1. Introduction
Energy storage devices with high energy and power densities are being developed for use as power sources for electric vehicles (EV) and hybrid electric vehicles (HEV) [
1,
2,
3]. Over the past few decades, the vast majority of relevant research has focused on upgrading the performance of conventional lithium-ion batteries for EV or HEV applications; however, their energy densities and specific charge capacities still fail to satisfy commercial requirements such as long-range driving, low cost, and fast charging [
1,
2,
4]. In recent years, rechargeable nonaqueous Li-air batteries have attracted much interest owing to their low cost, environmental friendliness, and high theoretical energy density (~3500 Wh·kg
−1), which is nearly equivalent to a nine-fold increase over conventional Li-ion batteries (~400 Wh·kg
−1) [
4,
5,
6,
7]. Despite these favorable characteristics, their practical applications are still hampered by several serious challenges including limited rate capability, poor cycling stability due to the instability of the electrode and electrolyte, and low round-trip efficiency induced by the rather large polarization, resulting in a wide charge–discharge voltage gap [
3,
8,
9,
10,
11,
12,
13,
14,
15]. These critical problems are highly attributable to the O
2 cathode.
A typical rechargeable Li-O
2 battery is constituted by a porous oxygen diffusion cathode, a lithium metal anode, and an Li
+-conducting electrolyte. In general, the O
2 cathode is an oxygen catalyst loaded with porous carbon material, which enables both Li
2O
2 deposition (oxygen reduction reactions, ORRs) and decomposition (oxygen evolution reactions, OERs) reactions to occur upon battery discharge and charge, respectively. Many reports [
1,
4,
5,
6,
8,
9,
11,
15,
16,
17,
18] have pointed out that the electrochemical performance of Li-O
2 batteries depends on many factors such as: the nature and microstructure of the O
2 electrode, electrolyte formula (especially, the composition of solvent), O
2 partial pressure, possible presence of reactive contaminants (e.g., trace water), and the choice of catalysts. In order to enhance the properties of rechargeable Li-O
2 batteries, several strategies have been followed over the years to explore the electrolyte formula, choice, and microstructure design of the O
2 electrode and optimization of the operating parameters [
1,
3,
5,
8,
9,
10,
11].
Carbon materials with various nanostructures have been developed and used as O
2 cathodes in Li-O
2 batteries [
4,
6,
10,
19]. It has been well demonstrated that the performance of Li-O
2 batteries is related to the properties of carbon, such as the morphology, surface area, porous structure, and conductivity [
6,
9,
20]. The design of porous carbon cathodes requires larger intraparticulate voids and open frameworks in their architecture structure to accommodate the insoluble discharge products. These voids and frameworks should help improve discharge capacity and cycling performance [
19,
20,
21]. Obviously, designing an optimum pore structure for carbon materials can effectively improve the electrochemical performance of Li-O
2 batteries. Although various porous carbon structures have been explored, some studies have demonstrated that hierarchically porous honeycomb-like carbon cathodes with mesoporous/macroporous pore size can increase the specific capacity of Li-O
2 batteries [
4,
5,
6,
15,
19,
20,
21,
22,
23,
24,
25,
26]. Moreover, it is well known that an ideal cathode catalyst can facilitate the complete reversibility of ORRs and OERs with low polarization in Li-O
2 batteries [
21]. Several potential catalysts have recently been proposed to promote ORRs and OERs, including nitrogen-doped carbon, metal oxides, metal nitrides, precious and nonprecious metals,
etc. [
1,
3,
8,
13,
15,
19,
27,
28,
29]. Among metal oxides, MnO
2 is a catalyst material of great interest because of its low cost, environmental friendliness, abundance, and electrocatalytic activity for ORRs in Li-O
2 batteries [
13,
28,
30,
31,
32]. This study of Li-O
2 batteries focuses on MnO
2-based catalysts.
In the first part of this work, we created a hierarchically mesoporous carbon-supported β-manganese oxide (MnO
2/C) as an O
2 cathode material. We present a detailed study of the Li-O
2 electrochemistry of the MnO
2/C material using an electrolyte of 1 M LiPF
6 in a propylene carbonate (PC, which was used in many of the initial works on Li-O
2 batteries) solvent. Although there have been many studies of MnO
2/C materials for Li-O
2 battery applications, few studies have examined the poor stability of the electrolyte due to its reaction with the superoxide radical (O
2•−) produced upon the discharge at the MnO
2/C electrode. In this work, the stability of the electrolyte against the O
2•− of the MnO
2/C electrode was first explored by the RRDE technique. The RRDE was developed about 50 years ago and has been verified to be a powerful tool for the study of electrochemical reactions. RRDE consists of two concentric electrodes (disk and ring electrodes) in a cylindrical holder with both of the electrodes facing downward into the solution. Products generated at the disk reaction are swept outward by the convection caused by rotation, and can be detected electrochemically at the ring by fixing the potential on the ring electrode. In this study, a disk electrode coated with MnO
2/C materials and a Pt ring electrode was fixed at an O
2•−/O
2 oxidation potential to collect the O
2•− ions in electrolytes. Therefore, in the second part, we emphasize aspects of the PC-based electrolyte reaction against O
2•− and the related kinetic information of O
2•− in the MnO
2/C electrode by studying rotating ring disk electrode (RRDE) experiments and using a lithium-free non-aqueous electrolyte due to the stability of the intermediate O
2•−. In addition, the oxygen solubility in the electrolyte and the oxygen diffusion velocity throughout the whole O
2 electrode play key roles in determining battery performance, especially at high current densities [
33]. In this work, the O
2 solubility, diffusion rates of O
2 and superoxide radical (O
2•−) coefficients (
and
), rate constant (
kf) for producing O
2•−, and PC-electrolyte decomposition rate constant (
k) of the MnO
2/C electrode were quantified.
