Oscillating Magnet Array−Based Nanomagnetic Gene Transfection: A Valuable Tool for Molecular Neurobiology Studies
Abstract
:1. Introduction
2. Results
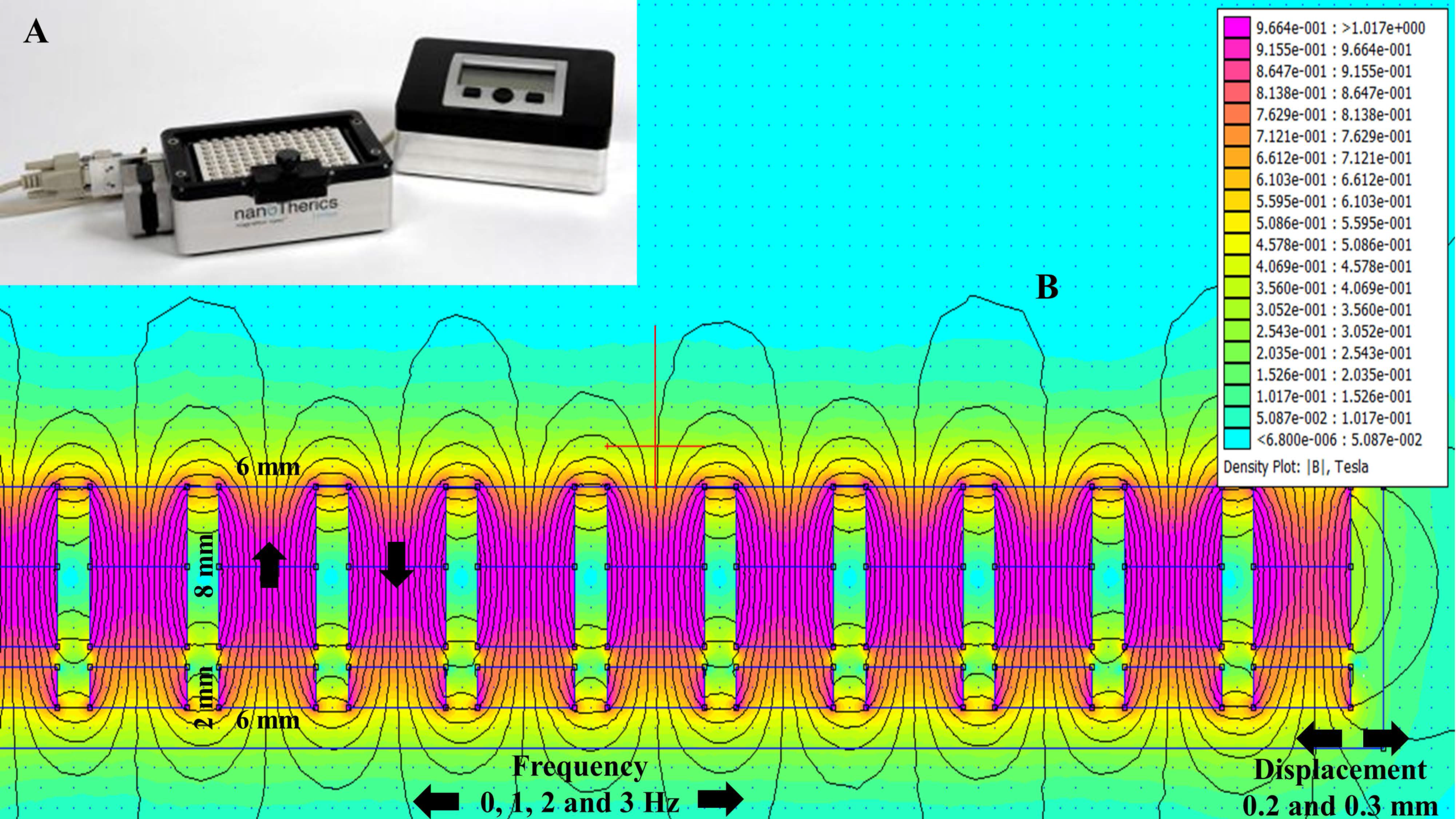
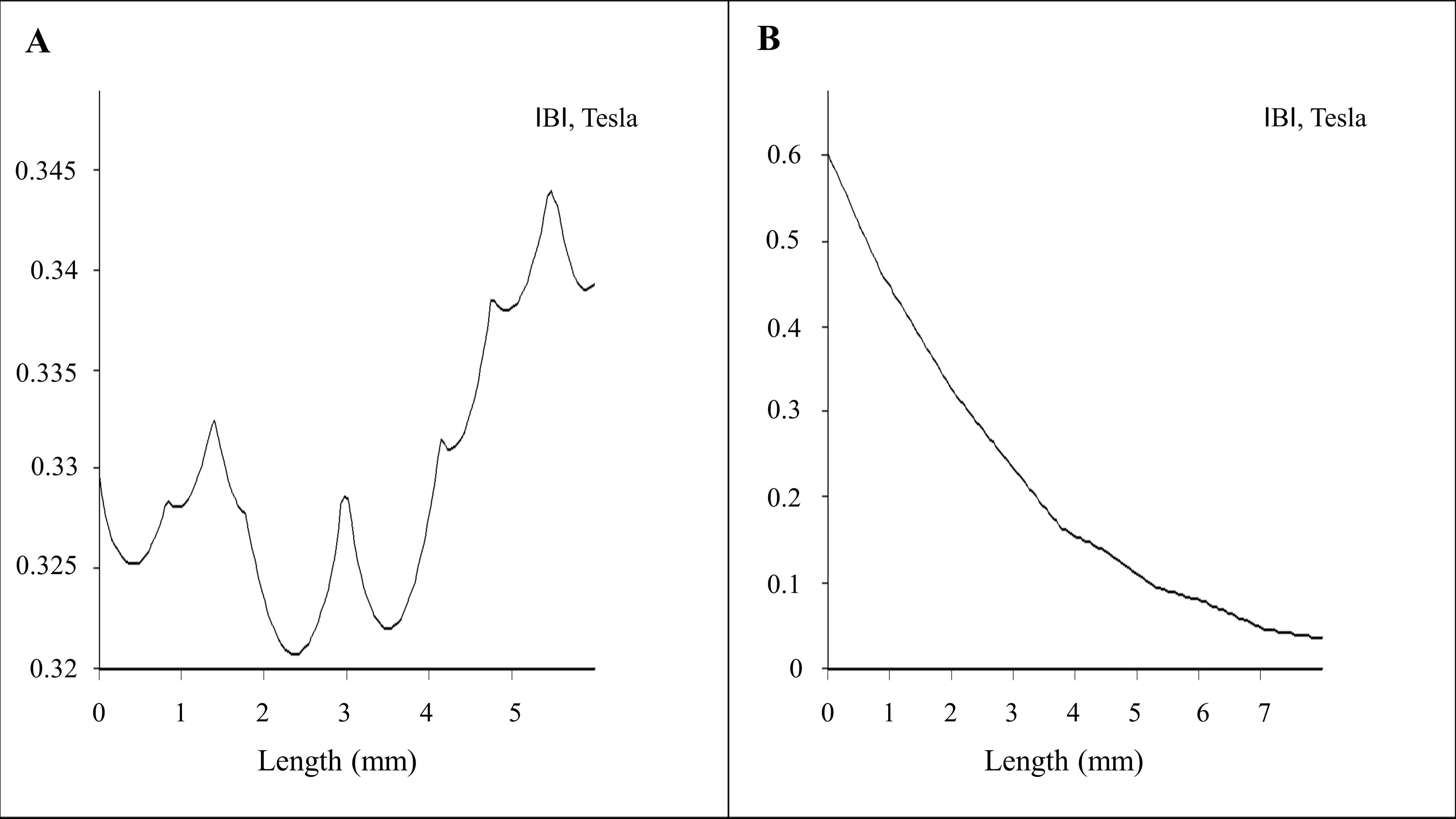
2.1. Magnetostatic Calculation
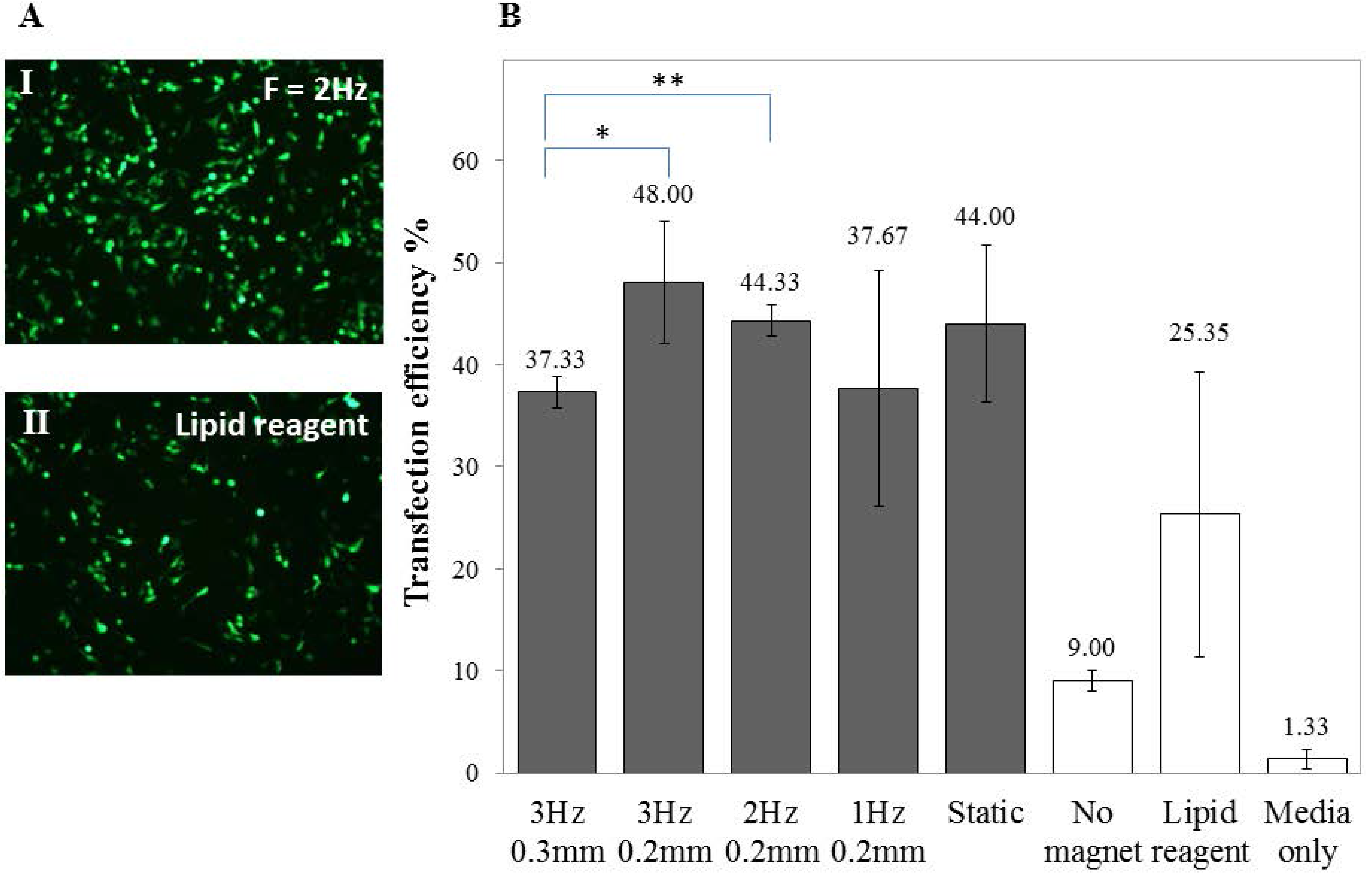
2.2. Transfection of Undifferentiated SH-SY5Y Cells
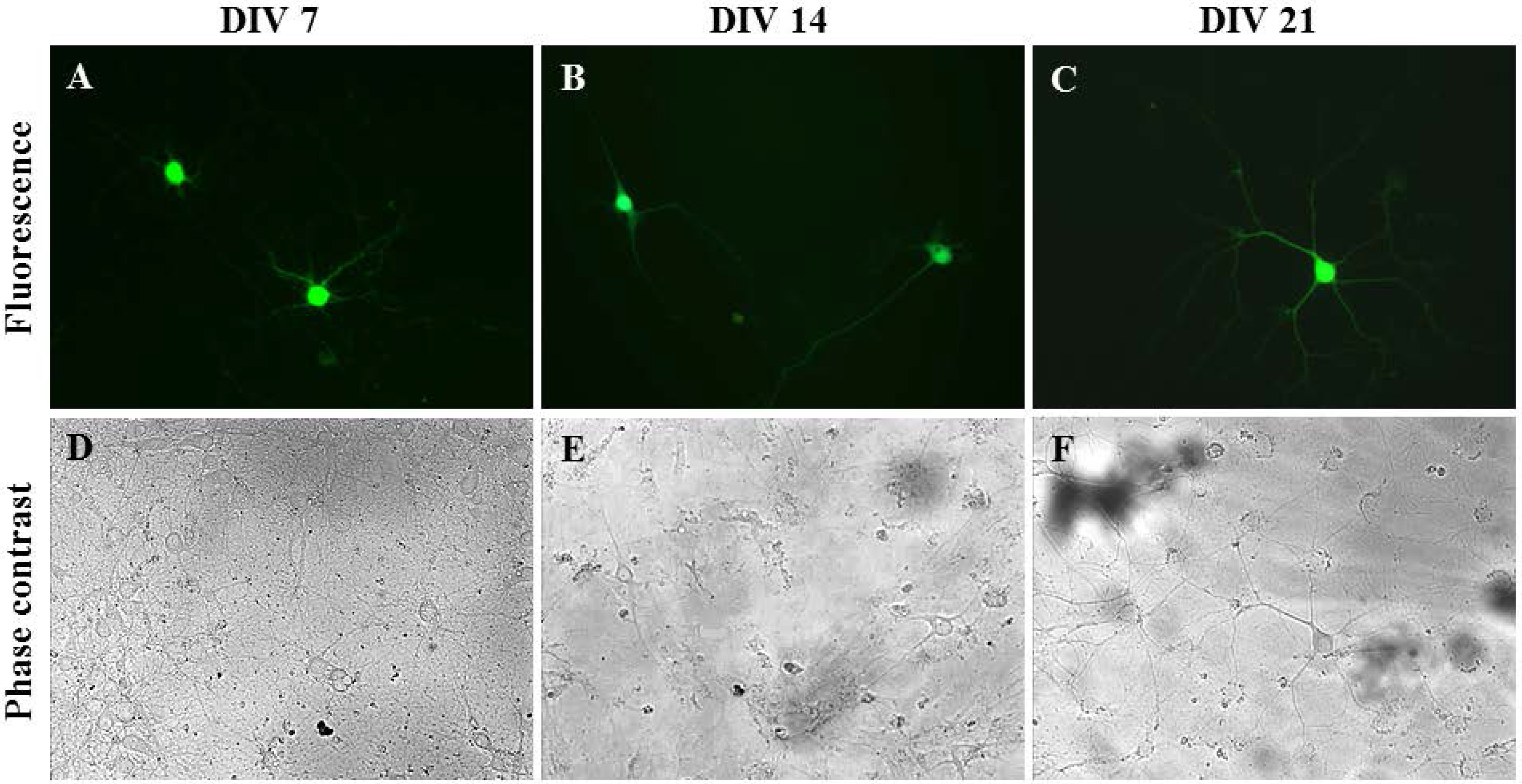
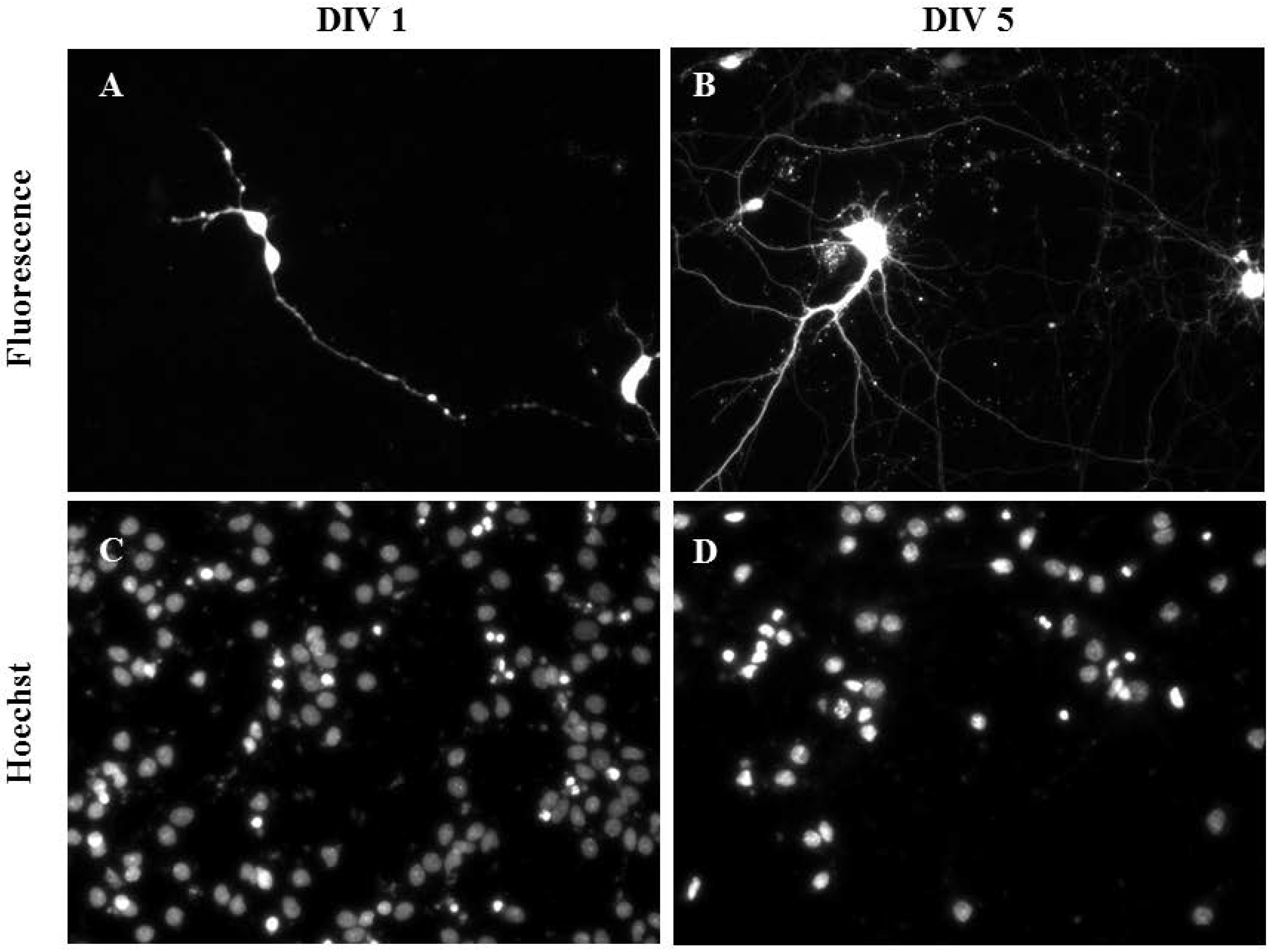
2.3. Gene Delivery and Prolonged Expression in Primary Hippocampal Neurons on Different Days In Vitro
2.4. Gene Delivery by Oscillating Nanomagnetic Gene Transfection in Primary Cortical Neurons
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. DNA Constructs
4.4. Transfection of Neurons (CN)
4.4.1. Nanomagnetic Gene Transfection
4.4.2. Lipid-Based Transfection
4.5. Flow Cytometry
4.6. Fluorescent Microscopy
4.7. Cell Viability Assay
4.8. Statistics
4.9. Numerical Model and Magnetic Field Calculation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- LaBerge, D.; Kasevich, R. The cognitive significance of resonating neurons in the cerebral cortex. Conscious. Cogn. 2013, 22, 1523–1550. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Williams, S.K.; Diem, R. Dysfunction of neuronal calcium signalling in neuroinflammation and neurodegeneration. Cell Tissue Res. 2013, 357, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kapogiannis, D.; Mattson, M.P. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011, 10, 187–198. [Google Scholar] [CrossRef]
