Morphology-Controlled High-Efficiency Small Molecule Organic Solar Cells without Additive Solvent Treatment
Abstract
:1. Introduction
2. Experimental Section
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Van der Poll, T.S.; Love, J.A.; Nguyen, T.Q.; Bazan, G.C. Non-Basic High-Performance Molecules for Solution-Processed Organic Solar Cells. Adv. Mater. 2012, 24, 3646–3649. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-processed small-molecule solar cells with 6.7% efficiency. Nat. Mater. 2012, 11, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, A.K.K.; Wang, D.H.; Gupta, V.; Leong, W.L.; Ke, L.; Bazan, G.C.; Heeger, A.J. Intensity dependence of current-voltage characteristics and recombination in high-efficiency solution-processed small-molecule solar cells. ACS Nano 2013, 7, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kyaw, A.K.K.; Wang, D.H.; Chand, S.; Bazan, G.C.; Heeger, A.J. Barium: An efficient cathode layer for bulk-heterojunction solar cells. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kan, B.; Liu, F.; Long, G.; Wan, X.; Chen, X.; Zuo, Y.; Ni, W.; Zhang, H.; Li, M. Small-molecule solar cells with efficiency over 9%. Nat. Photon. 2015, 9, 35–41. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1. [Google Scholar] [CrossRef]
