; Bachawal, S.; Machtaler, S.; Chowdhury, S.M.; Luong, R.; Tian, L.; Khuri-Yakub, B.; et al. Ultrasound-guided delivery of microrna loaded nanoparticles into cancer. J. Control. Release 2015, 203, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Ulapally, R.; Choe, J.W.; Abou Elkacem, L.; Yakub, B.K.; Wang, D.S.; Tian, L.; Paulmurugan, R.; et al. Ultrasound-guided therapeutic modulation of hepatocellular carcinoma using complementary micrornas. J. Control. Release 2016, 238, 272–280. [Google Scholar]
- Vandenbroucke, R.E.; Lentacker, I.; Demeester, J.; De Smedt, S.C.; Sanders, N.N. Ultrasound assisted siRNA delivery using PEG-siPlex loaded microbubbles. J. Control. Release 2008, 126, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Omata, D.; Negishi, Y.; Hagiwara, S.; Yamamura, S.; Endo-Takahashi, Y.; Suzuki, R.; Maruyama, K.; Nomizu, M.; Aramaki, Y. Bubble liposomes and ultrasound promoted endosomal escape of TAT-PEG liposomes as gene delivery carriers. Mol. Pharm. 2011, 8, 2416–2423. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, L.; Li, T.; Shen, X.; Yan, J.; Zuo, L.; Wu, C.; Liu, Y. Multifunctional PLGA nanobubbles as theranostic agents: Combining doxorubicin and P-gp siRNA co-delivery into human breast cancer cells and ultrasound cellular imaging. J. Biomed. Nanotechnol. 2015, 11, 2124–2136. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Lin, W.; Li, M.; Yang, Y.; Deng, J.; Liu, H.; Chen, Y.; Fu, X. Efficient siRNA delivery using novel cell-penetrating peptide-siRNA conjugate-loaded nanobubbles and ultrasound. Ultrasound Med. Biol. 2016, 42, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Qiao, Q.; Han, X.; Jing, H.; Zhang, H.; Liang, H.; Cheng, W. Targeted nanobubbles in low-frequency ultrasound-mediated gene transfection and growth inhibition of hepatocellular carcinoma cells. Tumour Biol. 2016, 37, 12113–12121. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Kawakami, S.; Higuchi, Y.; Maruyama, K.; Yamashita, F.; Hashida, M. Tumour-associated macrophages targeted transfection with NF-kB decoy/mannose-modified bubble lipoplexes inhibits tumour growth in tumour-bearing mice. J. Drug Target. 2014, 22, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Taghavi Pourianazar, N.; Gunduz, U. CpG oligodeoxynucleotide-loaded PAMAM dendrimer-coated magnetic nanoparticles promote apoptosis in breast cancer cells. Biomed. Pharmacother. 2016, 78, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Chiang, P.H.; Hsiao, W.C.; Chuang, C.C.; Chang, C.W. Redox-sensitive polymer/SPIO nanocomplexes for efficient magnetofection and MR imaging of human cancer cells. Langmuir 2015, 31, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Alvizo-Baez, C.A.; Luna-Cruz, I.E.; Vilches-Cisneros, N.; Rodriguez-Padilla, C.; Alcocer-Gonzalez, J.M. Systemic delivery and activation of the trail gene in lungs, with magnetic nanoparticles of chitosan controlled by an external magnetic field. Int. J. Nanomed. 2016, 11, 6449–6458. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Liu, C.; Ge, J.; Yang, W.; Liu, J.; Sun, W.; Yang, B.; Zheng, C.; Sun, H.; Hu, Q. Antitumor effect of trail on oral squamous cell carcinoma using magnetic nanoparticle-mediated gene expression. Cell Biochem. Biophys. 2014, 69, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, K.; Qiao, C.; Jin, X.; Zheng, C.; Yang, B.; Sun, H. Antitumor effect of human trail on adenoid cystic carcinoma using magnetic nanoparticle-mediated gene expression. Nanomedicine 2013, 9, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Talvensaari-Mattila, A.; Paakko, P.; Blanco-Sequeiros, G.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with the risk for a relapse in postmenopausal patients with node-positive breast carcinoma treated with antiestrogen adjuvant therapy. Breast Cancer Res. Treat. 2001, 65, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Talvensaari-Mattila, A.; Paakko, P.; Turpeenniemi-Hujanen, T. Matrix metalloproteinase-2 (MMP-2) is associated with survival in breast carcinoma. Br. J. Cancer 2003, 89, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Song, L.Y.; Ahkong, Q.F.; Rong, Q.; Wang, Z.; Ansell, S.; Hope, M.J.; Mui, B. Characterization of the inhibitory effect of PEG-lipid conjugates on the intracellular delivery of plasmid and antisense DNA mediated by cationic lipid liposomes. Biochim. Biophys. Acta BBA Biomembr. 2002, 1558, 1–13. [Google Scholar] [CrossRef]