- Bagetta, V.; Ghiglieri, V.; Sgobio, C.; Calabresi, P.; Picconi, B. Synaptic dysfunction in Parkinson’s disease. Biochem. Soc. Trans. 2010, 38, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Pavlov, I.; Kaila, K.; Kullmann, D.M.; Miles, R. Cortical inhibition, pH and cell excitability in epilepsy: What are optimal targets for antiepileptic interventions. J. Physiol. 2013, 59, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Ellwardt, E.; Zipp, F. Molecular mechanisms linking neuroinflammation and neurodegeneration in MS. Exp. Neurol. 2014, 262, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Buerli, T.; Pellegrino, C.; Baer, K.; Lardi-Studler, B.; Chudotvorova, I.; Fritschy, J.M.; Medina, I.; Fuhrer, C. Efficient transfection of DNA or shRNA vectors into neurons using nanomagnetic gene transfection. Nat. Protoc. 2007, 2, 3090–3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallini, C.; Bassell, G.J.; Rossoll, W. High-efficiency transfection of cultured primary motor neurons to study protein localization, trafficking, and function. Mol. Neurodegener. 2010, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Aoshima, Y.; Hokama, R.; Sou, K.; Sarker, S.R.; Iida, K.; Nakamura, H.; Inoue, T.; Takeoka, S. Cationic amino acid based lipids as effective nonviral gene delivery vectors for primary cultured neurons. ACS Chem. Neurosci. 2013, 4, 1514–1519. [Google Scholar] [CrossRef] [PubMed]
- Antkowiak, M.; Torres-Mapa, M.L.; Witts, E.C.; Miles, G.B.; Dholakia, K.; Gunn-Moore, F.J. Fast targeted gene transfection and optogenetic modification of single neurons using femtosecond laser irradiation. Sci. Rep. 2013, 3, 3281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hur, J.; Jeong, H.J.; Park, J.; Jeon, S. Chloride channel 