2. Experimental Methods
MnO
2/C composites were prepared by supramolecular self-assembly methods followed by a hydrothermal process. A modification of the mesoporous metal oxides and carbon nanocomposites procedure of Huang
et al. [
34] was applied to synthesize the MnO
2/C composites. The first step was to synthesize a 20 wt. % resol ethanolic solution according to an established method [
34,
35]. A solution was prepared by dissolving 1.5 g of triblock copolymer Pluronic F127 (OH(CH
2CH
2O)
n-(CH
2CH(CH
3)O)
m-(CH
2CH
2O)
nH, EO
106PO
70EO
106, Sigma Aldrich, St. Louis, MO, USA) in 10 g of anhydrous ethanol, then 5 g 20 wt. % resol ethanolic solution and 0.28 g MnCl
2·4H
2O (J.T. Baker, 99.8%) were added into the above solution slowly under stirring for 30 min at an ambient temperature. The homogeneous mixture was then transferred into a Petri dish at an ambient temperature for 24 h. After being dried, the films were heated at 100 °C for another 24 h to form orange transparent membranes. The as-made products were scraped from the Petri dish and ground into powders and then calcinated at 400 °C for 5 h under an Ar atmosphere with a heating rate of 1 °C·min
−1 to yield Mn/C powders. To obtain MnO
2/C composites, the as-prepared Mn/C powders were subjected to a hydrothermal process at 180 °C for 12 h with 0.22 g KMnO
4 (J.T. Baker) and 30 mL of deionized water in a Teflon-lined stainless steel autoclave.
A Rigaku-D/MaX-2550 diffractometer (Rigaku, Tokyo, Japan) with Cu Kα radiation (λ = 1.54 Å) was used to obtain X-ray diffraction (XRD) patterns for the samples. Small angle X-ray scattering (SAXS) measurements were taken on a Nanostar U small-angle X-ray scattering system (Bruker, Karlsruhe, Germany) using Cu Kα radiation (40 kV, 35 mA). The morphology of the sample was observed using a scanning electron microscope (SEM, Hitachi S-3400 (Hitachi Limited, Tokyo, Japan)) and transmission electron microscope (TEM, JEOL JEM-3010 (JEOL, Tokyo, Japan)). Selected area electron diffraction (SAED) was applied to examine samples’ crystallinity. The Brunauer–Emmett–Teller (BET) method was used to measure the specific surface area of the powders (ASAP2020). The residual carbon content of the samples was measured by an automatic elemental analyzer (EA, Elementar vario, EL III (Elementar Analysensysteme GmbH, Hanau, Germany)).
For electrochemical evaluation, the MnO2/C electrodes were prepared by wet coating, and were made from as-prepared MnO2/C composites with super P and a poly(vinylidene difluoride) (PVDF) binder (MKB-212C, Atofina, Serquigny, France) in a weight ratio of 64:16:20. The MnO2/C composites and super-P were first added to a solution of PVDF in N-methyl-2-pyrrolidone (NMP, Riedel-deHaen, Seelze, Germany). To make a slurry with an appropriate viscosity, the mixture was stirred for 20 min at room temperature using a magnetic bar, and then for 5 min using a turbine at 2000 rpm. The resulting slurry was coated onto a piece of separator (Celgard 2400, Charlotte, NC, USA) and dried at 60 °C under vacuum for 12 h. The coating had a thickness of ~100 μm with an active material mass loading of 8 ± 1 mg·cm−2. The quantity of active materials on the electrodes was kept constant. Electrodes were dried overnight at 100 °C under a vacuum before being transferred into an argon-filled glove box for cell assembly. The Li-O2 test cell (EQ-STC-LI-AIR, MTI Corporation, Richmond, CA, USA) was constructed with lithium metal as the negative electrode and the MnO2/C electrode as the positive electrode. A solution of 1 M LiPF6 in a PC solvent was used as the electrolyte in all cells. After assembly, the test cell was taken away from the Ar-filled glove box and attached to a gas pipe that was constantly purged with dry O2. Electrochemical tests were carried out after the cell was flushed with O2 for 6 h. The cells were cycled galvanostatically with a BAT-750B (Acu Tech System, Taipei, Taiwan) at a constant current of 100 mA/g with a voltage region of 2.0–4.3 V vs. Li/Li+ at room temperature.
For the RRDE experiments, the RRDE system (AFMT134DCPTT, Pine Research Instrumention, Durham, NC, USA) with interchangeable disk consisted of a 5 mm diameter glassy carbon electrode and a Pt ring electrode (1 mm width) with a 0.5 mm gap between them. The collection efficiency with this geometry is 0.24. The rotating ring-disk assembly was operated on a Pine AFMSRX rotator and CH705 Bipotentiostat (CH Instruments, Austin, TX, USA) with a computerized interface. Experiments were conducted using a three-electrode cell containing 10 mL of the electrolyte of interest and assembled in a dry Ar-filled AtmosBag (Sigma-Aldrich Z108450, St. Louis, MO, USA).
