Nano Copper Oxide-Modified Carbon Cloth as Cathode for a Two-Chamber Microbial Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
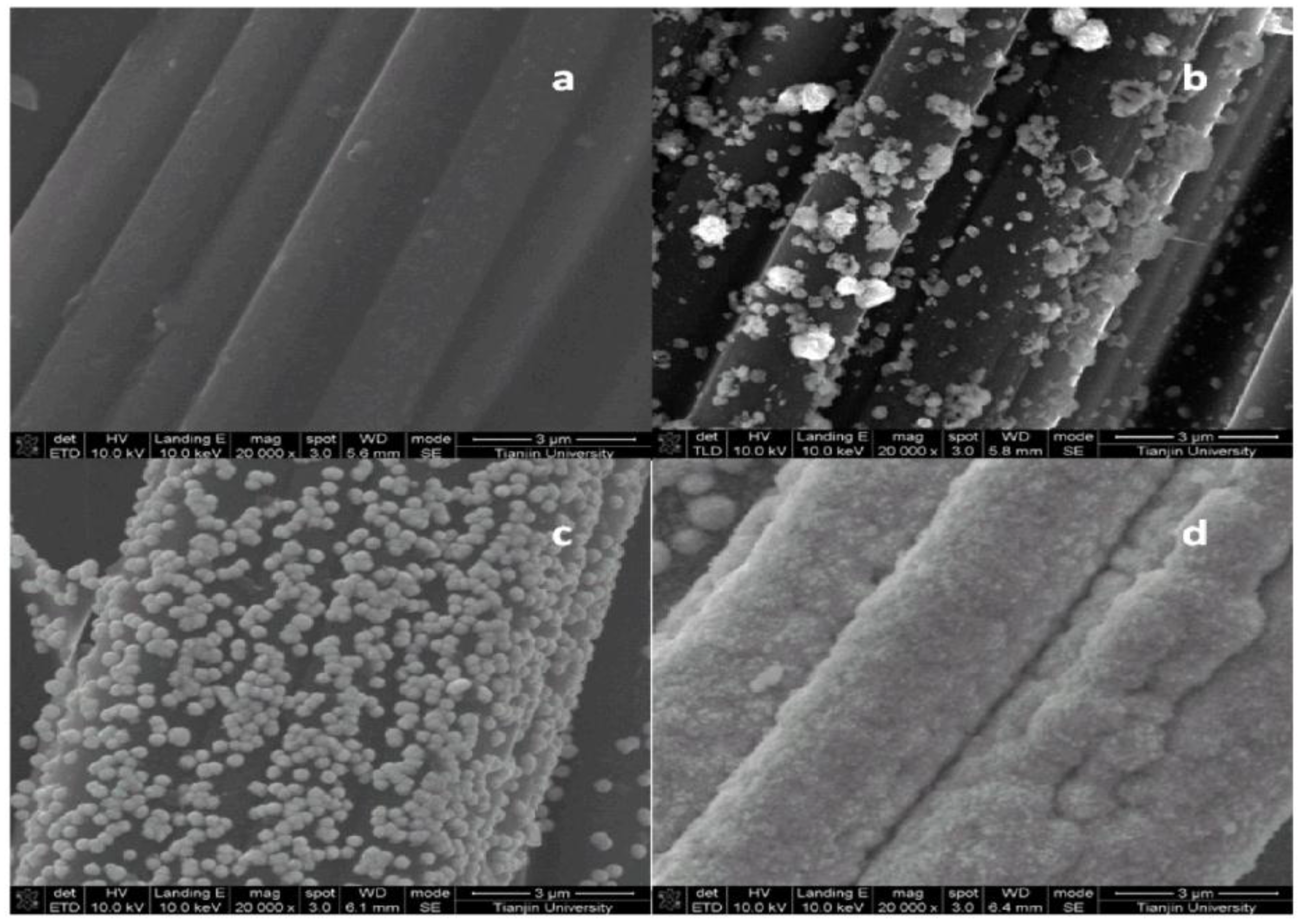
2.1. Scanning Electron Microscopy (SEM) and Brunauer-Emmett-Teller (BET) Characterization
2.2. X-ray Photoelectron Spectroscopy Results
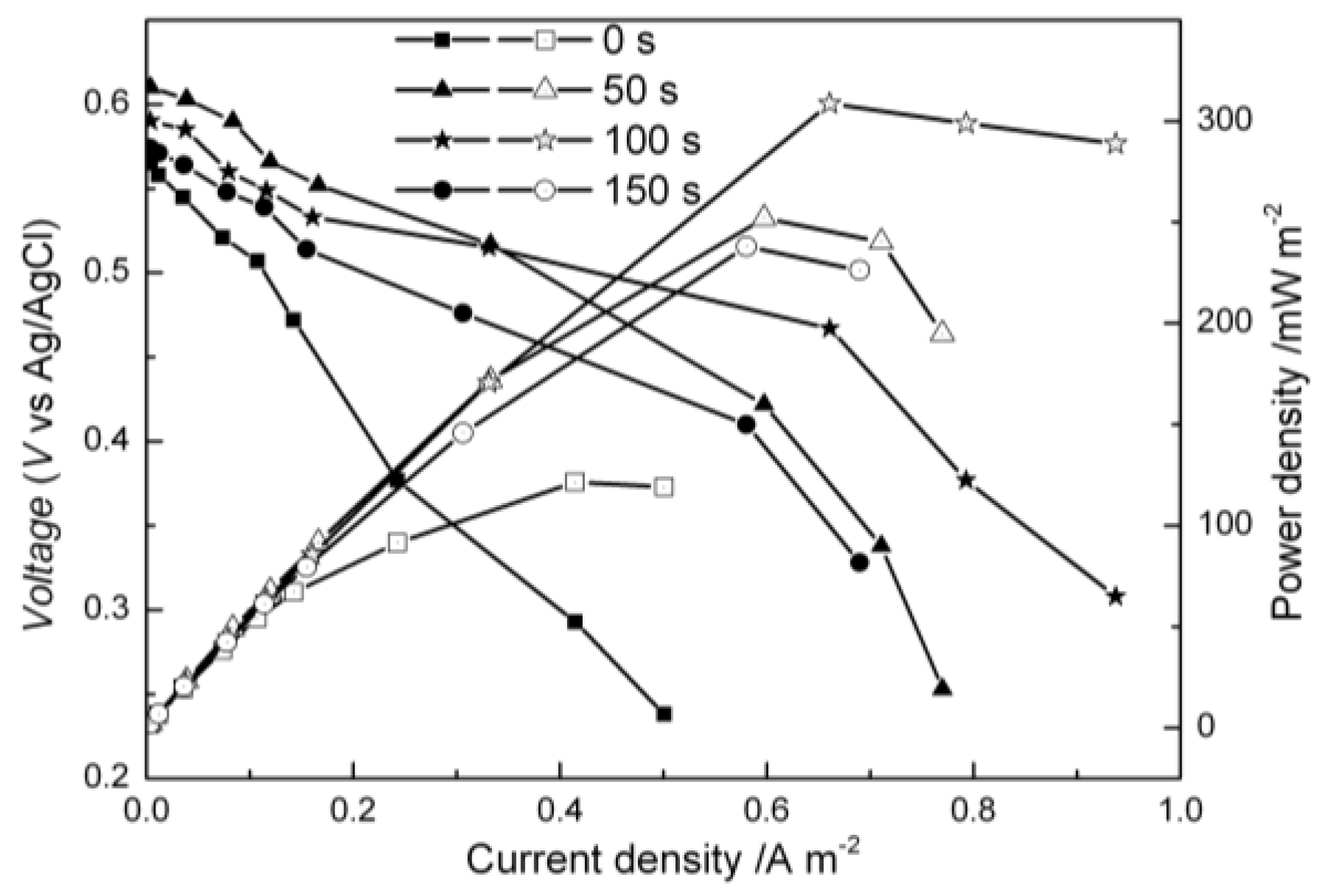
2.3. Performance of MFCs Equipped with the Modified Carbon Cloth Cathodes
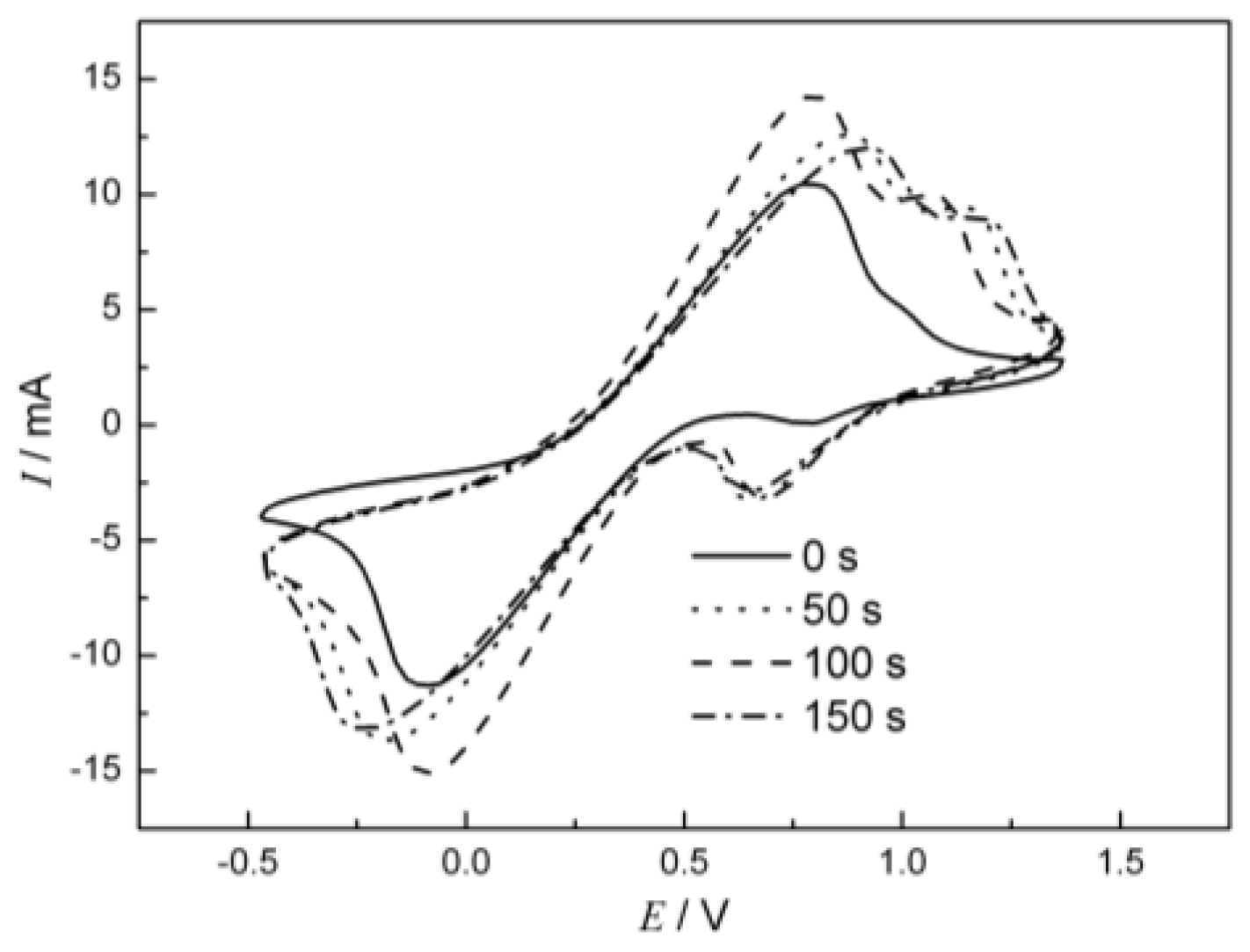
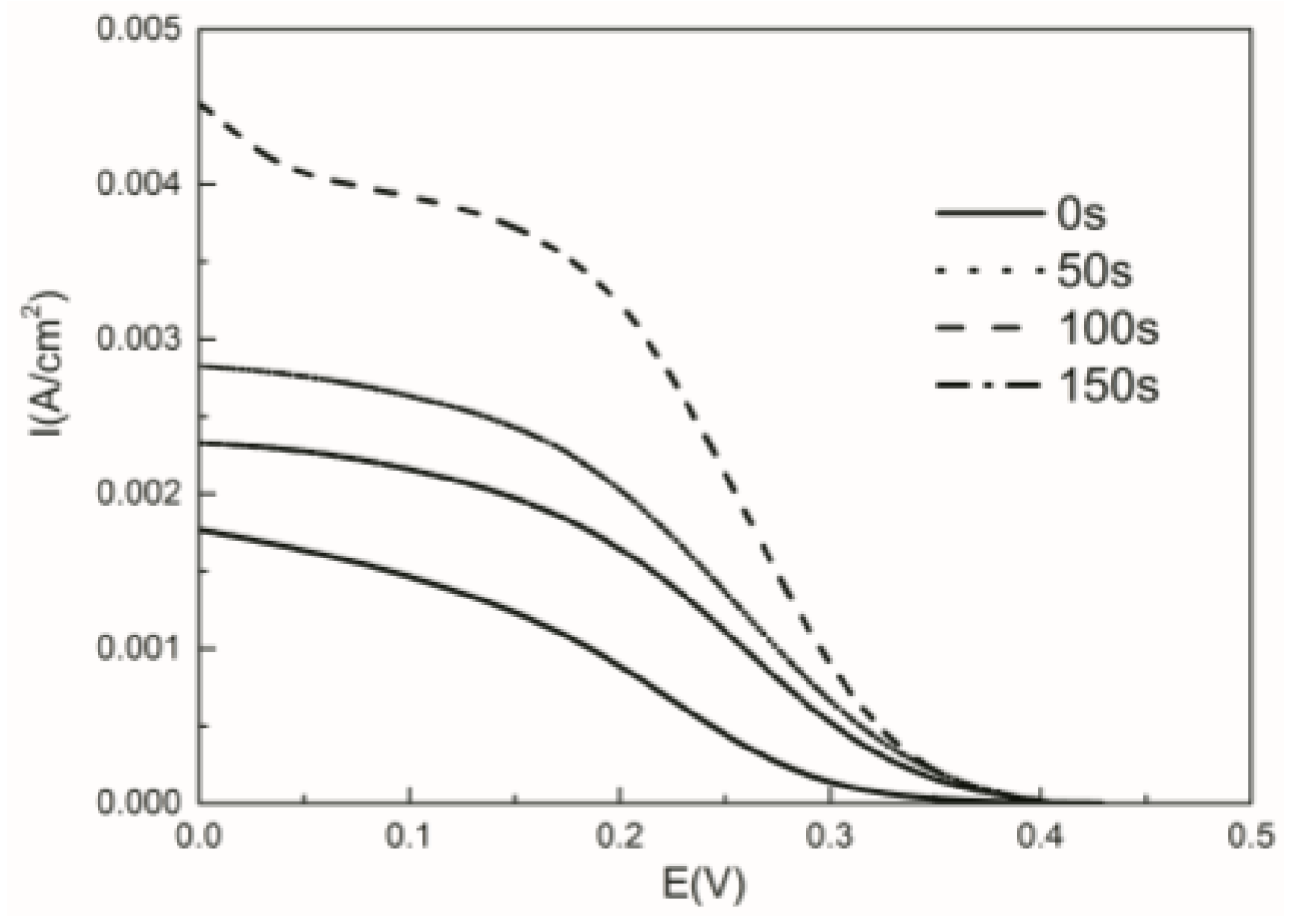
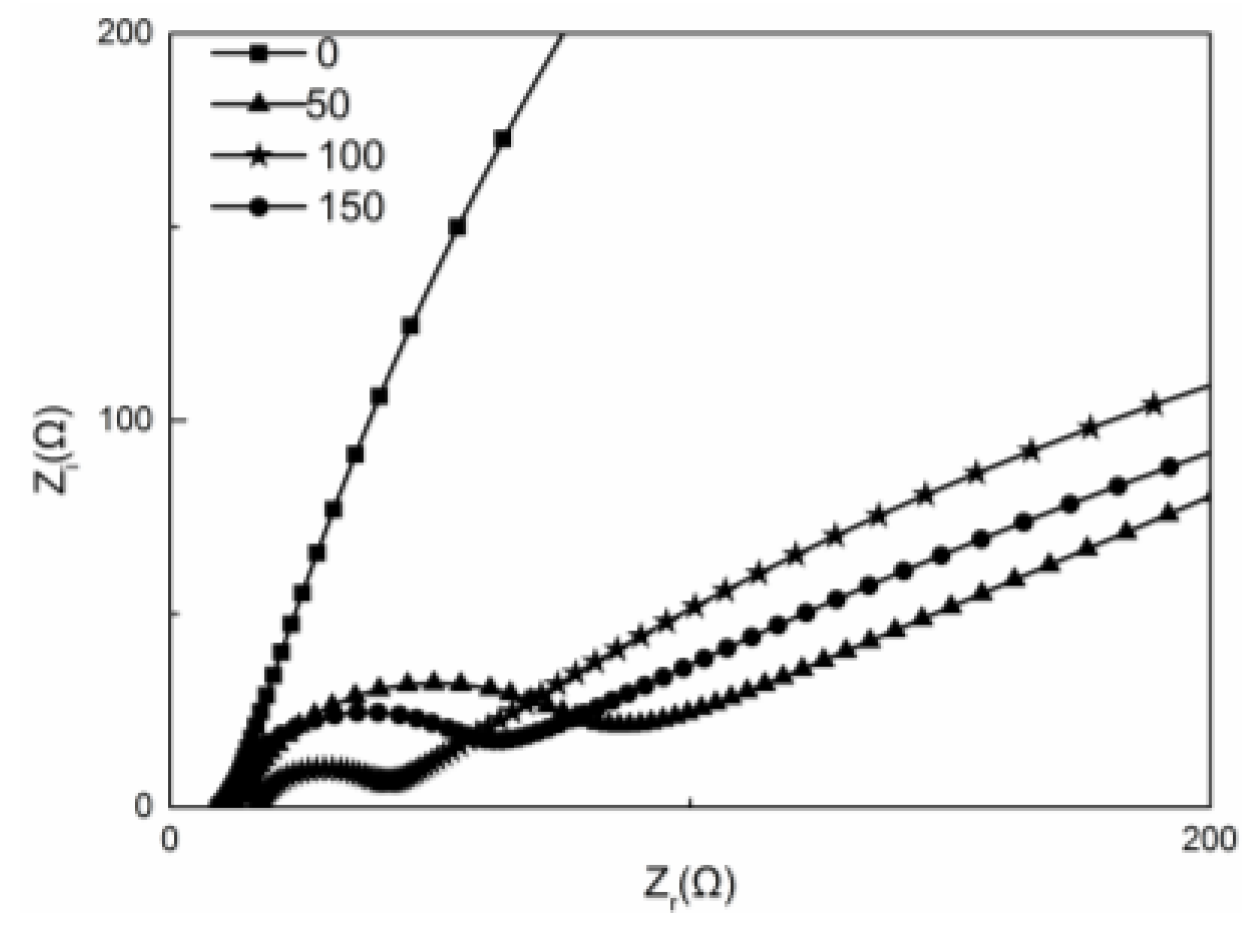
2.4. Electrochemical Characterization of Cu Oxide-Coated Carbon Cloth Cathode
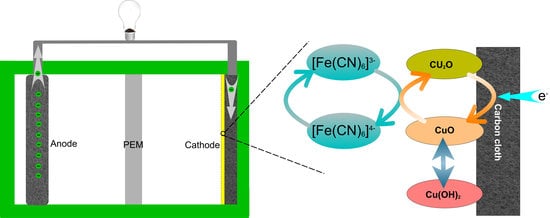
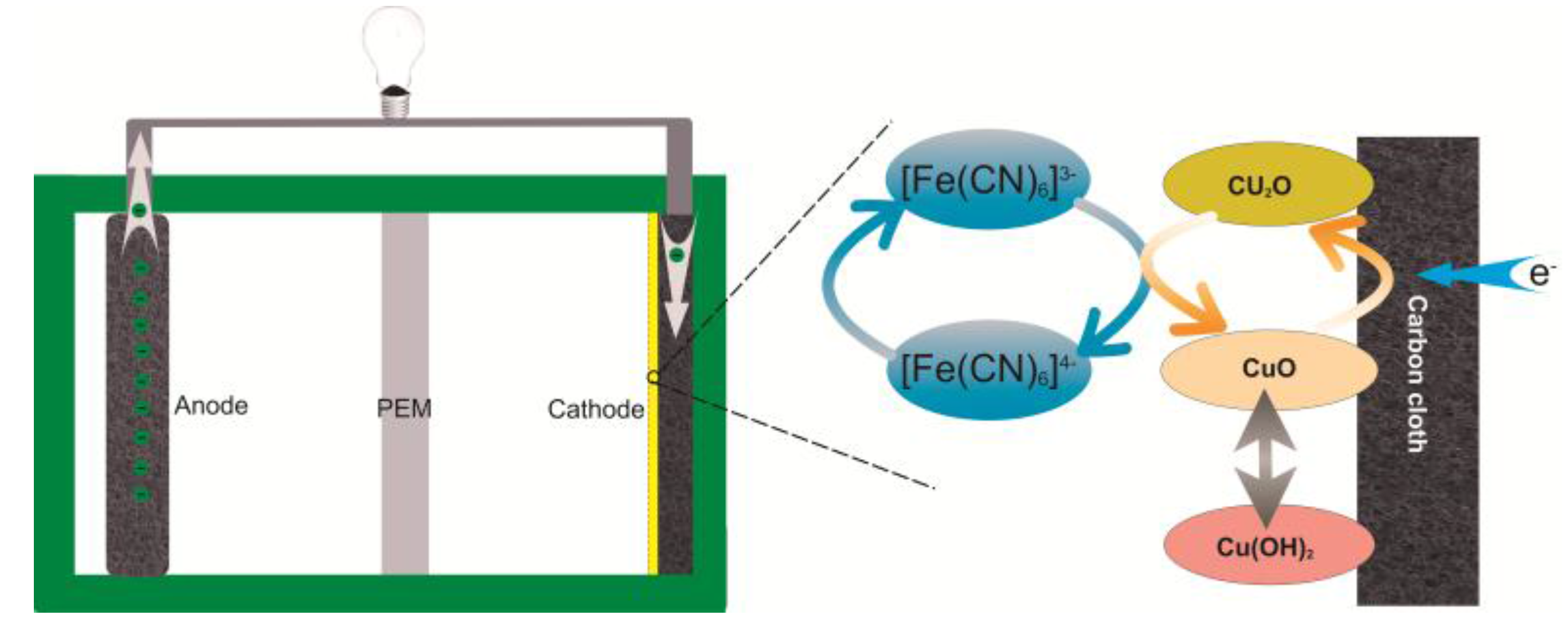
2.5. Catalytic Mechanism of Copper Oxide Nanocatalyst
3. Materials and Methods
3.1. Electrode Materials and Chemicals
3.2. MFC Construction and Operation
3.3. Electrochemical and Physical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MFC | microbial fuel cell |
| SEM | scanning electron microscopy |
| BET | Brunauer-Emmett-Teller |
| XPS | X-ray photoelectron spectroscopy |
| CV | cyclic voltammetry |
| LSV | linear sweep voltammetry |
| EIS | electrochemical impedance spectroscopy |
| OCV | open circuit voltage |
References
- Zhang, P.; Liu, X.H.; Li, K.X.; Song, Z.Q. Optimization of Fabrication Parameters to Enhance the Performance of Activated Carbon Air-Cathode. J. Electrochem. Soc. 2015, 162, F1347–F1355. [Google Scholar] [CrossRef]