- Harvie, P.; Wong, F.M.; Bally, M.B. Use of poly(ethylene glycol)-lipid conjugates to regulate the surface attributes and transfection activity of lipid-DNA particles. J. Pharm. Sci. 2000, 89, 652–663. [Google Scholar] [CrossRef]
- Bruun, J.; Larsen, T.B.; Jolck, R.I.; Eliasen, R.; Holm, R.; Gjetting, T.; Andresen, T.L. Investigation of enzyme-sensitive lipid nanoparticles for delivery of siRNA to blood-brain barrier and glioma cells. Int. J. Nanomed. 2015, 10, 5995–6008. [Google Scholar]
- Gjetting, T.; Jolck, R.I.; Andresen, T.L. Effective nanoparticle-based gene delivery by a protease triggered charge switch. Adv. Healthc. Mater. 2014, 3, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Rozema, D.B.; Blokhin, A.V.; Wakefield, D.H.; Benson, J.D.; Carlson, J.C.; Klein, J.J.; Almeida, L.J.; Nicholas, A.L.; Hamilton, H.L.; Chu, Q.; et al. Protease-triggered siRNA delivery vehicles. J. Control. Release 2015, 209, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shao, K.; Kuang, Y.; Liu, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; He, X.; Jiang, C. Tumor targeting and microenvironment-responsive nanoparticles for gene delivery. Biomaterials 2013, 34, 5294–5302. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.E.; Johnston, S.K.; Mrugala, M.M.; Beard, B.C.; Guyman, L.A.; Baldock, A.L.; Bridge, C.A.; Hawkins-Daarud, A.; Gori, J.L.; Born, D.E.; et al. Gene therapy enhances chemotherapy tolerance and efficacy in glioblastoma patients. J. Clin. Investig. 2014, 124, 4082–4092. [Google Scholar] [CrossRef] [PubMed]
- Burg, D.; Mulder, G.J. Glutathione conjugates and their synthetic derivatives as inhibitors of glutathione-dependent enzymes involved in cancer and drug resistance. Drug Metab. Rev. 2002, 34, 821–863. [Google Scholar] [CrossRef] [PubMed]
- Balendiran, G.K.; Dabur, R.; Fraser, D. The role of glutathione in cancer. Cell Biochem. Funct. 2004, 22, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [PubMed]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.P.; Carlson, J.L.; Samiec, P.S.; Sternberg, P., Jr.; Mody, V.C., Jr.; Reed, R.L.; Brown, L.A. Glutathione measurement in human plasma: Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef]
- Yoo, J.; Lee, D.; Gujrati, V.; Rejinold, N.S.; Lekshmi, K.M.; Uthaman, S.; Jeong, C.; Park, I.K.; Jon, S.; Kim, Y.C. Bioreducible branched poly(modified nona-arginine) cell-penetrating peptide as a novel gene delivery platform. J. Control. Release 2017, 246, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, S.; Bulleid, N.J. Glutathione is required to regulate the formation of native disulfide bonds within proteins entering the secretory pathway. J. Biol. Chem. 2004, 279, 39872–39879. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Y.; Xie, R.; Gong, S. Tumor-targeted pH/redox dual-sensitive unimolecular nanoparticles for efficient siRNA delivery. J. Control. Release 2017. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.S.; Rekha, M.R. Disulphide cross linked pullulan based cationic polymer for improved gene delivery and efflux pump inhibition. Colloids Surf. B 2016, 146, 879–887. [Google Scholar]
- Muthiah, M.; Che, H.L.; Kalash, S.; Jo, J.; Choi, S.Y.; Kim, W.J.; Cho, C.S.; Lee, J.Y.; Park, I.K. Formulation of glutathione responsive anti-proliferative nanoparticles from thiolated Akt1 siRNA and disulfide-crosslinked PEI for efficient anti-cancer gene therapy. Colloids Surf. B 2015, 126, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.W.; Son, S.; Jang, J.; Youn, H.; Lee, S.; Lee, D.; Lee, Y.S.; Jeong, J.M.; Kim, W.J.; Lee, D.S. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials 2011, 32, 4968–4975. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Hu, Y.; Xu, C.; Xu, F.J. Reducible polyrotaxane-based pseudo-comb polycations via consecutive ATRP processes for gene delivery. Acta Biomater. 2016, 32, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Prigodich, A.E.; Patel, P.C.; Mirkin, C.A. Gene regulation with polyvalent siRNA-nanoparticle conjugates. J. Am. Chem. Soc. 2009, 131, 2072–2073. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Kim, H.J.; Mi, P.; Zheng, M.; Takemoto, H.; Toh, K.; Kim, B.S.; Hayashi, K.; Naito, M.; Matsumoto, Y.; et al. Targeted systemic delivery of siRNA to cervical cancer model using cyclic RGD-installed unimer polyion complex-assembled gold nanoparticles. J. Control. Release 2016, 244, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Elbakry, A.; Zaky, A.; Liebl, R.; Rachel, R.; Goepferich, A.; Breunig, M. Layer-by-layer assembled gold nanoparticles for siRNA delivery. Nano Lett. 2009, 9, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.H.; Bae, K.H.; Hong, C.A.; Lee, Y.; Hahn, S.K.; Park, T.G. Multimerized siRNA cross-linked by gold nanoparticles. Bioconj. Chem. 2011, 22, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Lee, S.H.; Park, J.W.; Park, T.G. Multimeric small interfering ribonucleic acid for highly efficient sequence-specific gene silencing. Nat. Mater. 