4 is required for nerve growth factor-induced TrkAsignaling and neurite outgrowth in PC12 cells and cortical neurons. Neuroscience 2013, 253, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Kusakari, S.; Sato-Hashimoto, M.; Hayashi, Y.; Kotani, T.; Murata, Y.; Okazawa, H.; Oldenborg, P.A.; Kishi, S.; Matozaki, T.; et al. Hypothermia-induced tyrosine phosphorylation of SIRPα in the brain. J. Neurochem. 2012, 121, 891–902. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.E.; Ying, Z.; Baillie, L.D.; Zhai, R.; Mulligan, S.J.; Verge, V.M. Extracellular pH and neuronal depolarization serve as dynamic switches to rapidly mobilize trkA to the membrane of adult sensory neurons. J. Neurosci. 2013, 33, 8202–8215. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, V.A.; Salameh, A.I.; Boron, W.F.; Parker, M.D. Intracellular pH regulation by acid-base transporters in mammalian neurons. Front. Physiol. 2014, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Sariyer, I.K. Transfection of neuronal cultures. Methods Mol. Biol. 2013, 1078, 133–139. [Google Scholar] [PubMed]
- Agholme, L.; Lindström, T.; Kågedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimer’s Dis. 2010, 20, 1069–1082. [Google Scholar]
- Dwane, S.; Durack, E.; Kiely, P.A. Optimising parameters for the differentiation of SH-SY5Y cells to study cell adhesion and cell migration. BMC Res. Notes 2013, 6, 366. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili-Mahani, S.; Vazifekhah, S.; Pasban-Aliabadi, H.; Abbasnejad, M.; Sheibani, V. Protective effect of orexin-A on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y human dopaminergic neuroblastoma cells. Neurochem. Int. 2013, 63, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Chen, S.D.; Chuang, Y.C.; Lin, H.Y.; Huang, C.R.