Aggregation and Colloidal Stability of Commercially Available Al2O3 Nanoparticles in Aqueous Environments
Abstract
:1. Introduction
2. Results and Discussion
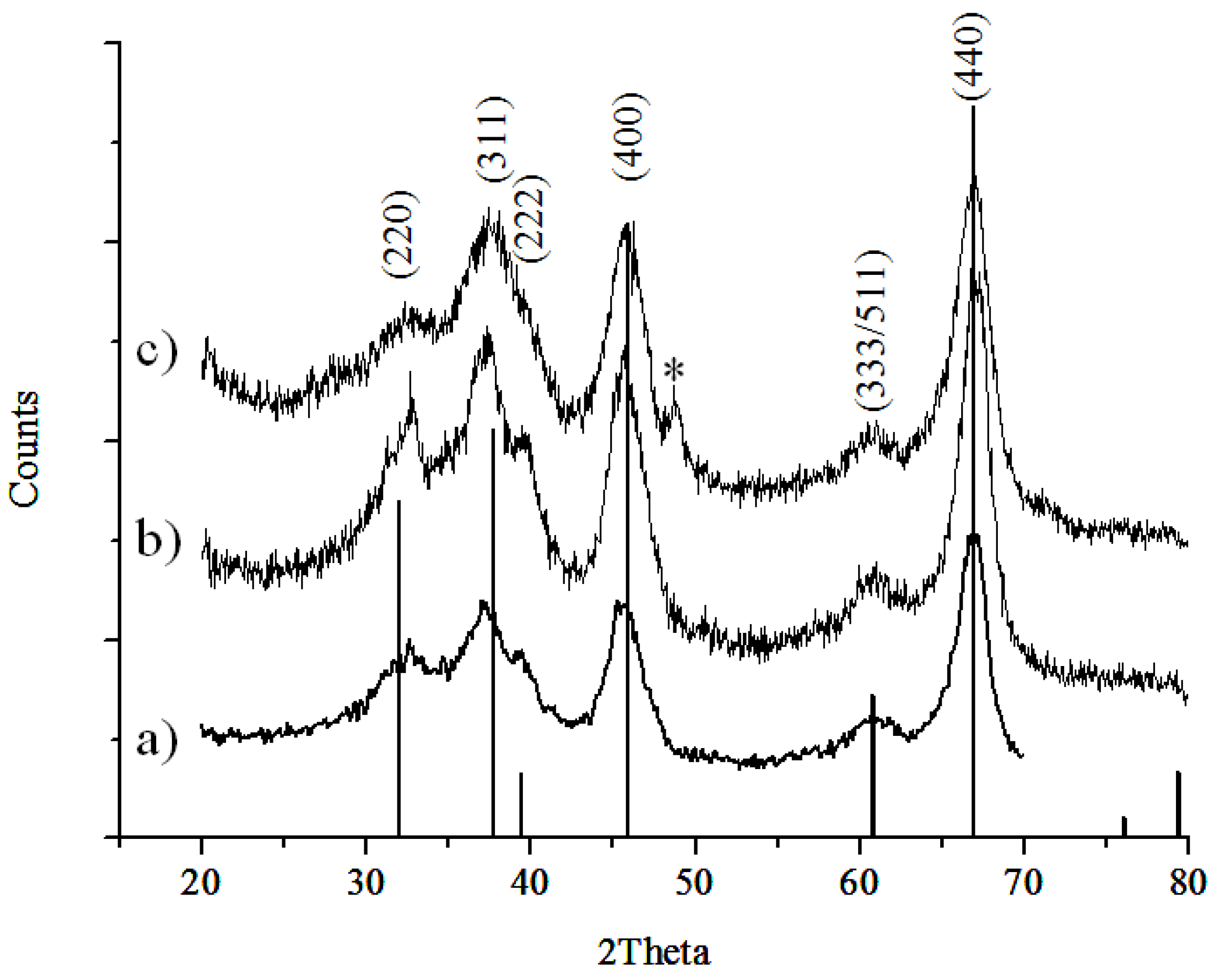
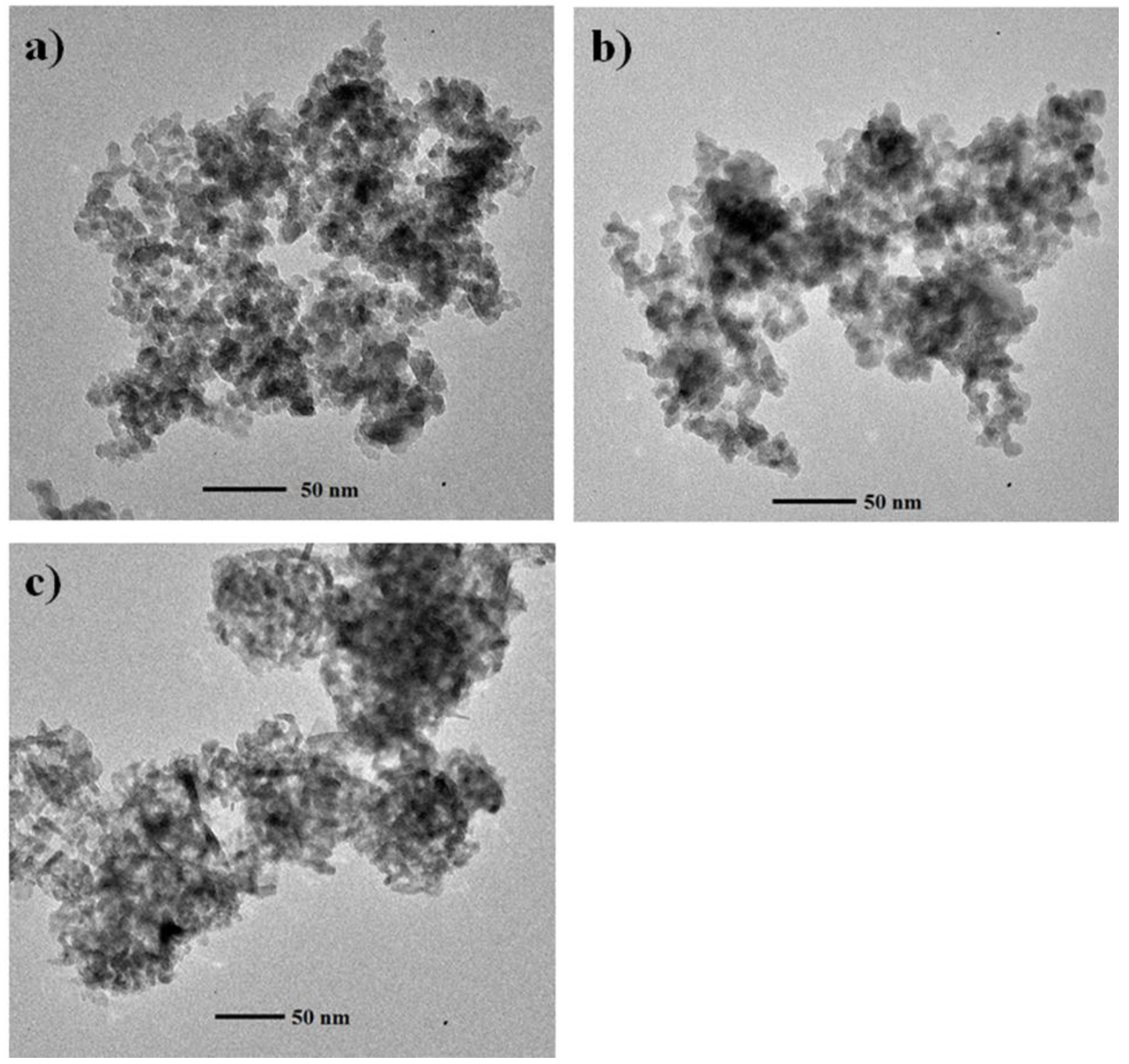
2.1. Material Characterization
2.2. Influence of Solution pH on Aggregation of Al2O3 NPs
2.3. Influence of Ionic Strength on Aggregation of Al2O3 NPs
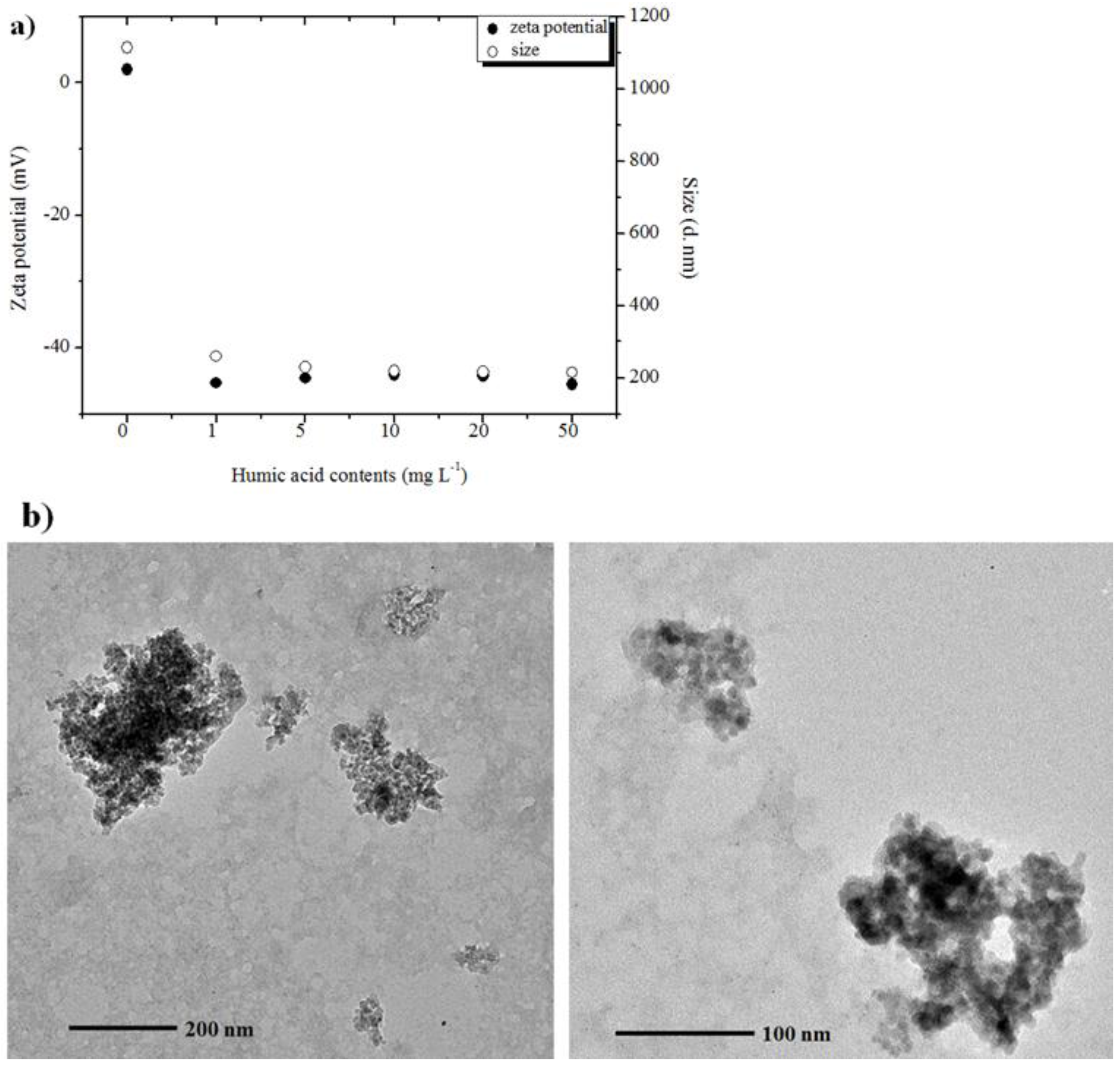
2.4. Influence of Humic Acid on Aggregation of Al2O3 NPs
2.5. Influence of Montmorillonite on Aggregation of Al2O3 NPs
3. Experimental Section
3.1. Materials
3.2. Powder XRD Measurements
3.3. Zeta Potential and Size Distribution Measurements
3.4. Ultrafiltration
3.5. TEM Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BF | Bright Field |
| DLS | Dynamic Light Scattering |
| HA | Humic Acid |
| ICP-OES | Inductively-Coupled Plasma Optical Emission Spectrometry |
| NPs | Nanoparticles |
| PZC | Point of Zero Charge |
| TEM | Transmission Electron Microscopy |
| XRD | X-ray diffraction |
References
- The Royal Society & the Royal Academy of Engineering. Nanoscience and Nanotechnologies: Opportunities and Uncertainties; Royal Society Publications: London, UK, 2004. [Google Scholar]
- U.S. Environmental Protection Agency. Nanotechnology White Paper, February 2007. Available online: https://www.epa.gov/sites/production/files/2015-01/documents/nanotechnology_whitepaper.pdf (accessed on 3 April 2016).