- Nabae, Y.; Yamanaka, I.; Otsuka, K. Electro-catalysis of the Cu/carbon cathode for the reduction of O2 during fuel-cell reactions. Appl. Catal. A 2005, 280, 149–155. [Google Scholar] [CrossRef]
- You, D.J.; Jin, S.A.; Lee, K.H.; Pak, C.; Choi, K.H.; Chang, H. Improvement of activity for oxygen reduction reaction by decoration of Ir on PdCu/C catalyst. Catal. Today 2012, 185, 138–142. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.M.; Liu, S.S.; Zhang, B.S.; Zhong, H.X.; Su, D.S. Facile synthesis of supported Pt-Cu nanoparticles with surface enriched Pt as highly active cathode catalyst for proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2012, 37, 17978–17983. [Google Scholar] [CrossRef]
- Xiong, L.; Kannan, A.M.; Manthiram, A. Pt-M (M = Fe, Co, Ni and Cu) electrocatalysts synthesized by an aqueous route for proton exchange membrane fuel cells. Electrochem. Commun. 2002, 4, 898–903. [Google Scholar] [CrossRef]
- Alia, S.M.; Jensen, K.; Contreras, C.; Garzon, F.; Pivovar, B.; Yan, Y. Platinum Coated Copper Nanowires and Platinum Nanotubes as Oxygen Reduction Electrocatalysts. ACS Catal. 2013, 3, 358–362. [Google Scholar] [CrossRef]
- Tsujimura, S.; Miura, Y.; Kano, K. CueO-immobilized porous carbon electrode exhibiting improved performance of electrochemical reduction of dioxygen to water. Electrochim. Acta 2008, 53, 5716–5720. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Guerriero, P.; Musiani, M.; Vazquez-Gomez, L.; Verlato, E. Electrochemical reduction of nitrate and nitrite in alkaline media at CuNi alloy electrodes. Electrochim. Acta 2013, 89, 488–496. [Google Scholar] [CrossRef]