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; et al. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic SH-SY5Y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014, 15, 1625–1646. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, L.; Zhu, X.; Zhang, K.; Huang, B.; Zhang, J.; Zhang, Y.; Zhu, L.; Zhou, B.; Zhou, F. Protective Effect of Paeoniflorin on Aβ25-35-Induced SH-SY5Y Cell Injury by Preventing Mitochondrial Dysfunction. Cell. Mol. Neurobiol. 2014, 34, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.D.; Refai, F.S.; Xie, S.P.; Ng, S.H.; Chan, C.H.; Ho, P.G.; Zhang, X.D.; Lim, T.M.; Tan, E.K. Mutant PINK1 upregulates tyrosine hydroxylase and dopamine levels, leading to vulnerability of dopaminergic neurons. Free Radic. Biol. Med. 2014, 68, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.Y.; Tang, B.L. Nogo/RTN4 isoforms and RTN3 expression protect SH-SY5Y cells against multiple death insults. Mol. Cell. Biochem. 2013, 384, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Vernon, M.M.; Dean, D.A.; Dobson, J. DNA Targeting Sequence Improves Magnetic Nanoparticle-Based Plasmid DNA Transfection Efficiency in Model Neurons. Int. J. Mol. Sci. 2015, 16, 19369–19386. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Lim, J.; Dobson, J. Enhanced nanomagnetic gene transfection of human prenatal cardiac progenitor cells and adult cardiomyocytes. PLoS ONE 2013, 8, e69812. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Clements, M.A.; Dobson, J. Delivery of Short Interfering Ribonucleic Acid-Complexed Magnetic Nanoparticles in an Oscillating Field Occurs via Caveolae-Mediated Endocytosis. PLoS ONE 2012, 7, e51350. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Dobson, J. Improved transfection efficiency of HUVEC and MEF cells using DNA-complexes with magnetic nanoparticles in an oscillating magnetic field. J. Genet. 2012, 91, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.R.; Chari, D.M. Enhancement of magnetic nanoparticle-mediated gene transfer to astrocytes by ‘magnetofection’: Effects of static and oscillating fields. Nanomedicine 2010, 5, 217–232. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Grienenbach, U.; Xenariou, S.; Keramane, A.; Batich, C.D.; Alton, E.W.; Dobson, J. Magnetic nanoparticles as gene delivery agents: Enhanced transfection in the presence of oscillating magnet arrays. Nanotechnology 2008, 19, 405102. [Google Scholar] [CrossRef] [PubMed]
- Fouriki, A.; Farrow, N.; Clements, M.A.; Dobson, J. Evaluation of the magnetic field requirements for nanomagnetic gene transfection. Nano Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.I.; Pickard, M.R.; Granger, N.; Chari, D.M. Magnetic nanoparticle- mediated gene transfer to oligodendrocyte precursor cell transplant populations is enhanced by nanomagnetic gene transfection strategies. ACS Nano 2011, 5, 6527–6538. [Google Scholar] [CrossRef] [PubMed]