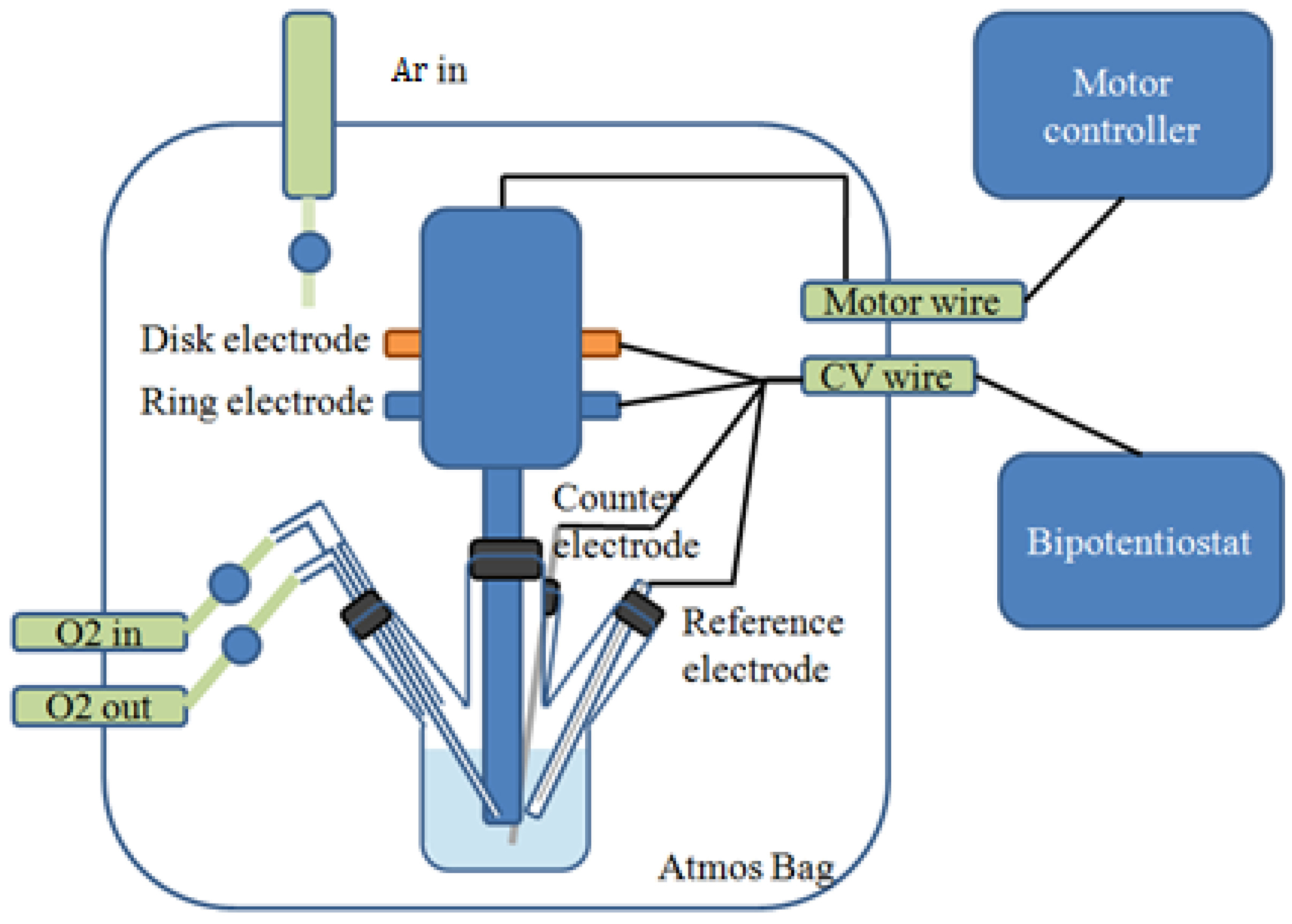
Figure 1 shows the schematic of a four-neck, jacketed glass cell with the RRDE system. The counter electrode was a Li foil connected to a Ni wire, which was isolated by a layer of Celgard 2400 separator to prevent convective oxygen transport to the electrode. The Ag/Ag
+ reference electrode consisted of an Ag wire immersed into 0.1 M AgNO
3 in CH
3CN and sealed with a vycor frit at its tip. All potentials in this study were referenced to the Li/Li
+ potential scale (volts
vs. Li
+/Li or V
Li+), obtained by calibration of the reference electrode against a fresh lithium wire before the experiments (0 V
Li = −3.46 ± 0.01 V
vs. Ag/Ag
+). The working electrode consisted of a catalyst-covered glassy carbon disk and was immersed into the Ar or O
2-purged electrolyte for 30 min before each experiment. Prior to the RRDE measurements, Alternating current (AC) impedance measurements were carried out to determine the uncompensated ohmic electrolyte drop between working and reference electrodes by applying a 10 mV perturbation (0.1 MHz to 10 mHz) at the open circuit. IR (drop) correction to remove ohmic losses was performed by considering a total cell resistance of ~293 Ω measured by AC impedance. The capacitive-corrected ORR currents were calculated by subtracting the current measurement under Ar from that obtained in pure O
2 under identical scan rates, rotation speeds, and catalyst loadings.
Figure 1.
Schematic of a four-neck, jacketed glass cell with a rotating ring-disk electrode (RRDE) system.
Figure 1.
Schematic of a four-neck, jacketed glass cell with a rotating ring-disk electrode (RRDE) system.
3. Results and Discussion
The phase composition and structure of the prepared MnO
2/C composites were examined by the wide-angle XRD and SAXS patterns given in
Figure 2a,b. As shown in
Figure 2a, all peaks can be identified as a pure and well-crystallized β-MnO
2 phase (JCPDS 24-0735) with an ordered tetragonal structure indexed to the P42/mnm space group. Moreover, the XRD curves did not show any evidence of the formation of crystalline or amorphous carbon. It appears that when using resol/Pluronic F127 templates as a carbon source, the final product is most likely to remain amorphous or in a low crystalline carbon state. The appearance of the scattering peak in the SAXS pattern, as shown in
Figure 2b, indicates the long-range regularity and highly ordered nature of the mesoporous structures of the prepared MnO
2/C composite.
The morphology of the prepared MnO
2/C composite was observed using SEM and TEM, as shown in
Figure 3a–f. From the SEM images of the MnO
2/C composite (
Figure 3a,b), it is clear that the oriented tetragonal MnO
2 nanorods are arranged on the surface of the carbon matrix. The prepared β-MnO
2 nanorods, typically 2–3 μm in length, have a square cross-section with an edge length in the range of 200–300 nm.
Figure 3c,d show the TEM images of the MnO
2/C composite at different magnifications. Large domains of highly ordered stripe-like 1D channels are clearly observed.
Figure 3e displays a TEM image of a typical nanorod with a smooth surface, and a SAED pattern based on a single nanorod (
Figure 3f), indicating single-crystalline nature. The SEM and TEM analysis suggest that hollow MnO
2 nanorods grow homogeneously on the ordered mesoporous carbon frameworks to form the structure of the hierarchically mesoporous MnO
2/C composite.
Figure 2.
(a) Wide-angle X-ray diffraction (XRD) patterns; (b) small angle X-ray scattering (SAXS) patterns of MnO2/C composites.
Figure 2.
(a) Wide-angle X-ray diffraction (XRD) patterns; (b) small angle X-ray scattering (SAXS) patterns of MnO2/C composites.
Figure 3.
Scanning electron microscope (SEM) images (a) MnO2/C composites; (b) high magnification of the region marked with a square in (a); and transmission electron microscope (TEM) images (c,e) MnO2/C composites; (d) high magnification of the region marked with a square in (c); and (f) selected area electron diffraction (SAED) pattern of the region marked with a square in (e).
Figure 3.
Scanning electron microscope (SEM) images (a) MnO2/C composites; (b) high magnification of the region marked with a square in (a); and transmission electron microscope (TEM) images (c,e) MnO2/C composites; (d) high magnification of the region marked with a square in (c); and (f) selected area electron diffraction (SAED) pattern of the region marked with a square in (e).