- Lu, W.; Lieber, C.M. Nanoelectronics from the bottom up. Nat. Mater. 2007, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The use of zero-valent iron for groundwater remediation and wastewater treatment: A review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Future Markets Inc. The Global Market for Metal Oxide Nanoparticles to 2020 (2013): 322. Available online: http://www.researchandmarkets.com/research/jkjd5k/the_global_market (accessed on 28 January 2016).
- Rittner, M.N.; Abraham, T. Nanostructured materials: An overview and commercial analysis. JOM 1998, 50, 37–38. [Google Scholar] [CrossRef]
- Abraham, T. Healthy Prospects for Nanoceramic Powders; Ceramic Industry: Norwalk, CT, USA, 2000. [Google Scholar]
- Frey, A.; Neutra, M.R.; Robey, F.A. Peptomer aluminum oxide nanoparticle conjugates as systemic and mucosal vaccine candidates: Synthesis and characterization of a conjugate derived from the C4 domain of HIV-1MN Gp120. Bioconjugate Chem. 1997, 8, 424–433. [Google Scholar] [CrossRef] [PubMed]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Sawyer, W.G.; Freudenberg, K.D.; Bhimaraj, P.; Schadler, L.S. A study on the friction and wear behavior of PTFE filled with alumina nanoparticles. Wear 2003, 254, 573–580. [Google Scholar] [CrossRef]
- De Luca, L.T.; Galfetti, L.; Severini, F.; Meda, L.; Marra, G.; Vorozhtsov, A.B.; Sedoi, V.S.; Babuk, V.A. Burning of Nano-Aluminized Composite Rocket Propellants. Combust. Explos. Shock Waves 2005, 41, 680–692. [Google Scholar] [CrossRef]
- Khanna, A.S. Nanotechnology in High Performance Paint Coatings. Asian J. Exp. Sci. 2008, 21, 25–32. [Google Scholar]
- Landry, V.; Riedl, B.; Blanchet, P. Alumina and zirconia acrylate nanocomposites coatings for wood flooring: Photocalorimetric characterization. Prog. Org. Coat. 2008, 61, 76–82. [Google Scholar] [CrossRef]
- Wong, K.F.; Kurma, T. Transport properties of alumina nanofluids. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Lewis, W.K.; Harruff, B.A.; Gord, J.R.; Rosenberger, A.T.; Sexton, T.M.; Guliants, E.A.; Bunker, C.E. Chemical Dynamics of Aluminum Nanoparticles in Ammonium Nitrate and Ammonium Perchlorate Matrices: Enhanced Reactivity of Organically Capped Aluminum. J. Phys. Chem. C 2011, 115, 70–77. [Google Scholar] [CrossRef]
- Jolivet, J.-P.; Barron, A.R. Nanomaterials Fabrication. In Environmental Nanotechnology: Applications and Impacts of Nanomaterials; Wiesner, M.R., Bottero, J.-Y., Eds.; McGraw-Hill: New York, NY, USA, 2007; Chapter 3; pp. 29–103. [Google Scholar]
- Griffitt, R.J.; Luo, J.; Gao, J.; Bonzongo, J.-C.; Barber, D.S. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ. Toxicol. Chem. 2008, 27, 1972–1978. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.M.; Pakrashi, S.; Chandrasekaran, N.; Mukherjee, A. Studies on toxicity of aluminum oxide (Al2O3) nanoparticles to microalgae species: Scenedesmus sp. and Chlorella sp. J. Nanopart. Res. 2011, 13, 3287–3299. [Google Scholar] [CrossRef]
- Pakrashi, S.; Dalai, S.; Prathna, T.C.; Trivedi, S.; Myneni, R.; Raichur, A.M.; Chandrasekaran, N.; Mukherjee, A. Cytotoxicity of aluminum oxide nanoparticles towards fresh water algal isolate at low exposure concentrations. Aquat. Toxicol. 2013, 132–133, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Czymmek, K.J.; Huang, C.P. Responses of Ceriodaphnia dubia to TiO2 and Al2O3 nanoparticles: A dynamic nano-toxicity assessment of energy budget distribution. J. Hazard. Mater. 2011, 187, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Pakrashi, S.; Dalai, S.; Humayun, A.; Chakravarty, S.; Chandrasekaran, N.; Mukherjee, A. Ceriodaphnia dubia as a potential bio-indicator for assessing acute aluminum oxide nanoparticle toxicity in fresh water environment. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, L.; Duan, Z.; Qi, R.; Li, Y. Comparative toxicity of several metal oxide nanoparticle aqueous suspensions to Zebrafish (Danio rerio) early development stage. J. Environ. Sci. Health A 2008, 43, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, I.M.; Chowdhury, B.; Chandrasekaran, N.; Mukherjee, A. Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yu, S.; Wu, Q.; Tang, M.; Pu, Y.; Wang, D. Chronic Al2O3-nanoparticle exposure causes neurotoxic effects on locomotion behaviors by inducing severe ROS production and disruption of ROS defense mechanisms in nematode Caenorhabditis elegans. J. Hazard. Mater. 2012, 219–220, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Stayton, I.; Huang, Y.-W.; Zhou, X.-D.; Ma, Y. Cytotoxicity and cell membrane depolarization induced by aluminum oxide nanoparticles in human lung epithelial cells A549. Toxicol. Environ. Chem. 2008, 90, 983–996. [Google Scholar] [CrossRef]
- Di Virgilio, A.L.; Reigosa, M.; Arnal, P.M.; De Mele, M.F.L. Comparative study of the cytotoxic and genotoxic effects of titanium oxide and aluminum oxide nanoparticles in Chinese hamster ovary (CHO-K1) cells. J. Hazard. Mater. 2010, 177, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mashayekhi, H.; Pan, B.; Bhowmik, P.; Xing, B. Colloidal behavior of aluminum oxide nanoparticles as affected by pH and natural organic matter. Langmuir 2008, 24, 12385–12391. [Google Scholar] [CrossRef] [PubMed]
- Hochella, M.F., Jr.; Aruguete, D.; Kim, B.; Madden, A.S. Naturally occurring inorganic nanoparticles: General assessment and a global budget for one of earth’s last unexplored geochemical components. In Nature’s Nanostructures; Barnard, A.S., Guo, H., Eds.; Pan Stanford Publishing: Singapore, Singapore, 2012; pp. 1–42. [Google Scholar]
- Chen, G.; Liu, X.; Su, C. Distinct effects of humic acid on transport and retention of TiO2 rutile nanoparticles in saturated sand columns. Environ. Sci. Technol. 2012, 46, 7142–7150. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Le Coustumer, P.; Stoll, S. TiO2 nanoparticles aggregation and disaggregation in presence of alginate and Suwannee River humic acids. pH and concentration effects on nanoparticle stability. Water Res. 2013, 47, 6052–6063. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Vitorazi, L.; Berret, F.-F.; Stoll, S. Towards a better understanding on agglomeration mechanisms and thermodynamic properties of TiO2 nanoparticles interacting with natural organic matter. Water Res. 2015, 80, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Tong, M.P.; Li, H.Y.; Yang, K. Deposition kinetics of zinc oxide nanoparticles on natural organic matter coated silica surfaces. J. Colloid Interface Sci. 2011, 350, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-F.; Zhao, Z.-S.; Jiang, G.-B. Coating Fe3O4 magnetic nanoparticles with humic acid for high efficient removal of heavy metals in water. Environ. Sci. Technol. 2008, 42, 6949–6954. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Mashayekhi, H.; Bhowmik, P.; Xing, B. Colloidal stability of Al2O3 nanoparticles as affected by coating of structurally different humic acids. Langmuir 2010, 26, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Lagaly, G.; Ziesmer, S. Colloid chemistry of clay minerals: The coagulation of montmorillonite dispersions. Adv. Colloid Interface Sci. 2003, 100, 105–128. [Google Scholar] [CrossRef]
- Tombácz, E. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Zhou, D.; Abdel-Fattah, A.I.; Keller, A.A. Clay minerals destabilize engineered nanoparticles in aqueous environments. Environ. Sci. Technol. 2012, 46, 7520–7526. [Google Scholar] [CrossRef] [PubMed]
- Lippens, B.C.; De Boer, J.H. Study of phase transformations during calcination of aluminum hydroxides by selected area electron diffraction. Acta Crystallogr. 1964, 17. [Google Scholar] [CrossRef]