- Li, W.; Hendriks, K.H.; Roelofs, W.; Kim, Y.; Wienk, M.M.; Janssen, R.A. Efficient small bandgap polymer solar cells with high fill factors for 300 nm thick films. Adv. Mater. 2013, 25, 3182–3186. [Google Scholar] [CrossRef] [PubMed]
- Kyaw, A.K.K.; Wang, D.H.; Gupta, V.; Zhang, J.; Chand, S.; Bazan, G.C.; Heeger, A.J. Efficient Solution-Processed Small-Molecule Solar Cells with Inverted Structure. Adv. Mater. 2013, 25, 2397–2402. [Google Scholar] [CrossRef] [PubMed]
- Sharenko, A.; Proctor, C.M.; van der Poll, T.S.; Henson, Z.B.; Nguyen, T.Q.; Bazan, G.C. A High-Performing Solution-Processed Small Molecule: Perylene Diimide Bulk Heterojunction Solar Cell. Adv. Mater. 2013, 25, 4403–4406. [Google Scholar] [CrossRef] [PubMed]
- Koster, L.; Smits, E.; Mihailetchi, V.; Blom, P. Device model for the operation of polymer/fullerene bulk heterojunction solar cells. Phys. Rev. B 2005, 72. [Google Scholar] [CrossRef]
- Street, R.; Cowan, S.; Heeger, A. Experimental test for geminate recombination applied to organic solar cells. Phys. Rev. B 2010, 82. [Google Scholar] [CrossRef]
- Cowan, S.R.; Street, R.; Cho, S.; Heeger, A. Transient photoconductivity in polymer bulk heterojunction solar cells: competition between sweep-out and recombination. Phys. Rev. B 2011, 83. [Google Scholar] [CrossRef]
- Walker, B.; Kim, C.; Nguyen, T.Q. Small molecule solution-processed bulk heterojunction solar cells. Chem. Mater. 2010, 23, 470–482. [Google Scholar] [CrossRef]