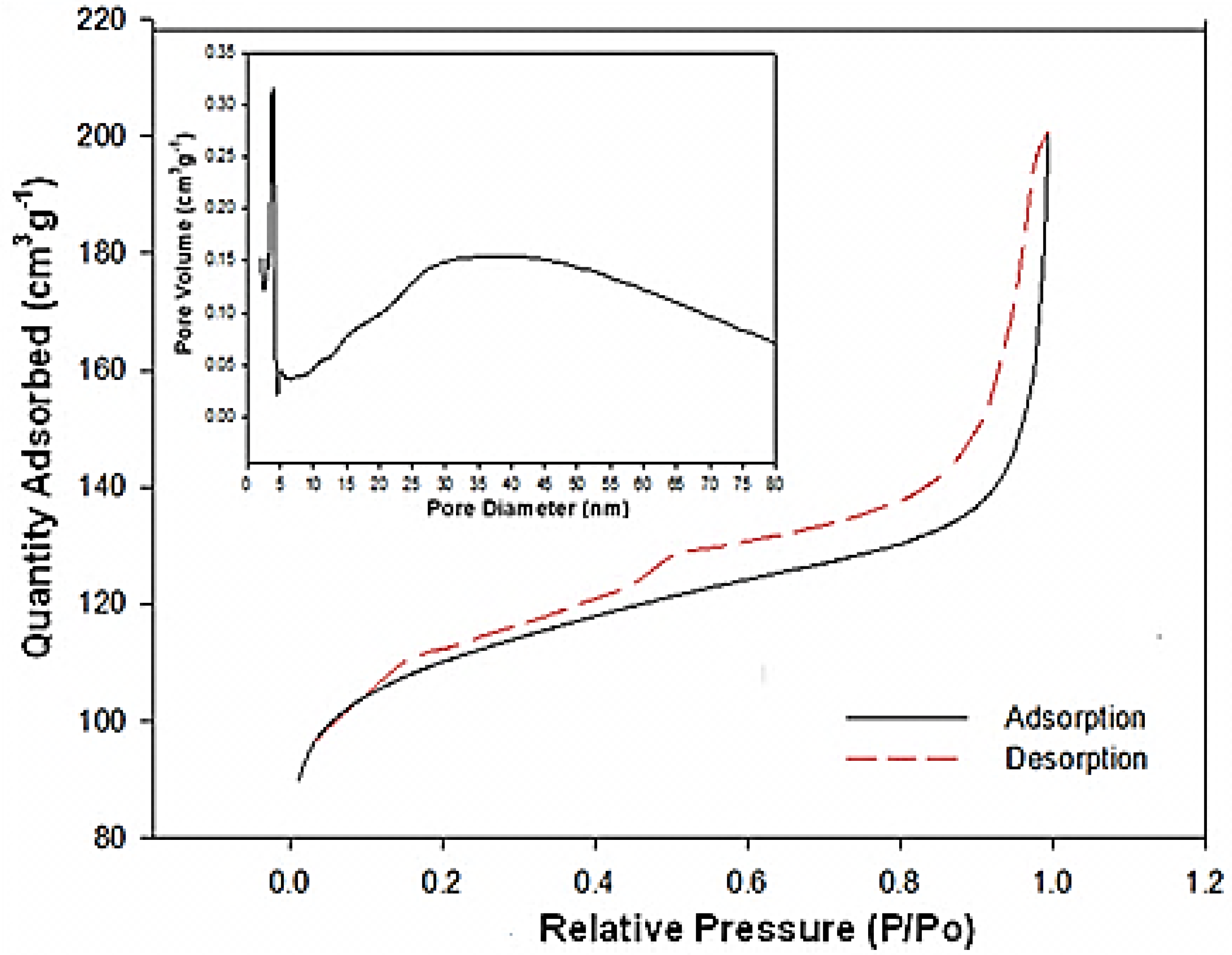
The pore structure of the mesoporous MnO
2/C composite was determined by nitrogen adsorption-desorption isothermal measurements. As shown in
Figure 4, the adsorption isothermal curve of the MnO
2/C composite has a well-defined step as in typical IV classification with a H
1-type hysteretic loop in the p/p
o range of 0.40–1.0, indicating mesoporous material character. These findings suggest that the MnO
2/C composite sample does not contain framework-confined pores but is rather made up of individual nanorods. This is in agreement with the results from the SEM and TEM images. The Barrett–Joyner–Halenda (BJH) pore size distribution for the mesoporous MnO
2/C composite, shown in the insert of
Figure 4, reveals peaks centering at 4.8 and 35 nm. This result confirms that most of the pore channels in the ordered mesoporous carbon are not blocked by the loading of MnO
2 nanorods. The nanoarchitecture of ordered mesoporous channels is maintained, which is desirable for the O
2 electrode in Li-O
2 batteries. Moreover, the measured BET surface area of the MnO
2/C composite is relatively high, at about 424 m
2·g
−1. The hierarchical microstructure of the MnO
2/C composite results in a large specific surface area. This is important for enhancing the electrochemical properties of an O
2 cathode material.
Figure 4.
Nitrogen sorption isotherms of MnO2/C composites. The insert is the Barrett–Joyner–Halenda (BJH) desorption pore size distribution.
Figure 4.
Nitrogen sorption isotherms of MnO2/C composites. The insert is the Barrett–Joyner–Halenda (BJH) desorption pore size distribution.