- Schoch, S.; Cibelli, G.; Thiel, G. Neuron-specific gene expression of synapsin I. Major role of a negative regulatory mechanism. J. Biol. Chem. 1996, 271, 3317–3323. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Shiroshima, T.; Lee, S.J.; Yasumura, M.; Uemura, T.; Chen, X.; Iwakura, Y.; Mishina, M. Interleukin-1 receptor accessory protein organizes neuronal synaptogenesis as a cell adhesion molecule. J. Neurosci. 2012, 32, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Espada, S.; Ortega, F.; Molina-Jijón, E.; Rojo, A.I.; Pérez-Sen, R.; Pedraza-Chaverri, J.; Miras-Portugal, M.T.; Cuadrado, A. The purinergic P2Y13 receptor activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Free Radic. Biol. Med. 2010, 49, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Beltrán, S.; Espada, S.; Orozco-Ibarra, M.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic acid activates the antioxidant pathway Nrf2/HO-1 and protects cerebellar granule neurons against oxidative stress. Neurosci. Lett. 2008, 447, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Y.; Song, H.; Liu, Y.; Liu, M.; Yuan, Y.; Ding, F.; Gu, X.; Wang, Y. Involvement of gecko SNAP25b in spinal cord regeneration by promoting outgrowth and elongation of neurites. Int. J. Biochem. Cell Biol. 2012, 44, 2288–2298. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, K.; Im, H. α-Synuclein modulates neurite outgrowth by interacting with SPTBN1. Biochem. Biophys. Res. Commun. 2012, 424, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Picard, K.L.; Nyska, A.; Tischler, A.S. Adrenergic differentiation and ret expression in rat pheochromocytomas. Endocr. Pathol. 