2010, 9, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Mok, H.; Jo, S.; Hong, C.A.; Park, T.G. Dual gene targeted multimeric siRNA for combinatorial gene silencing. Biomaterials 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Weyergang, A.; Prasmickaite, L.; Bonsted, A.; Hogset, A.; Strand, M.T.; Wagner, E.; Selbo, P.K. Photochemical internalization (PCI): A technology for drug delivery. Methods Mol. Biol. 2010, 635, 133–145. [Google Scholar] [PubMed]
- Smith, A.M.; Mancini, M.C.; Nie, S. Bioimaging: Second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710–711. [Google Scholar] [CrossRef] [PubMed]
- Kalash, R.S.; Lakshmanan, V.K.; Cho, C.S.; Park, I.K. Theranostics, 1st ed.; William Andrew: Norwich, NY, USA, 2016; p. 347. [Google Scholar]
- Huschka, R.; Zuloaga, J.; Knight, M.W.; Brown, L.V.; Nordlander, P.; Halas, N.J. Light-induced release of DNA from gold nanoparticles: Nanoshells and nanorods. J. Am. Chem. Soc. 2011, 133, 12247–12255. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, G.; Zhang, R.; Flores, L.G., 2nd; Huang, Q.; Gelovani, J.G.; Li, C. Tumor site-specific silencing of NF-kB p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010, 70, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, T.; Xie, Y.; Sun, Z.; Liu, H.; Lin, J.; Liu, C.; Mao, Z.W.; Nie, S. Chitosan layered gold nanorods as synergistic therapeutics for photothermal ablation and gene silencing in triple-negative breast cancer. Acta Biomater. 2015, 25, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kim, H.C.; Mu, C.; Gentile, E.; Mai, J.; Wolfram, J.; Ji, L.N.; Ferrari, M.; Mao, Z.W.; Shen, H. Multifunctional gold nanorods for siRNA gene silencing and photothermal therapy. Adv. Healthc. Mater. 2014, 3, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.K.; Yu, X.F.; Wang, J.H.; Li, Z.B.; Li, P.H.; Wang, H.; Song, L.; Chu, P.K.; Li, C. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 2016, 78, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.K.; Lee, Y.K.; Hong, J.; Ghandehari, H.; Yun, C.O. Mild hyperthermia induced by gold nanorod-mediated plasmonic photothermal therapy enhances transduction and replication of oncolytic adenoviral gene delivery. ACS Nano 2016, 10, 10533–10543. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Yang, X.; Shi, X.; Tan, X.; Peng, R.; Wang, J.; Liu, Z. Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery. Small 2013, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed]
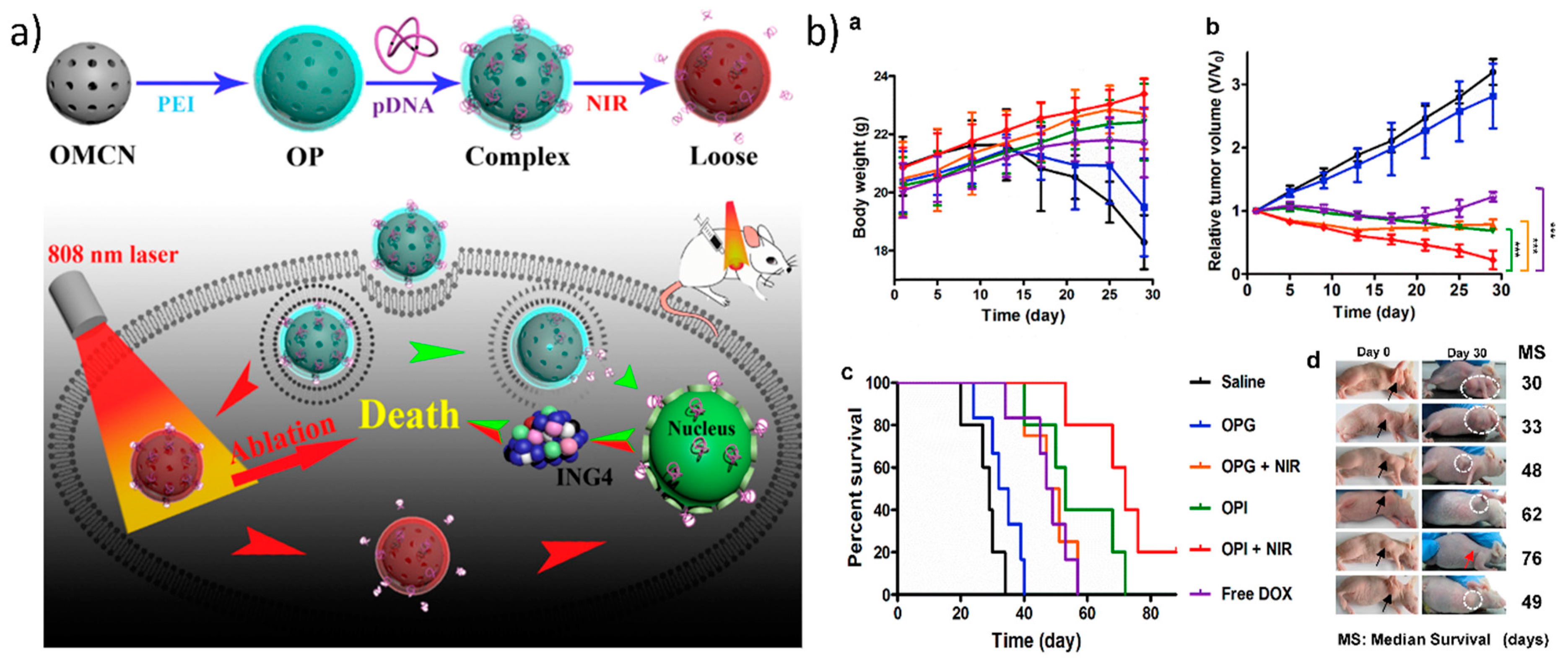
- Meng, Y.; Wang, S.; Li, C.; Qian, M.; Yan, X.; Yao, S.; Peng, X.; Wang, Y.; Huang, R. Photothermal combined gene therapy achieved by polyethyleneimine-grafted oxidized mesoporous carbon nanospheres. Biomaterials 2016, 100, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.; Lee, M.; Choi, H.C.; Kim, W.J. Photothermal gene delivery: Stimuli-regulated enzymatically degradable smart graphene-oxide-polymer nanocarrier facilitating photothermal gene delivery. Adv. Healthc. Mater. 2016, 5, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Chng, E.L.; Sofer, Z.; Pumera, M. Cytotoxicity of exfoliated transition-metal dichalcogenides (MoS2, WS2, and WSe2) is lower than that of graphene and its analogues. Chemistry 2014, 20, 9627–9632. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Wang, X.; Yuan, R.; Chen, H.; Zhi, Q.; Gao, L.; Wang, B.; Guo, Z.; Xue, X.; Cao, W.; et al. A promising gene delivery system developed from PEGylated MoS2 nanosheets for gene therapy. Nanoscale Res. Lett. 2014, 9, 587. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Kim, W.J. Single-layered MoS2-PEI-PEG nanocomposite-mediated gene delivery controlled by photo and redox stimuli. Small 2016, 12, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Duran, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Shen, H.; Tu, X.; Zhang, Z. Assessing in vivo toxicity of graphene materials: Current methods and future outlook. Nanomedicine (London) 2014, 9, 1565–1580. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.T.; Mu, X.Y.; Wu, X.L.; Meng, L.X.; Guan, W.B.; Ma, Y.Q.; Sun, H.; Wang, C.J.; Li, X.F. Toxicity of multi-walled carbon nanotubes, graphene oxide, and reduced graphene oxide to zebrafish embryos. Biomed. Environ. Sci. 2014, 27, 676–683. [Google Scholar] [PubMed]