MnO
2 has been known as a highly active ORR catalyst for some time [
30,
32,
36] and has recently been applied as an O
2 cathode catalyst in Li-O
2 batteries. Due to the studies of the electrocatalytic activity of MnO
2, the following discussions regarding electrochemical tests make comparisons between Super-P carbon (SP) and MnO
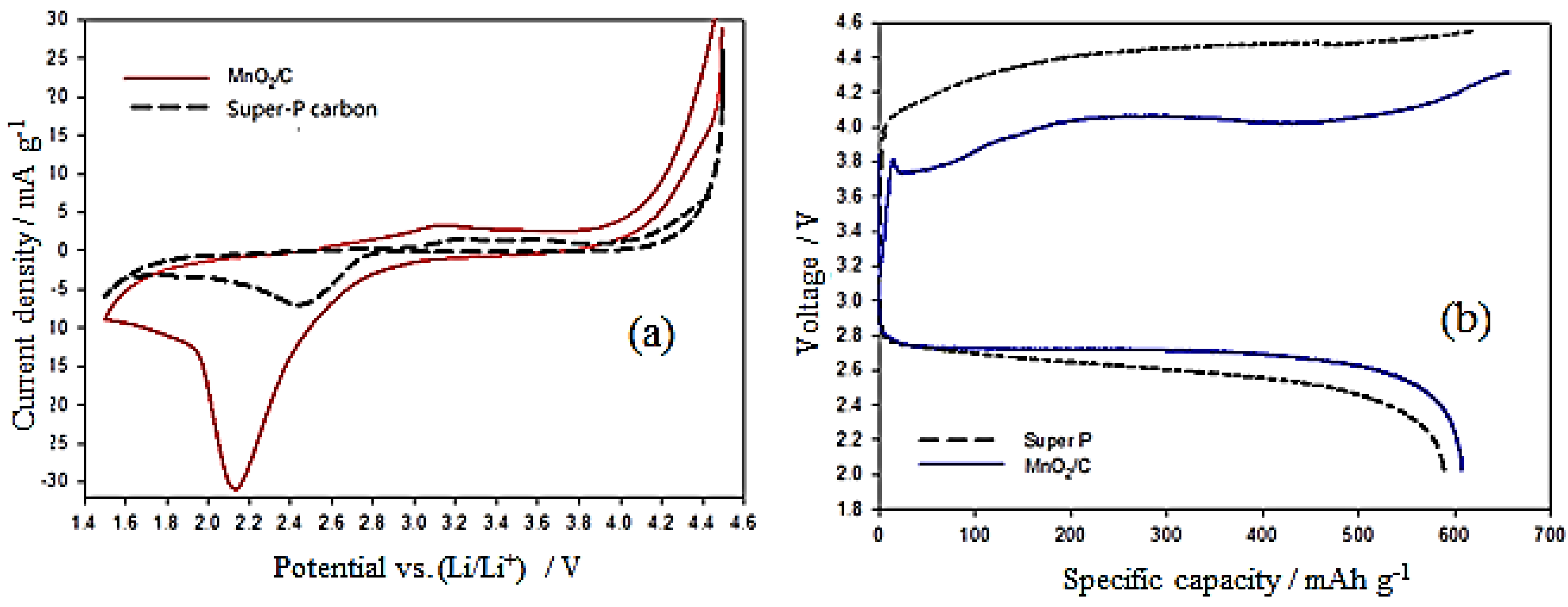
2/C materials. To better study the catalytic activity of the electrodes, cyclic voltammetry (CV) and charge-discharge voltage measurements were carried out. At first, CV was carried out in the Ar-purged electrolyte and subsequently in the same solution saturated with O
2. The capacitively-corrected CV curves derived from both measurements are shown in
Figure 5a. The CV plots of the O
2 electrodes prepared from MnO
2/C and SP cycled between 1.5 and 4.5 V with 2 mV·s
−1 and the O
2-saturated 1 M LiPF
6/PC electrolyte are shown in
Figure 5a. From the CV curves, the reduction peak voltage is shifted toward positive voltage, exhibiting electrocatalytic activity in the ORR of both samples. However, the MnO
2/C offers more positive onset reduction peak potential and a larger peak current, which clearly indicate the superior electrocatalytic activity of MnO
2/C compared to SP. Furthermore, the onset oxidation peaks appearing in the CV curves are about 2.7 and 2.9 V for MnO
2/C and SP, respectively. This demonstrates that MnO
2/C, with its lower onset oxidation peak, is more efficient for Li
2O
2 decomposition and has higher catalytic activity for the OER. The initial charge–discharge voltage profiles for both samples are shown in
Figure 5b. The charge–discharge profiles of the MnO
2/C electrode exhibit much lower charge overpotential than do those of the SP electrode, although the reduction of the total overpotential is only about 25%. The round-trip efficiencies of the Li-O
2 batteries with a MnO
2/C electrode were lower than those with the SP electrode. These results indicate that the MnO
2/C composite can facilitate the complete reversibility of ORR and OER with low polarization for a Li-O
2 battery. This finding is in good agreement with the CV measurement. The initial discharge capacities of the MnO
2/C and SP electrodes were 612 mAh·g
−1 and 589 mAh·g
−1, respectively. The good electrochemical performance of the MnO
2/C electrode may be due to the hierarchical mesostructure and large specific surface area, and the catalytic activity of the MnO
2/C composite.
Figure 5.
(a) CV curves were recorded at a scanning rate of 2 mV·s−1 for MnO2/C and Super-P carbon samples; (b) initial charge–discharge profiles for MnO2/C and Super P samples at a current density of 0.2 mA·cm−2.
Figure 5.
(a) CV curves were recorded at a scanning rate of 2 mV·s−1 for MnO2/C and Super-P carbon samples; (b) initial charge–discharge profiles for MnO2/C and Super P samples at a current density of 0.2 mA·cm−2.
The rotating ring disk electrode (RRDE) technique was also used to investigate the kinetics of ORR since the ORR current is strongly relevant to hydrodynamic conditions [
31]. Here, we used a glassy carbon (GC) electrode and an as-prepared MnO
2/C composite coated on the GC (MnO
2/C-GC) electrode as the working electrodes to study the stability of the electrolyte at the MnO
2/C electrode. Many reports [
1,
5,
9] have shown the O
2•− produced in the first step of the ORR upon battery discharge:
The reaction between the O
2•− and the electrolyte is the critical problem that causes poor Li-O
2 battery cyclability. In the PC-based electrolyte, the ethereal carbon atom in PC suffers from nucleophilic attacks by O
2•−, yielding carbonate, acetate, and formate species (among others), according to Equation (2) [
37,
38]:
Here, we applied rotating disk electrode (RDE) voltammetry to measure the rate constant (
kf) when reducing O
2 to O
2•− for Equation (1) in the 0.1 M TBAPF
6/PC electrolyte. The reaction rate constant,
kf, can be evaluated via the Koutecky–Levich (K–L) equation for a first-order reaction as follows [
31]:
where
ik and
id represent kinetics and diffusion limiting current density (A·m
−2), respectively;
n is the number of electrons exchanged in the electrochemical reaction;
F is Faraday’s constant (96,485 C·mol
−1);
kf is the rate constant for Equation (1);
is the diffusion coefficient of O
2 in the solution; ν is the kinematic viscosity; ω is the angular frequency of the rotation; and
is the saturation concentration of O
2 in the solution. Additionally, knowing the values of ν and
for an electrolyte, one can obtain the concentration of oxygen (
) and rate constant (
kf) for Equation (1) by linearly fitting the K-L plots of
i−1 vs. ω
−0.5, as follows
Prior to estimating the value of
kf from the K–L equation, the kinematic viscosity (ν) of the electrolyte and the diffusion coefficients of O
2 and O
2•− (
and
), need to be quantified. The value of ν for PC with 0.1 M TBAPF
6 is 2.59 × 10
−2 cm
2·s
−1 at 25 °C (ρ = 1.2 g·mL
−1 and η = 3.13 mPa·s) and was measured by a Rheometer (Malvern Gemini, Malvern Instruments Ltd., Malvern, UK). For a known viscosity, the diffusion coefficients can be directly determined from the transit-time (
Ts) measurement by the RRDE technique, as reported previously [
37,
39].