2008, 19, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fouriki, A.; Clements, M.A.; Farrow, N.; Dobson, J. Efficient transfection of MG-63 osteoblasts using magnetic nanoparticles and oscillating magnetic fields. J. Tissue Eng. Regen. Med. 2012, 8, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Fouriki, A.; Dobson, J. Oscillating magnet array-based nanomagnetic gene transfection of human mesenchymal stem cells. Nanomedicine 2013, 9, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.F.; Pickard, M.R.; Chari, D.M. Magnetic nanoparticle mediated transfection of neural stem cell suspension cultures is enhanced by applied oscillating magnetic fields. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Kenan, R.F.B.; Osakada, Y.; Xu, W.; Sinit, R.S.; Chen, L.; Zhao, X.; Chen, J.; Cui, B.; Wu, C. Defective Axonal Transport of Rab7 GTPase Results in Dysregulated Trophic Signaling. J. Neurosci. 2013, 33, 7451–7462. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J.J. Applications of MNPs in biomedicine. J. Phys. D 2003, 36, R167. [Google Scholar] [CrossRef]
- Zhang, H.; Lee, M.Y.; Hogg, M.G.; Dordick, J.S.; Sharfstein, S.T. Gene delivery in three-dimensional cell cultures by superparamagnetic nanoparticles. ACS Nano 2010, 4, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Zhang, M. Superparamagnetic iron oxide nanoparticle-based delivery systems for biotherapeutics. Expert Opin. Drug Deliv. 2013, 10, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Delyagina, E.; Li, W.; Ma, N.; Steinhoff, G. Magnetic targeting strategies in gene delivery. Nanomedicine 2011, 6, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Sapet, C.; Laurent, N.; de Chevigny, A.; Le Gourrierec, L.; Bertosio, E.; Zelphati, O.; Béclin, C. High transfection efficiency of neural stem cells with magnetofection. Biotechniques 2011, 50, 187–189. [Google Scholar] [PubMed]