- Macova, Z.; Bouzek, K.; Serak, J. Electrocatalytic activity of copper alloys for NO3− reduction in a weakly alkaline solution-Part 2: Copper-tin. J. Appl. Electrochem. 2007, 37, 557–566. [Google Scholar] [CrossRef]
- Li, M.; Feng, C.; Zhang, Z.; Shen, Z.; Sugiura, N. Electrochemical reduction of nitrate using various anodes and a Cu/Zn cathode. Electrochem. Commun. 2009, 11, 1853–1856. [Google Scholar] [CrossRef]
- Fan, N.; Li, Z.; Zhao, L.; Wu, N.; Zhou, T. Electrochemical denitrification and kinetics study using Ti/IrO2-TiO2-RuO2 as the anode and Cu/Zn as the cathode. Chem. Eng. J. 2013, 214, 83–90. [Google Scholar] [CrossRef]
- Gauthard, F.; Epron, F.; Barbier, J. Palladium and platinum-based catalysts in the catalytic reduction of nitrate in water: effect of copper, silver, or gold addition. J. Catal. 2003, 220, 182–191. [Google Scholar] [CrossRef]
- Comisso, N.; Cattarin, S.; Fiameni, S.; Gerbasi, R.; Mattarozzi, L.; Musiani, M.; Vazquez-Gomez, L.; Verlato, E. Electrodeposition of Cu-Rh alloys and their use as cathodes for nitrate reduction. Electrochem. Commun. 2012, 25, 91–93. [Google Scholar] [CrossRef]
- Huang, T.-J.; Chou, C.-L. Electrochemical CO2 reduction with power generation in SOFCs with Cu-added LSCF-GDC cathode. Electrochem. Commun. 2009, 11, 1464–1467. [Google Scholar] [CrossRef]