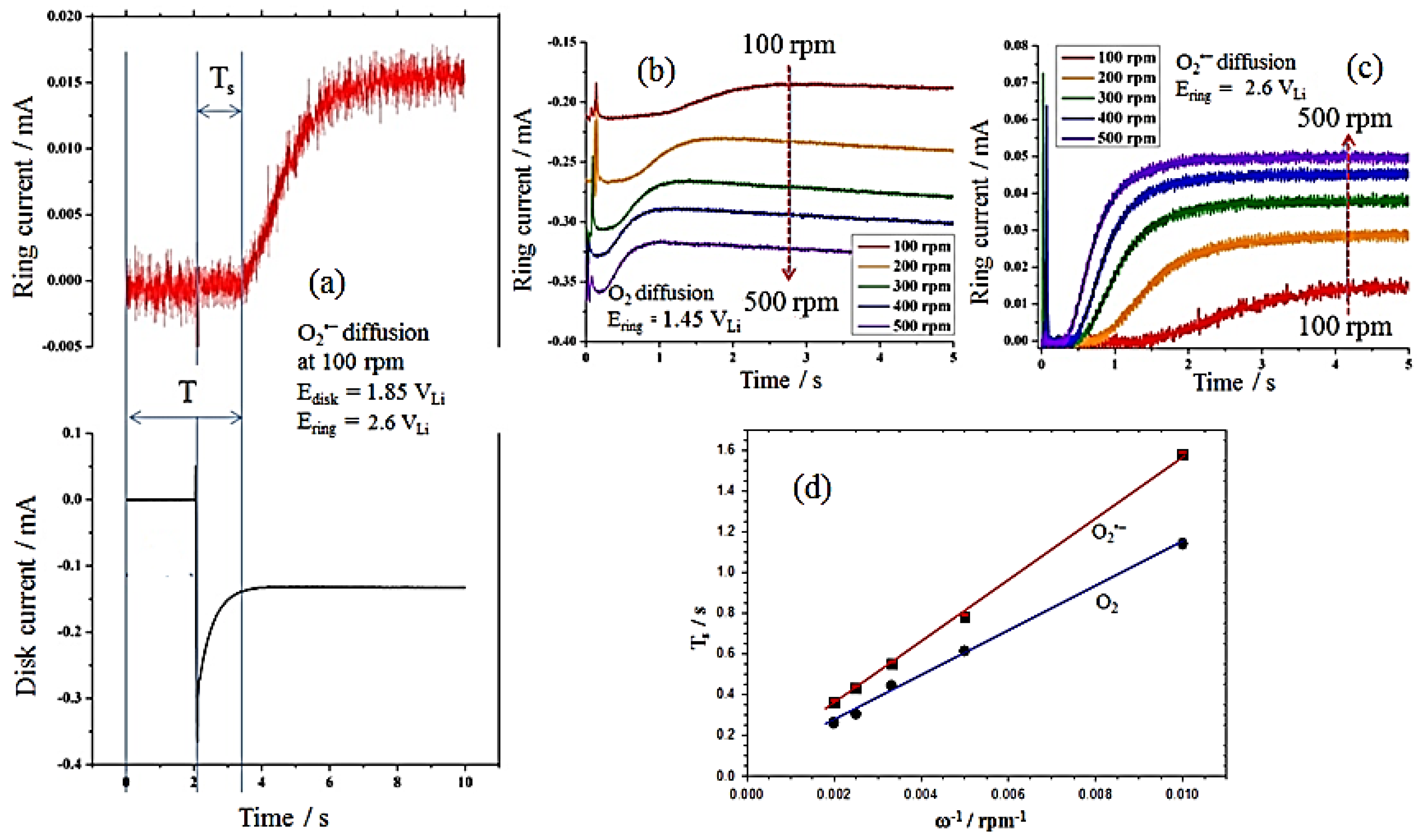
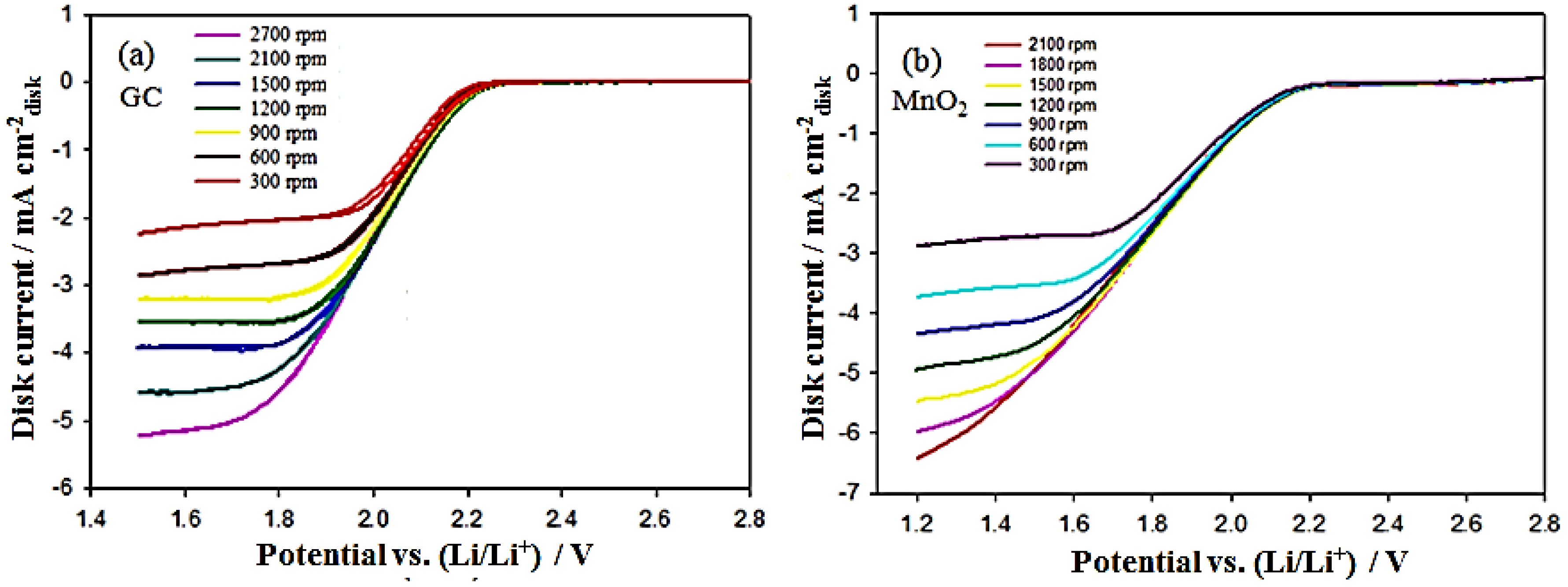
Figure 6a shows an example of
Ts measurement in O
2-saturated solutions of 0.1 M TBAPF
6 in PC at ω = 100 rpm;
Ts, the origin of which is taken at time = 2 s (the time at which the disk is conducted cathodic potential at 1.85 V
Li), is measured graphically from the intercept of the base steady ring current and the fast attenuate ring current line.