- Coffin, R.C.; Peet, J.; Rogers, J.; Bazan, G.C. Streamlined microwave-assisted preparation of narrow-bandgap conjugated polymers for high-performance bulk heterojunction solar cells. Nat. Chem. 2009, 1, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Cho, S.; Rogers, J.T.; Schmidt, K.; Hsu, B.B.Y.; Moses, D.; Coffin, R.C.; Kramer, E.J.; Bazan, G.C.; Heeger, A.J. Higher molecular weight leads to improved photoresponsivity, charge transport and interfacial ordering in a narrow bandgap semiconducting polymer. Adv. Func. Mater. 2010, 20, 3959–3965. [Google Scholar] [CrossRef]
- Ye, L.; Jing, Y.; Guo, X.; Sun, H.; Zhang, S.; Zhang, M.; Huo, L.; Hou, J. Remove the residual additives toward enhanced efficiency with higher reproducibility in polymer solar cells. J. Phys. Chem. C 2013, 117, 14920–14928. [Google Scholar] [CrossRef]
- Burkhard, G.F.; Hoke, E.T.; McGehee, M.D. Accounting for interference, scattering, and electrode absorption to make accurate internal quantum efficiency measurements in organic and other thin solar cells. Adv. Mater. 2010, 22, 3293–3297. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Z.; Zhu, T.; Mi, B.; Gao, Z.; Huang, W. Structure optimization of organic planar heterojunction solar cells. J. Phys. D 2013, 46. [Google Scholar] [CrossRef]