- Kortlever, R.; Tan, K.H.; Kwon, Y.; Koper, M.T.M. Electrochemical carbon dioxide and bicarbonate reduction on copper in weakly alkaline media. J. Solid State Electrochem. 2013, 17, 1843–1849. [Google Scholar] [CrossRef]
- Chen, S.L.; Chen, Y.; He, G.H.; He, S.J.; Schroder, U.; Hou, H.Q. Stainless steel mesh supported nitrogen-doped carbon nanofibers for binder-free cathode in microbial fuel cells. Biosens. Bioelectron. 2012, 34, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Tamakloe, R.Y. Effect of COD and H2O2 concentration on DC-MFC. Renew Energ. 2015, 83, 1299–1304. [Google Scholar] [CrossRef]
- Wetser, K.; Sudirjo, E.; Buisman, C.J.N.; Strik, D.P.B.T.B. Electricity generation by a plant microbial fuel cell with an integrated oxygen reducing biocathode. Appl. Energ. 2015, 137, 151–157. [Google Scholar] [CrossRef]
- Kaneshiro, H.; Takano, K.; Takada, Y.; Wakisaka, T.; Tachibana, T.; Azuma, M. A milliliter-scale yeast-based fuel cell with high performance. Biochem. Eng. J. 2014, 83, 90–96. [Google Scholar] [CrossRef]
- Raghavulu, S.V.; Goud, R.K.; Sarma, P.N.; Mohan, S.V. Saccharomyces cerevisiae as anodic biocatalyst for power generation in biofuel cell: Influence of redox condition and substrate load. Bioresource Technol. 2011, 102, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Sayed, E.T.; Tsujiguchi, T.; Nakagawa, N. Catalytic activity of baker’s yeast in a mediatorless microbial fuel cell. Bioelectrochemistry 2012, 86, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Poulston, S.; Parlett, P.; Stone, P.; Bowker, M. Surface oxidation and reduction of CuO and Cu2O studied using XPS and XAES. Surf. Interface Anal. 1996, 24, 811–820. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Chusuei, C.C.; Brookshier, M.A.; Goodman, D.W. Correlation of relative X-ray photoelectron spectroscopy shake-up intensity with CuO particle size. Langmuir 1999, 15, 2806–2808. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Okazaki, M.; Kapoor, M.P.; Osaki, T.; Ohashi, F. Oxidative steam reforming of methanol over CuZnAl(Zr)-oxide catalysts for the selective production of hydrogen for fuel cells: Catalyst characterization and performance evaluation. J. Catal. 2000, 194, 373–384. [Google Scholar] [CrossRef]
- Dubal, D.P.; Dhawale, D.S.; Salunkhe, R.R.; Jamdade, V.S.; Lokhande, C.D. Fabrication of copper oxide multilayer nanosheets for supercapacitor application. J. Alloys Compd. 2010, 492, 26–30. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F. Characterization of copper oxide thin films deposited by the thermal evaporation of cuprous oxide (Cu2O). Vacuum 2008, 82, 623–629. [Google Scholar] [CrossRef]
- Goh, S.W.; Buckley, A.N.; Lamb, R.N.; Rosenberg, R.A.; Moran, D. The oxidation states of copper and iron in mineral sulfides, and the oxides formed on initial exposure of chalcopyrite and bornite to air. Geochim. Cosmochim. Acta 2006, 70, 2210–2228. [Google Scholar] [CrossRef]