- Zhou, R.-S.; Snyder, R.L. Structures and transformation mechanisms of the η, γ, and θ transition aluminas. Acta Crystallogr. 1991, 47, 617–630. [Google Scholar] [CrossRef]
- Digne, M.; Sautet, P.; Raybaud, P.; Euzen, P.; Toulhoat, H. Use of DFT to achieve a rational understanding of acid-basic properties of γ-alumina surfaces. J. Catal. 2004, 226, 54–68. [Google Scholar] [CrossRef]
- Handy, R.D.; von der Kammer, F.; Lead, J.R.; Hassellöv, M.; Owen, R.; Crane, M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology 2008, 17, 287–314. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Hotze, E.M.; Bernhardt, E.S.; Dionysiou, D.D.; Pedersen, J.A.; Wiesner, M.R.; Xing, B. Environmental occurrences, behavior, fate and ecological effects of nanomaterials. J. Environ. Qual. 2010, 39, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Ottofuelling, S.; von der Kammer, F.; Hofmann, T. Commercial titanium dioxide nanoparticles in both natural and synthetic water: Comprehensive multidimensional testing and prediction of aggregation behavior. Environ. Sci. Technol. 2011, 45, 10045–10052. [Google Scholar] [CrossRef] [PubMed]
- Godymchuk, A.; Karepina, E.; Yunda, E.; Bozhko, I.; Lyamina, G.; Kuznetsov, D.; Gusev, A.; Kosova, N. Aggregation of manufactured nanoparticles in aqueous solutions of mono- and bivalent electrolytes. J. Nanopart. Res. 2015, 17. [Google Scholar] [CrossRef]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P.C. Influence of ionic strength, pH, and cation valence on aggregation kinetics of titanium dioxide nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Thio, B.J.R.; Zhou, D.; Keller, A.A. Influence of natural organic matter on the aggregation and deposition of titanium dioxide nanoparticles. J. Hazard. Mater. 2011, 189, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Buettner, K.M.; Rinciog, C.I.; Mylon, S.E. Aggregation kinetics of cerium oxide nanoparticles in monovalent and divalent electrolyte. Colloids Surf. A 2010, 366, 74–79. [Google Scholar] [CrossRef]
- Avena, M.J.; Koopal, L.K. Kinetics of humic acid adsorption at solid-water interfaces. Environ. Sci. Technol. 1999, 33, 2739–2744. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry; Interscience: New York, NY, USA, 1963. [Google Scholar]
- Johnston, C.T.; Tombácz, E. Soil Mineralogy with Environmental Applications; Dixon, J.B., Schulze, D.G., Eds.; Soil Science Society of America: Madison, WI, USA, 2002; pp. 37–67. [Google Scholar]
- Delhorme, M.; Labbez, C.; Caillet, C.; Thomas, F. Acid-Base Properties of 2:1 Clays. I. Modeling the Role of Electrostatics. Langmuir 2010, 26, 9240–9249. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Hochella, M.F., Jr. Analytical Transmission Electron Microscopy and Scanning Transmission Electron Microscopy Techniques for the Characterization of Nanomaterial Composition, Phase, and Crystallinit. In Characterization of Nanomaterials in Complex Environmental and Biological Media; Baalousha, M., Saleh, N., Lead, J., Eds.; Elsevier Ltd.: Waltham, MA, USA, 2015; pp. 123–152. [Google Scholar]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mui, J.; Ngo, J.; Kim, B. Aggregation and Colloidal Stability of Commercially Available Al2O3 Nanoparticles in Aqueous Environments. Nanomaterials 2016, 6, 90. https://doi.org/10.3390/nano6050090
Mui J, Ngo J, Kim B. Aggregation and Colloidal Stability of Commercially Available Al2O3 Nanoparticles in Aqueous Environments. Nanomaterials. 2016; 6(5):90. https://doi.org/10.3390/nano6050090
Chicago/Turabian StyleMui, Julie, Jennifer Ngo, and Bojeong Kim. 2016. "Aggregation and Colloidal Stability of Commercially Available Al2O3 Nanoparticles in Aqueous Environments" Nanomaterials 6, no. 5: 90. https://doi.org/10.3390/nano6050090