- Takei, Y. Phosphorylation of Nogo receptors suppresses Nogo signaling, allowing neurite regeneration. Sci. Signal. 2009, 2, ra14. [Google Scholar] [CrossRef] [PubMed]
- Jämsä, A.; Hasslund, K.; Cowburn, R.F.; Bäckström, A.; Vasänge, M. The retinoic acid and brain-derived neurotrophic factor differentiated SH-SY5Y cell line as a model for Alzheimer’s disease-like tau phosphorylation. Biochem. Biophys. Res. Commun. 2004, 319, 993–1000. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subramanian, M.; Tyler, A.-J.; Luther, E.M.; Daniel, E.D.; Lim, J.; Dobson, J. Oscillating Magnet Array−Based Nanomagnetic Gene Transfection: A Valuable Tool for Molecular Neurobiology Studies. Nanomaterials 2017, 7, 28. https://doi.org/10.3390/nano7020028
Subramanian M, Tyler A-J, Luther EM, Daniel ED, Lim J, Dobson J. Oscillating Magnet Array−Based Nanomagnetic Gene Transfection: A Valuable Tool for Molecular Neurobiology Studies. Nanomaterials. 2017; 7(2):28. https://doi.org/10.3390/nano7020028
Chicago/Turabian StyleSubramanian, Mahendran, Aimee-Jayne Tyler, Eva Maria Luther, Elena Di Daniel, Jenson Lim, and Jon Dobson. 2017. "Oscillating Magnet Array−Based Nanomagnetic Gene Transfection: A Valuable Tool for Molecular Neurobiology Studies" Nanomaterials 7, no. 2: 28. https://doi.org/10.3390/nano7020028
APA StyleSubramanian, M., Tyler, A.-J., Luther, E. M., Daniel, E. D., Lim, J., & Dobson, J. (2017). Oscillating Magnet Array−Based Nanomagnetic Gene Transfection: A Valuable Tool for Molecular Neurobiology Studies. Nanomaterials, 7(2), 28. https://doi.org/10.3390/nano7020028