- Le, M.; Ren, M.; Zhang, Z.; Sprunger, P.T.; Kurtz, R.L.; Flake, J.C. Electrochemical Reduction of CO2 to CH3OH at Copper Oxide Surfaces. J. Electrochem. Soc. 2011, 158, E45–E49. [Google Scholar] [CrossRef]
- Patake, V.D.; Joshi, S.S.; Lokhande, C.D.; Joo, O.-S. Electrodeposited porous and amorphous copper oxide film for application in supercapacitor. Mater. Chem. Phys. 2009, 114, 6–9. [Google Scholar] [CrossRef]
- Gunawardena, A.; Fernando, S.; To, F. Performance of a Yeast-mediated Biological Fuel Cell. Int. J. Mol. Sci. 2008, 9, 1893–1907. [Google Scholar] [CrossRef] [PubMed]
- Hubenova, Y.V.; Rashkov, R.S.; Buchvarov, V.D.; Arnaudova, M.H.; Babanova, S.M.; Mitov, M.Y. Improvement of Yeast–Biofuel Cell Output by Electrode Modifications. Ind. Eng. Chem. Res. 2010, 50, 557–564. [Google Scholar] [CrossRef]
- Hubenova, Y.; Rashkov, R.; Buchvarov, V.; Babanova, S.; Mitov, M. Nanomodified NiFe- and NiFeP-carbon felt as anode electrocatalysts in yeast-biofuel cell. J. Mater. Sci. 2011, 46, 7074–7081. [Google Scholar] [CrossRef]
- Yin, M.; Wu, C.K.; Lou, Y.B.; Burda, C.; Koberstein, J.T.; Zhu, Y.M.; O’Brien, S. Copper oxide nanocrystals. J. Am. Chem. Soc. 2005, 127, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Ping, J.F.; Ru, S.P.; Fan, K.; Wu, J.A.; Ying, Y.B. Copper oxide nanoparticles and ionic liquid modified carbon electrode for the non-enzymatic electrochemical sensing of hydrogen peroxide. Microchim. Acta 2010, 171, 117–123. [Google Scholar] [CrossRef]