- Xue, P.; Bao, J.N.; Zhang, L.; Xu, Z.G.; Xu, C.J.; Zhang, Y.L.; Kang, Y.J. Functional magnetic prussian blue nanoparticles for enhanced gene transfection and photothermal ablation of tumor cells. J. Mater. Chem. B 2016, 4, 4717–4725. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Z.; Wang, Z.; Li, W.; Ju, E.; Yan, Z.; Liu, Z.; Ren, J.; Qu, X. A graphitic hollow carbon nitride nanosphere as a novel photochemical internalization agent for targeted and stimuli-responsive cancer therapy. Nanoscale 2016, 8, 12570–12578. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Moan, J. Lysosomes as photochemical targets. Int. J. Cancer 1994, 59, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Prydz, K.; Moan, J. Photochemical treatment with the lysosomally localized dye tetra(4-sulfonatophenyl)porphine results in lysosomal release of the dye but not of β-N-acetyl-d-glucosaminidase activity. Biochim. Biophys. Acta 1993, 1158, 300–306. [Google Scholar] [CrossRef]
- Berg, K.; Selbo, P.K.; Prasmickaite, L.; Tjelle, T.E.; Sandvig, K.; Moan, J.; Gaudernack, G.; Fodstad, O.; Kjolsrud, S.; Anholt, H.; et al. Photochemical internalization: A novel technology for delivery of macromolecules into cytosol. Cancer Res. 1999, 59, 1180–1183. [Google Scholar] [PubMed]
- Selbo, P.K.; Weyergang, A.; Bonsted, A.; Bown, S.G.; Berg, K. Photochemical internalization of therapeutic macromolecular agents: A novel strategy to kill multidrug-resistant cancer cells. J. Pharmacol. Exp. Ther. 2006, 319, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.; Fretz, M.M.; Hogset, A.; Storm, G.; Schiffelers, R.M. Photochemical internalization enhances silencing of epidermal growth factor receptor through improved endosomal escape of siRNA. Biochim. Biophys. Acta 2007, 1768, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Zamora, G.; Wang, F.; Sun, C.H.; Trinidad, A.; Kwon, Y.J.; Cho, S.K.; Berg, K.; Madsen, S.J.; Hirschberg, H. Photochemical internalization-mediated nonviral gene transfection: Polyamine core-shell nanoparticles as gene carrier. J. Biomed. Opt. 2014, 19, 105009. [Google Scholar] [CrossRef] [PubMed]
- Mathews, M.S.; Shih, E.C.; Zamora, G.; Sun, C.H.; Cho, S.K.; Kwon, Y.J.; Hirschberg, H. Glioma cell growth inhibition following photochemical internalization enhanced non-viral PTEN gene transfection. Lasers Surg. Med. 2012, 44, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Raemdonck, K.; Naeye, B.; Hogset, A.; Demeester, J.; De Smedt, S.C. Prolonged gene silencing by combining siRNA nanogels and photochemical internalization. J. Control. Release 2010, 145, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Gargouri, M.; Sapin, A.; Arica-Yegin, B.; Merlin, J.L.; Becuwe, P.; Maincent, P. Photochemical internalization for pDNA transfection: Evaluation of poly(d,l-lactide-co-glycolide) and poly(ethylenimine) nanoparticles. Int. J. Pharm. 2011, 403, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Shieh, M.J.; Peng, C.L.; Lou, P.J.; Chiu, C.H.; Tsai, T.Y.; Hsu, C.Y.; Yeh, C.Y.; Lai, P.S. Non-toxic phototriggered gene transfection by pamam-porphyrin conjugates. J. Control. Release 2008, 129, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.H.; Chang, E.L.; Ting, C.Y.; Lin, Y.C.; Liao, E.C.; Huang, C.Y.; Chang, Y.C.; Chan, H.L.; Wei, K.C.; Yeh, C.K. Folate-conjugated gene-carrying microbubbles with focused ultrasound for concurrent blood-brain barrier opening and local gene delivery. Biomaterials 2016, 106, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Marentis, T.C.; Kusler, B.; Yaralioglu, G.G.; Liu, S.; Haeggstrom, E.O.; Khuri-Yakub, B.T. Microfluidic sonicator for real-time disruption of eukaryotic cells and bacterial spores for DNA analysis. Ultrasound Med. Biol. 2005, 31, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.K.; Zhang, W.; Hu, B.; Ying, T. Liposome-mediated transfection of wild-type p53 DNA into human prostate cancer cells is improved by low-frequency ultrasound combined with microbubbles. Oncol. Lett. 2016, 11, 3829–3834. [Google Scholar] [PubMed]
- Chen, F.; Li, Y.; Feng, Y.; He, X.; Wang, L. Evaluation of antimetastatic effect of lncRNA-ATB siRNA delivered using ultrasound-targeted microbubble destruction. DNA Cell Biol. 2016, 35, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yin, T.; Li, J.; Zheng, B.; Qiu, C.; Wang, P. Feasibility of integrating tumor therapy with therapeutic effect evaluation using siRNA-loaded microbubbles. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2015, 35, 874–878. [Google Scholar]