Figure 6b,c show measurements of steady ring currents at the rotation rates (ω) of different electrodes, yielding
Ts values for O
2 and O
2•−. Then, the obtained
Ts is related to the ω and the ratio of ν and the diffusion coefficient (
D), according to Equation (7) [
37,
39]:
where K is proportionally constant depending on the RRDE’s geometry; K = 43.1[log(
r2/
r1)]
2/3 (for
Ts, reported in s and ω in rpm). For the RRDE used here, with
r1 = 0.25 cm and
r2 = 0.325 cm, the value of K is 10.1 rpm·s.
Table 1 shows the estimated values of the diffusion coefficients of O
2 and O
2•− calculated from Equation (7) based on the slopes of
Ts vs. ω
−1 obtained from
Figure 6d.
Figure 7a shows that well-defined O
2 diffusion-limited currents are obtained for the ORR on a GC electrode in an O
2-saturated 0.1 M TBAPF
6/PC solution. The K–L plot for the disk current values at 1.50 V
Li reveals the expected linear relation between the inverse of the limiting current and ω
−0.5 (see Equation (6)). As shown in
Table 1, the concentration of oxygen (
) on the GC electrode was estimated from the slope of the K–L plot using the prior measured values of ν and
, where
n = 1 (according to the reaction of Equation (1)). The value of
is 6.1 M, which is higher than the finding of a previous report (4.8 M) [
37]. This can be attributed to the larger O
2 flow rate in this experiment. The estimated value of
was also applied in the following calculations of the MnO
2/C-GC electrode since the same operation parameters (
i.e., O
2 flow rate, electrolyte composition, and amount) were used, as listed in
Table 1. The rate constant for producing O
2•−,
kf for GC and the MnO
2/C-GC electrodes can be obtained by linearly fitting the K–L plots of
i−1 vs. ω
−0.5 (see Equation (6)), as shown in
Figure 7a,b. The values of
kf for GC and the MnO
2/C-GC electrodes are 1.92 × 10
−2 cm·s
−1 and 4.29 × 10
−2 cm·s
−1, respectively. This result indicates that the MnO
2/C cathode catalyst exhibits a larger
kf value, resulting from higher electrocatalytic activity for the first step of the ORR (see Equation (1)) which produces a higher concentration of O
2•−.
Figure 6.
(a) Example of determination of the superoxide radical (O2•−) transit-time (Ts) in O2-saturated solutions of 0.1 M TBAPF6 in propylene carbonate (PC) at ω = 100 rpm, Edisk = 1.85 V and Ering = 2.6 V. Transit time (Ts) values at different rotation rates for the diffusion of (b) O2 and (c) O2•−; (d) relation between the inverse of the rotation speed and the transient time for O2 and O2•−.
Figure 6.
(a) Example of determination of the superoxide radical (O2•−) transit-time (Ts) in O2-saturated solutions of 0.1 M TBAPF6 in propylene carbonate (PC) at ω = 100 rpm, Edisk = 1.85 V and Ering = 2.6 V. Transit time (Ts) values at different rotation rates for the diffusion of (b) O2 and (c) O2•−; (d) relation between the inverse of the rotation speed and the transient time for O2 and O2•−.
Table 1.
Summary of the electrolyte properties estimated with the proposed RRDE-based methodology and comparison with findings reported in the literature.
Table 1.
Summary of the electrolyte properties estimated with the proposed RRDE-based methodology and comparison with findings reported in the literature.
| Disk Material/Electrolyte | ν (cm2·s−1) | (cm2·s−1) | (cm2·s−1) | (mM) | Reference |
|---|
| GC/0.1 M TBAPF6, PC | 2.6 × 10−2 | 1.9 × 10−5 | 8.6 × 10−6 | 6.1 | This work |
| MnO2/C-GC/0.1 M TBAPF6, PC | 2.6 × 10−2 | 1.9 × 10−5 | 1.8 × 10−6 | 6.1 | This work |
| GC/0.2M TBATFSI, PC | 2.6 × 10−2 | 2.5 × 10−5 | 6.8 × 10−6 | 4.8 | [37] |
Figure 7.
(a) Steady-state CV curves of a glassy carbon rotating disk electrode (RDE) in an O2-saturated 0.1 M TBAPF6/PC solution at a scan rate of 50 mV/s between 1.5 and 2.8 VLi with different rotation rates. The insert is the Koutecky–Levich plot derived from the disc current values at 1.50 VLi; (b) steady-state CV curves of a MnO2/C RDE in an O2-saturated 0.1 M TBAPF6/PC solution at a scan rate of 50 mV/s between 1.2 and 2.8 VLi with different rotation rates.
Figure 7.
(a) Steady-state CV curves of a glassy carbon rotating disk electrode (RDE) in an O2-saturated 0.1 M TBAPF6/PC solution at a scan rate of 50 mV/s between 1.5 and 2.8 VLi with different rotation rates. The insert is the Koutecky–Levich plot derived from the disc current values at 1.50 VLi; (b) steady-state CV curves of a MnO2/C RDE in an O2-saturated 0.1 M TBAPF6/PC solution at a scan rate of 50 mV/s between 1.2 and 2.8 VLi with different rotation rates.