- Wang, C.-H.; Shih, H.-C.; Tsai, Y.-T.; Du, H.-Y.; Chen, L.-C.; Chen, K.-H. High methanol oxidation activity of electrocatalysts supported by directly grown nitrogen-containing carbon nanotubes on carbon cloth. Electrochim. Acta 2006, 52, 1612–1617. [Google Scholar] [CrossRef]
- Abbaspour, A.; Ghaffarinejad, A. Copper Oxide Nanoparticle Modified Sol-Gel-Derived Carbon Ceramic by Microwave Irradiation, and Its Application for Determination of Adenine at Very Low Potential. Electroanalysis 2011, 23, 651–661. [Google Scholar] [CrossRef]
- Aita, T.; Watanabe, Y.; Higuchi, T.; Sato, S. Electrocatalytic Properties of CuO Immobilized on Conductive Carbon Particles in Alkaline Surfactant Solution. J. Chem. Eng. Jpn. 2008, 41, 279–283. [Google Scholar] [CrossRef]
- Guzman-Vargas, A.; Oliver-Tolentino, M.A.; Lima, E.; Flores-Moreno, J. Efficient electrocatalytic reduction of nitrite species on zeolite modified electrode with Cu-ZSM-5. Electrochim. Acta 2013, 108, 583–590. [Google Scholar] [CrossRef]
- Joo, S.; Liang, H. Tribo-electrochemical characterization of copper thin films. Electrochim. Acta 2013, 99, 133–137. [Google Scholar] [CrossRef]
- Matsushima, H.; Bund, A.; Plieth, W.; Kikuchi, S.; Fukunaka, Y. Copper electrodeposition in a magnetic field. Electrochim. Acta 2007, 53, 161–166. [Google Scholar] [CrossRef]
- Hao, M.Q.; Liu, X.H.; Feng, M.N.; Zhang, P.P.; Wang, G.Y. Generating power from cellulose in an alkaline fuel cell enhanced by methyl viologen as an electron-transfer catalyst. J. Power Sources 2014, 251, 222–228. [Google Scholar] [CrossRef]