- Wang, P.; Yin, T.; Li, J.; Zheng, B.; Wang, X.; Wang, Y.; Zheng, J.; Zheng, R.; Shuai, X. Ultrasound-responsive microbubbles for sonography-guided siRNA delivery. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Panje, C.M.; Wang, D.S.; Pysz, M.A.; Paulmurugan, R.; Ren, Y.; Tranquart, F.; Tian, L.; Willmann, J.K. Ultrasound-mediated gene delivery with cationic versus neutral microbubbles: Effect of DNA and microbubble dose on in vivo transfection efficiency. Theranostics 2012, 2, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.P.; Wang, L.F.; Guo, Y.L.; Li, L.; Fan, X.Z.; Ding, J.; Huang, H.Y. Preparation of protamine cationic nanobubbles and experimental study of their physical properties and in vivo contrast enhancement. Ultrasound Med. Biol. 2013, 39, 2147–2157. [Google Scholar] [CrossRef] [PubMed]
- He, S.N.; Li, Y.L.; Yan, J.J.; Zhang, W.; Du, Y.Z.; Yu, H.Y.; Hu, F.Q.; Yuan, H. Ternary nanoparticles composed of cationic solid lipid nanoparticles, protamine, and DNA for gene delivery. Int. J. Nanomed. 2013, 8, 2859–2869. [Google Scholar]
- Florinas, S.; Kim, J.; Nam, K.; Janat-Amsbury, M.M.; Kim, S.W. Ultrasound-assisted siRNA delivery via arginine-grafted bioreducible polymer and microbubbles targeting VEGF for ovarian cancer treatment. J. Control. Release 2014, 183, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Dellian, M.; Fukumura, D.; Leunig, M.; Berk, D.A.; Torchilin, V.P.; Jain, R.K. Vascular permeability in a human tumor xenograft: Molecular size dependence and cutoff size. Cancer Res. 1995, 55, 3752–3756. [Google Scholar] [PubMed]
- Rapoport, N.Y.; Nam, K.H.; Gao, Z.; Kennedy, A. Application of ultrasound for targeted nanotherapy of malignant tumors. Acoust. Phys. 2009, 55, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kawakami, S.; Kono, Y.; Un, K.; Higuchi, Y.; Maruyama, K.; Yamashita, F.; Hashida, M. Enhancement of the anti-tumor effect of DNA vaccination using an ultrasound-responsive mannose-modified gene carrier in combination with doxorubicin-encapsulated PEGylated liposomes. Int. J. Pharm. 2014, 475, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Xu, M.; Cao, Z.; Gao, J.; Chen, Y.; Li, Y.; Yang, Z.; Xie, X.; Jiang, Q.; Wang, W.; et al. Ultrasound-triggered phase-transition cationic nanodroplets for enhanced gene delivery. ACS Appl. Mater. Interfaces 2015, 7, 13524–13537. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, N.; McHale, A.P. Microbubble-enhanced ultrasound-mediated gene transfer--towards the development of targeted gene therapy for cancer. Int. J. Hyperth. 2012, 28, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Alter, J.; Sennoga, C.A.; Lopes, D.M.; Eckersley, R.J.; Wells, D.J. Microbubble stability is a major determinant of the efficiency of ultrasound and microbubble mediated in vivo gene transfer. Ultrasound Med. Biol. 2009, 35, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Horie, S.; Watanabe, Y.; Ono, M.; Mori, S.; Kodama, T. Evaluation of antitumor effects following tumor necrosis factor-alpha gene delivery using nanobubbles and ultrasound. Cancer Sci. 2011, 102, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Wang, P.; Li, J.; Wang, Y.; Zheng, B.; Zheng, R.; Cheng, D.; Shuai, X. Tumor-penetrating codelivery of siRNA and paclitaxel with ultrasound-responsive nanobubbles hetero-assembled from polymeric micelles and liposomes. Biomaterials 2014, 35, 5932–5943. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yang, Y.; Lin, W.; Liu, H.; Chen, Y.; Fu, X.; Deng, J. Cell-penetrating peptide-siRNA conjugate loaded YSA-modified nanobubbles for ultrasound triggered siRNA delivery. Colloids Surf. B Biointerfaces 2015, 136, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Castro, R.; Rodrigues, J.; Shi, X.; Tomas, H. Pamam dendrimer/pDNA functionalized-magnetic iron oxide nanoparticles for gene delivery. J. Biomed. Nanotechnol. 2015, 11, 1370–1384. [Google Scholar] [CrossRef] [PubMed]