- Jo, J.H.; Chun, Y.-S.; Kim, I. Optical Modeling-Assisted Characterization of Polymer: Fullerene Photodiodes. IEEE Photonics J. 2014, 6, 1–7. [Google Scholar]
- Liu, Y.; Zhao, J.; Li, Z.; Mu, C.; Ma, W.; Hu, H.; Jiang, K.; Lin, H.; Ade, H.; Yan, H. Aggregation and morphology control enables multiple cases of high-efficiency polymer solar cells. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
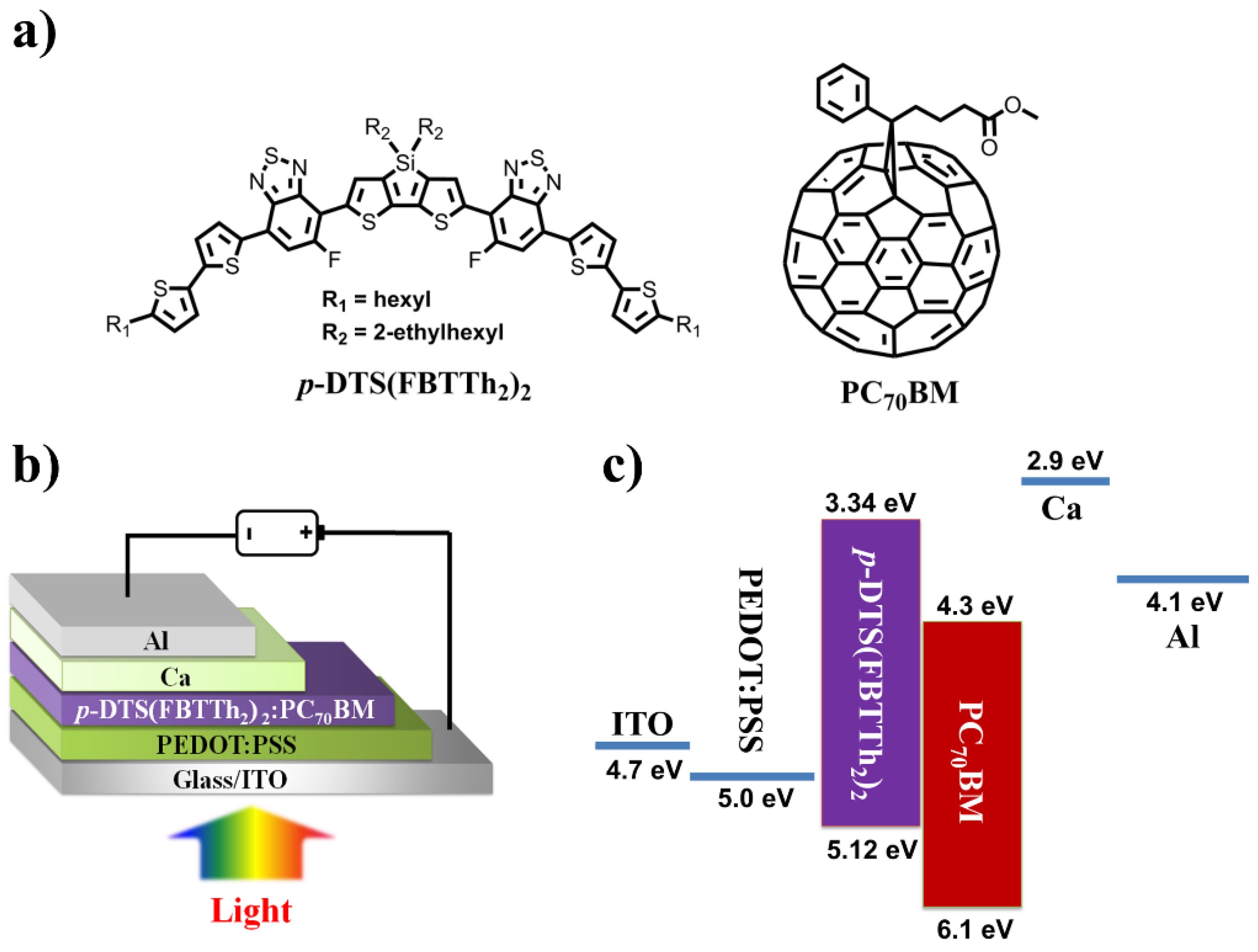

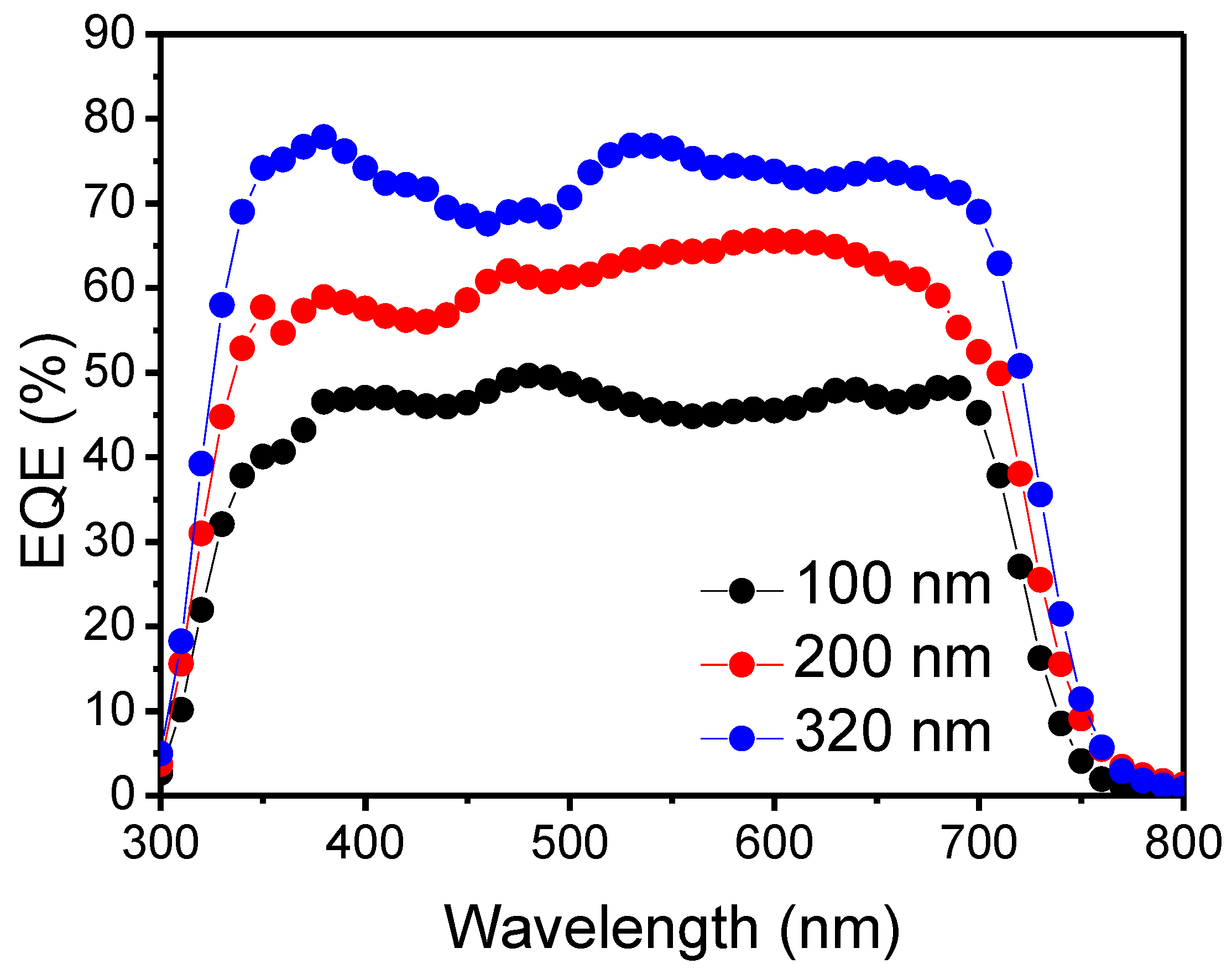

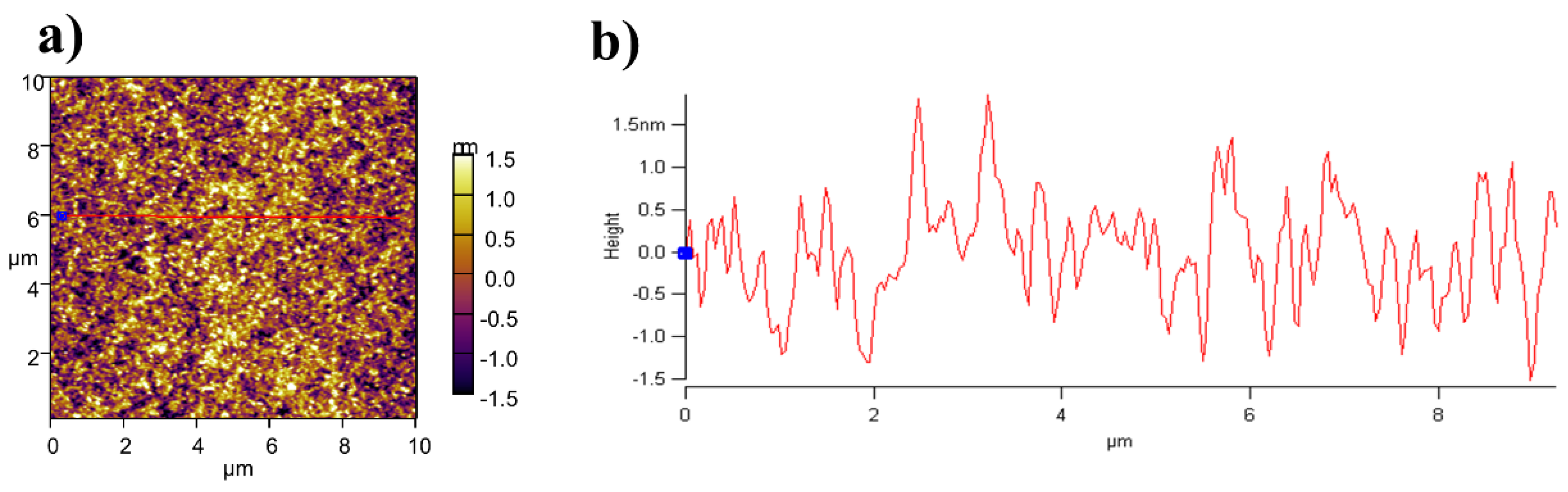
| BHJ Thickness | Voc (V) | Jsc (mA/cm2) | Fill Factor | PCE (%) |
|---|---|---|---|---|
| 100 nm | 0.78 | 9.2 | 0.39 | 2.78 |
| 200 nm | 0.80 | 11.1 | 0.41 | 3.64 |
| 320 nm | 0.74 | 17.8 | 0.52 | 6.83 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, I.K.; Jo, J.H.; Yun, J.-H. Morphology-Controlled High-Efficiency Small Molecule Organic Solar Cells without Additive Solvent Treatment. Nanomaterials 2016, 6, 64. https://doi.org/10.3390/nano6040064
Kim IK, Jo JH, Yun J-H. Morphology-Controlled High-Efficiency Small Molecule Organic Solar Cells without Additive Solvent Treatment. Nanomaterials. 2016; 6(4):64. https://doi.org/10.3390/nano6040064
Chicago/Turabian StyleKim, Il Ku, Jun Hyung Jo, and Jung-Ho Yun. 2016. "Morphology-Controlled High-Efficiency Small Molecule Organic Solar Cells without Additive Solvent Treatment" Nanomaterials 6, no. 4: 64. https://doi.org/10.3390/nano6040064