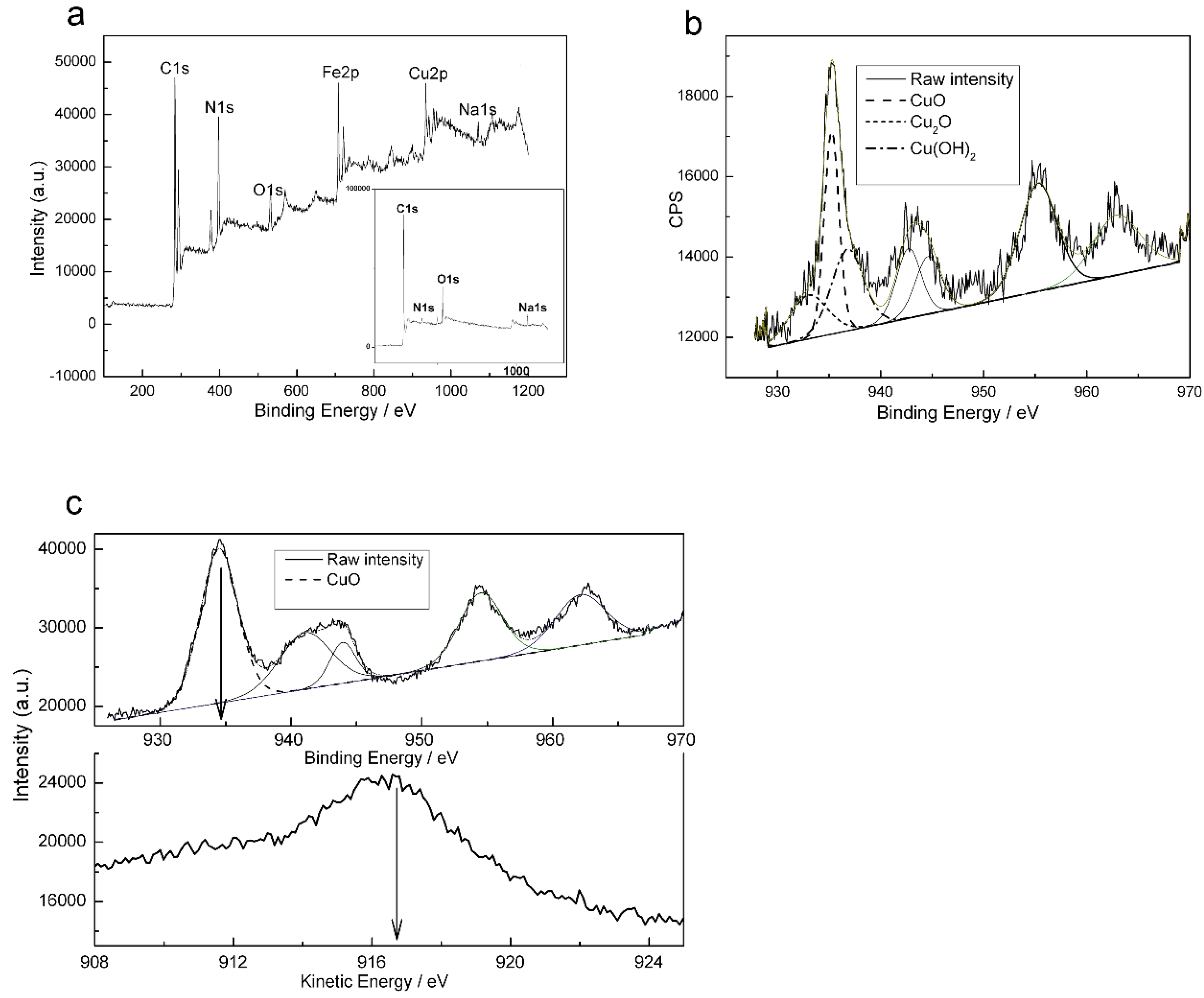
| Element | Bare Carbon Cloth | Modified Carbon Cloth | ||
|---|---|---|---|---|
| BE (eV) | AC (%) | BE (eV) | AC (%) | |
| O 1s | 532 | 11.58 | 532 | 10.15 |
| C 1s | 284 | 85.30 | 284 | 41.54 |
| N 1s | 399 | 1.97 | 398 | 30.13 |
| Na 1s | 1071 | 1.15 | 1071 | 1.48 |
| Fe 2p | 708 | 5.14 | ||
| Cu 2p | 933.00 | 2.64 | ||
| Cu 2p | 934.70 | 4.75 | ||
| Cu 2p | 935.78 | 4.18 | ||
| Total Cu | 11.57 | |||
| Cu LMM (L-inner level-M-inner level-M-inner level electron transition) | 569.89 | |||
| Samples | 0 s | 50 s | 100 s | 150 s |
|---|---|---|---|---|
| Ro (Ω) | 10.74 | 11.38 | 10.41 | 8.533 |
| Rct (Ω) | 1309 | 84.36 | 25.72 | 37.53 |
| Rd (Ω) | 1145 | 1140 | 932.5 | 1171 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Zhang, P.; Li, K.; Liu, X.; Zhang, P. Nano Copper Oxide-Modified Carbon Cloth as Cathode for a Two-Chamber Microbial Fuel Cell. Nanomaterials 2016, 6, 238. https://doi.org/10.3390/nano6120238
Dong F, Zhang P, Li K, Liu X, Zhang P. Nano Copper Oxide-Modified Carbon Cloth as Cathode for a Two-Chamber Microbial Fuel Cell. Nanomaterials. 2016; 6(12):238. https://doi.org/10.3390/nano6120238
Chicago/Turabian StyleDong, Feng, Peng Zhang, Kexun Li, Xianhua Liu, and Pingping Zhang. 2016. "Nano Copper Oxide-Modified Carbon Cloth as Cathode for a Two-Chamber Microbial Fuel Cell" Nanomaterials 6, no. 12: 238. https://doi.org/10.3390/nano6120238