- Prosen, L.; Hudoklin, S.; Cemazar, M.; Stimac, M.; Lampreht Tratar, U.; Ota, M.; Scancar, J.; Romih, R.; Sersa, G. Magnetic field contributes to the cellular uptake for effective therapy with magnetofection using plasmid DNA encoding against mcam in B16F10 melanoma in vivo. Nanomedicine (London) 2016, 11, 627–641. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.; Lausier, J.; Gersting, S.W.; Rudolph, C.; Plank, C.; Welsch, U.; Rosenecker, J. Insights into the mechanism of magnetofection using pei-based magnetofectins for gene transfer. J. Gene Med. 2004, 6, 923–936. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.D.; Selomulya, C.; Ho, J.; Apostolopoulos, V.; Plebanski, M. Delivery of DNA vaccines: An overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, T.; Kawashima, H.; Tanaka, T.; Hirose, M.; Toyama-Sorimachi, N.; Matsuzawa, Y.; Miyasaka, M. Cd44 binds a chondroitin sulfate proteoglycan, aggrecan. Int. Immunol. 2001, 13, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.M.; Wang, J.; Yan, Z.; You, Y.P.; Li, C.Y.; Qian, X.; Yin, Y.; Zhao, P.; Wang, Y.Y.; Wang, X.F.; et al. Mir-128 inhibits tumor growth and angiogenesis by targeting p70s6k1. PLoS ONE 2012, 7, e32709. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, A.M.; Gabriel, S.A.; Sukhorukov, G.B.; Gould, D.J. Improved and targeted delivery of bioactive molecules to cells with magnetic layer-by-layer assembled microcapsules. Nanoscale 2015, 7, 9686–9693. [Google Scholar] [CrossRef] [PubMed]
- Banobre-Lopez, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. J. Greatpol. Cancer Cent. Pozn. Pol. Soc. Radiat. Oncol. 2013, 18, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Atterwill, C.K.; Poole, A.; Jones, C.; Jones, R.; Brown, C. Mechanistic investigation of species-specific thyroid lesions induced by treatment with the histamine h1 antagonist temelastine (sk&f 93944) in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1989, 27, 681–690. [Google Scholar]
- Lin, M.; Huang, J.; Zhang, J.; Wang, L.; Xiao, W.; Yu, H.; Li, Y.; Li, H.; Yuan, C.; Hou, X.; et al. The therapeutic effect of PEI-Mn0.5Zn0.5Fe2O4 nanoparticles/pEgr1-HSV-TK/GCV associated with radiation and magnet-induced heating on hepatoma. Nanoscale 2013, 5, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.T.; Shah, B.P.; Lee, K.B. Combined magnetic nanoparticle-based microrna and hyperthermia therapy to enhance apoptosis in brain cancer cells. Small 2014, 10, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.H.; Lima, E., Jr.; Mansilla, M.V.; Zysler, R.D.; Troiani, H.; Pisciotti, M.L.; Locatelli, C.; Benech, J.C.; Oddone, N.; Zoldan, V.C.; et al. Superparamagnetic iron-oxide nanoparticles mPEG350- and mPEG2000-coated: Cell uptake and biocompatibility evaluation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Yiu, H.H.; McBain, S.C.; Lethbridge, Z.A.; Lees, M.R.; Dobson, J. Preparation and characterization of polyethylenimine-coated Fe3O4-MCM-48 nanocomposite particles as a novel agent for magnet-assisted transfection. J. Biomed. Mater. Res. Part A 2010, 92, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Eltoukhy, A.A.; Love, K.T.; Langer, R.; Anderson, D.G. Lipidoid-coated iron oxide nanoparticles for efficient DNA and siRNA delivery. Nano Lett. 2013, 13, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Chen, J.; Zhang, Z.; Zheng, G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine (London) 2014, 9, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Taratula, O.; Garbuzenko, O.; Savla, R.; Wang, Y.A.; He, H.; Minko, T. Multifunctional nanomedicine platform for cancer specific delivery of siRNA by superparamagnetic iron oxide nanoparticles-dendrimer complexes. Curr. Drug Deliv. 2011, 8, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Hsieh, T.Y.; Chiang, C.S.; Chen, P.J.; Chen, Y.Y.; Chiu, T.L.; Chen, S.Y. Surfactant-free, lipo-polymersomes stabilized by iron oxide nanoparticles/polymer interlayer for synergistically targeted and magnetically guided gene delivery. Adv. Healthc. Mater. 2014, 3, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Li, K.; Li, J.; Wen, S.; Chen, Q.; Shen, M.; Zheng, L.; Zhang, G.; Shi, X. Dendrimer-assisted formation of Fe3O4/Au nanocomposite particles for targeted dual mode CT/MR imaging of tumors. Small 2015, 11, 4584–4593. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, X.; Wei, C.; Xu, Z.J.; Sim, S.S.; Liu, L.; Xu, C. Micro-optical coherence tomography tracking of magnetic gene transfection via Au-Fe3O4 dumbbell nanoparticles. Nanoscale 2015, 7, 17249–17253. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L.G. Impact of carbon nanotubes and graphene on immune cells. J. Transl. Med. 2014, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- El-Yamany, N.A.; Mohamed, F.F.; Salaheldin, T.A.; Tohamy, A.A.; Abd El-Mohsen, W.N.; Amin, A.S. Graphene oxide nanosheets induced genotoxicity and pulmonary injury in mice. Exp. Toxicol. Pathol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, N.; Yang, J.; Choi, J. Differential genotoxic and epigenotoxic effects of graphene family nanomaterials (GFNs) in human bronchial epithelial cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.; Lee, S.H.; Park, H. Biofunctionalized nanoparticles: An emerging drug delivery platform for various disease treatments. Drug Discov. Today 2016, 21, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.; Lee, S.H.; Park, H. Lipid-based surface engineering of PLGA nanoparticles for drug and gene delivery applications. Biomater. Res. 2016, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- C, N.D.; Tate, J.A.; Kett, W.C.; Batra, J.; Demidenko, E.; Lewis, L.D.; Hoopes, P.J.; Gerngross, T.U.; Griswold, K.E. Tumor cell targeting by iron oxide nanoparticles is dominated by different factors in vitro versus in vivo. PLoS ONE 2015, 10, e0115636. [Google Scholar]
- Huang, H.Y.; Liu, H.L.; Hsu, P.H.; Chiang, C.S.; Tsai, C.H.; Chi, H.S.; Chen, S.Y.; Chen, Y.Y. A multitheragnostic nanobubble system to induce blood-brain barrier disruption with magnetically guided focused ultrasound. Adv. Mater. 2015, 27, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.W.; Liu, S.W.; Liou, Y.R.; Wu, Y.H.; Yang, Y.C.; Wang, C.R.; Li, P.C. Nanodroplet-vaporization-assisted sonoporation for highly effective delivery of photothermal treatment. Sci. Rep. 2016, 6, 24753. [Google Scholar] [CrossRef] [PubMed]


| Trigger | Gene Transporter | Gene | Trigger’s Outcome | Reference |
|---|---|---|---|---|
| Enzyme-triggered gene release (Enz-TGR) | ||||
| Glutathione-dependent enzyme-triggered gene release | Glycol chitosan conjugated to low molecular weight polyethylenimine (PEI) via a disulfide bond (GCS-ss-PEI) | GFP plasmid DNA | Low cytotoxicity, higher transgene expression, GSH responsive. | [52] |
| Cationic folic acid and camptothecin conjugated four-arm PEG micelle | Tumor necrosis factor-α (TNFα)-encoded plasmid | GSH-mediated TNFα plasmid DNA release, increased survival rate, reduced tumor metastasis, suppressed 4T1 tumor growth. | [53] | |
| Fluorinated bioreducible N,N-dimethyldipropylenetriamine polymer | Luciferase silencing RNA (LucsiRNA) | Low cytotoxicity, high gene silencing efficiency, GSH-mediated siRNA release, high cell internalization and buffering capacity. | [54] | |
| Surface charge-switchable folate modified co-delivery system and tumor-targeting polypeptide (FK)/PEG-2,3-dimethylmaleic anhydride-modified-PLL | P53-expressing plasmid | GSH-mediated release of proapoptotic peptide C-KLA (TPP) and p53 plasmid, high particle accumulation in tumor. | [55] | |
| Protease-triggered gene release | MMP2-sensitive self-assembling copolymer, polyethylene glycol-peptide-polyethylenimine-1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (PEG-pp-PEI-PE) | Anti-survivin siRNA | Successful cancer cell-selective co-delivery of siRNA and paclitaxel, higher cellular uptake and exposure of hidden PEI by MMP2 cleavage. | [56] |
| MMP2-sensitive peptide-CPP arginine (R9) peptide conjugated in between PEG and poly(ε-caprolactone) (PCL) in a micelle | Anti-Plk1 siRNA | Effective gene silencing, selective uptake of micelle in MMP2-overexpressing cancer cells. | [57] | |
| MMP2-cleavable substrate peptide conjugated cationic β-cyclodextrin-polyethylenimine conjugates (En-CNP) | microRNA miR-34a | High transfection of miR-34a inhibited 4T1 tumor growth. Increase in particle accumulation in tumor along with reduced accumulation in the liver. | [58] | |
| siRNA complexed cationic liposome consisting of PEG2000-peptidyl lipids with peptidyl moieties sensitive to MMP2 | Anti-luciferase siRNA | Increase in knockdown of luciferase expression in the stable luciferase-expressing cells MCF-7-luc and HT1080-luc. | [59] | |
| Light-triggered gene release (L-TGR) | ||||
| Photothermally triggered gene release | Gold nanorod-embedded large-pore mesoporous organosilica (GNR@LPMO) nanospheres | PLK1 siRNA | Effective gene release by photothermal irradiation, released PLK1 siRNA lowered PLK1 gene expression, induced early apoptosis, reduced tumor volume. | [60] |
| Chitosan-functionalized copper sulfide nanoparticles (CuS@CS NPs ) | Luciferase plasmid | Increase in luciferase expression after irradiation compared with PEI transfected cells. | [61] | |
| Single-walled carbon nanotube (SWCNT) wrapped with poly(ethylenimine)-cholesterol (PCS) | TP53 plasmid | Increase in TP53 expression, three-fold reduction in tumor volume compared to non-irradiated tumor. | [62] | |
| SWCNT conjugated PEI | hTERT siRNA | hTERT expression reduced in PC-3 tumor, resulted in decrease in tumor growth after Near infrared (NIR) irradiation. | [63] | |
| Photochemical internalization/photosensitizer-triggered gene release | Photosensitizer (TatU1A-dye)-labeled cell penetrating peptide (TAT) conjugated with RNA binding protein | EGFP shRNA | EGFP silencing efficiency after irradiation is 80% in the stable EGFP-expressing CHO cell line compared to non-irradiated cells. | [64] |
| Dendrimer phthalocyanine micelle coated over gold nanorods | Venus, yellow fluorescent protein (YFP)-expressing plasmid | YFP expression increased 5 times more in HeLa tumor than in non-irradiated tumor | [65] | |
| Pheophorbide-a (PhA)-conjugated chondroitin sulfate complexed PEI polyplex | EGFR-shRNA | HCT116 tumor growth drastically reduced with an increase in EGFR gene silencing after irradiation. | [66] | |
| Pegylated oligoethylenimine (OEI) conjugated to TPECM via an aminoacrylate (AA) linker | EGFP plasmid | After irradiation, enhanced gene expression in HeLa cells with higher cell viability. | [67] | |
| Ultrasound-triggered gene release (US-TGR) | ||||
| Microbubble ultrasound-triggered gene release | Lipid-based microbubble conjugated with polystyrene nanospheres and mRNA lipoplexes. | Luciferase mRNA | Increase in diffusion of mRNA lipoplexes into the cells through the membrane pores caused by cavitation microbubbles upon US irradiation. | [68] |
| PLGA/PEG nanoparticles delivered along with microbubble | miR-122 microRNA | Increase in the accumulation of miR-122 after US irradiation. | [69,70] | |
| PEGylated siRNA/lipid complexes conjugated over lipid-based microbubble via biotin-avidin conjugate. | Luciferase siRNA | Decrease in luciferase expression in HUH7eGFPLuc cells after US irradiation. | [71] | |
| TAT peptide-labeled PEG-modified liposomes (TAT-PEG liposomes) along with bubble liposomes | Luciferase plasmid | Increase in luciferase expression in HeLa cells upon US exposure. | [72] | |
| Nanobubble ultrasound triggered gene release | DOX-PLGA/PEI/P-gp shRNA nanobubbles | P-gp shRNA | Decrease in P-gp expression, and increased in DOX-mediated cell toxicity in MCF-7/ADR after US irradiation. | [73] |
| Cell penetrating peptide-siRNA conjugate loaded in liposome nanobubbles | Anti-c-myc siRNA | Inhibition of HT-1080 tumor due to the silencing of c-Myc by siRNA delivered through US exposure. | [74] | |
| siRNA/cationic liposome conjugated with glypican-3 (GPC3) antibody via biotin-avidin nanobubble (siRNA TNB) complexes | Neuroepithelial transforming protein 1 (NET-1) siRNA | Substantial increase in gene silencing efficiency after exposing the nanoparticles to low-frequency US. | [75] | |
| Mannosylated PEG nanobubble lipoplexes | Nf-KappaB decoy oligonucleotide (NKBDO) | Increase in transfection of oligonucleotide due to the US exposure, reduced solid tumor growth. | [76] | |
| Magnetic-triggered gene release (M-TGR) | ||||
| PAMAM dendrimer-coated magnetic nanoparticles (DcMNP) | CpG oligonucleotide | Higher cell apoptosis in MDA-MB-231 and SKBR3 cells. | [77] | |
| Disulfide PEI-coated SPION (PSPIO) | pcDNA3.Luciferase plasmid DNA | High gene transfection efficiency in the presence of serum after magnetic field exposure. | [78] | |
| Chitosan magnetic nanoparticles | TNF-related apoptosis-inducing ligand (TRAIL)-expressing plasmid | Increase in TRAIL gene expression after magnetofection caused apoptosis in cancer cells. | [79] | |
| PEI-modified Fe3O4 nanoparticle | pACTERT-TRAIL plasmid | Increase in apoptosis induced in SACC-83 cells and Tca83 cells by TRAIL gene expression after magnetic field application. | [80,81] | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendrakumar, S.K.; Uthaman, S.; Cho, C.S.; Park, I.-K. Trigger-Responsive Gene Transporters for Anticancer Therapy. Nanomaterials 2017, 7, 120. https://doi.org/10.3390/nano7060120
Rajendrakumar SK, Uthaman S, Cho CS, Park I-K. Trigger-Responsive Gene Transporters for Anticancer Therapy. Nanomaterials. 2017; 7(6):120. https://doi.org/10.3390/nano7060120
Chicago/Turabian StyleRajendrakumar, Santhosh Kalash, Saji Uthaman, Chong Su Cho, and In-Kyu Park. 2017. "Trigger-Responsive Gene Transporters for Anticancer Therapy" Nanomaterials 7, no. 6: 120. https://doi.org/10.3390/nano7060120