Recently, Herranz
et al. [
37] used RRDE voltammetry to quantify the stability of an electrolyte against O
2•− by the rate constant (
k) for Equation (2) According to their methods, the O
2•− produced at the disk electrode in Equation (1) and the amount of O
2•− were quantified at the ring electrode. The amount of O
2•− consumed depends on the effective transient time,
Ts, between the disk and the ring and the rate constant,
k, for Equation (2). Longer
Ts and larger
k values cause increasing consumption of O
2•− due to its reaction with the electrolyte, resulting in a lower O
2•− oxidation current at the ring. Therefore, the collection efficiency,
Nk, for O
2•− at the ring electrode decreases with increasing transient time, which, in turn, depends on the geometry of ring and disk electrode, the diffusion coefficient of O
2•− in the electrolyte,
, and the electrode rotation speed, ω. The correlation with the collection efficiency is the absolute ratio of ring and disk currents and can be characterized by the following equation [
37,
40]:
where
A1 = 1.288,
A2 = 0.643 ν
1/6 1/3, β = 3ln(
r3/
r2),
U =
k−1tanh(
A1k) and
T2 = 0.718ln(
r2/
r1), whereby
r1–
r3 refer to the radius of the disk and internal and external ring radii, respectively; ν is the kinematic viscosity; ω is the rotation rate;
k is the rate constant for Equation (2); and
is the diffusion coefficient of O
2•−.
Ngeometrical is the geometrical collection efficiency of the RRDE corresponding to the fraction of a species electrochemically generated at the disk. This species is detected at the ring due to the lack of side-reactions with the electrolyte. Equation (8) shows the variation of
Nk where the rotation rate and the rate constant (
k) can be calculated at higher rotation rates, which show that the
Nk is close to a constant value.
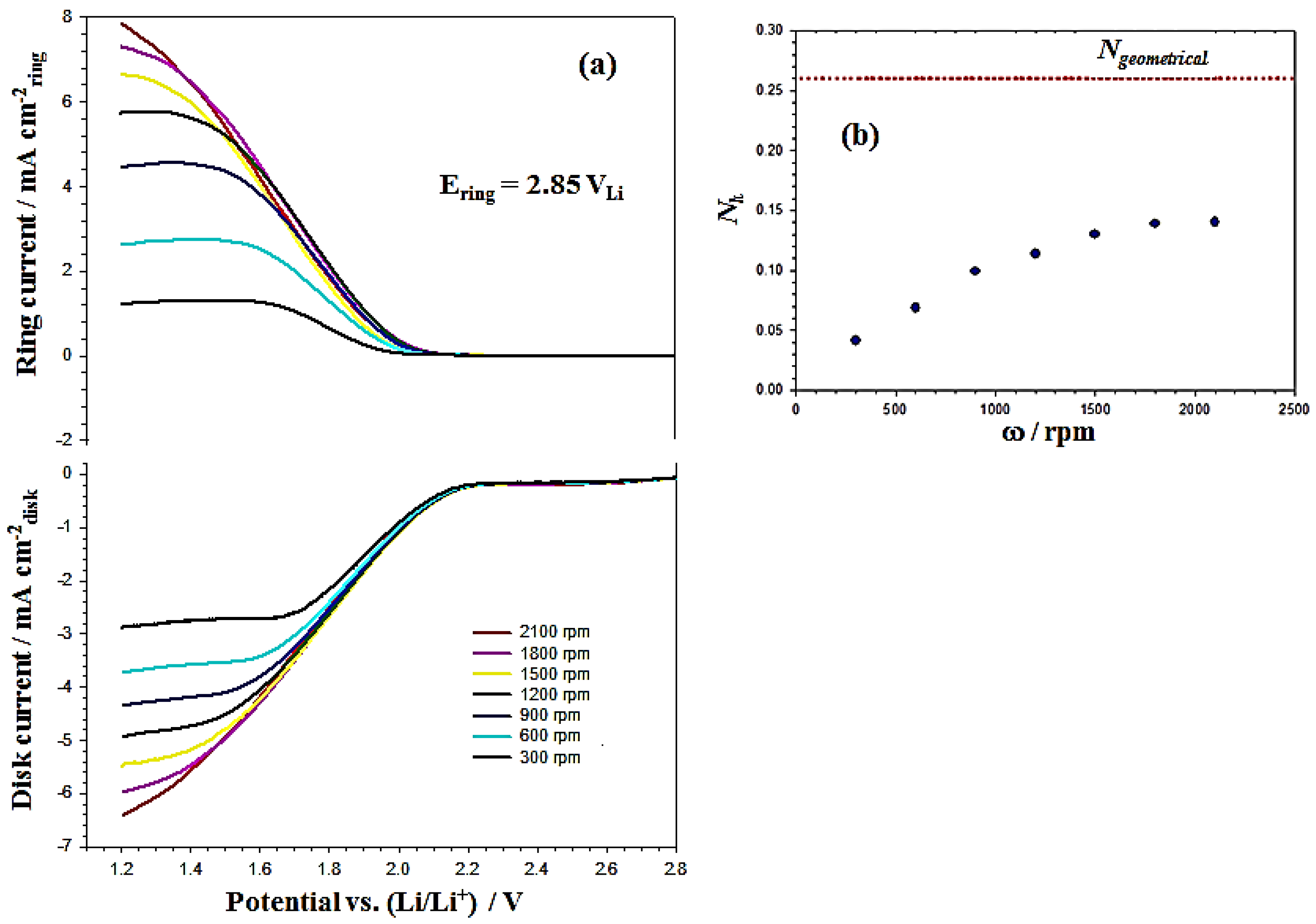
Figure 8a shows the RRDE profiles of the MnO
2/C sample coating on the disk electrode. The disk and ring currents are recorded in an O
2-saturated 0.1 M TBAPF
6/PC solution at rotation rates between 300 and 2100 rpm, with continuous holding of the Pt ring at 2.85 V
Li. The ring current increases with the rotation rates because the shorter transient time at higher rotation rates reduces the reaction time between O
2•− and the PC electrolyte so that a higher concentration of superoxide radical can be oxidized at the ring. Also, the
Nk increases with rotation rates (ω) and is close to a constant value (0.14) at ω = 2100 rpm, as shown in
Figure 8b. The PC-electrolyte decomposition rate constant (
k) can be calculated by Equation (8) using the
Nk value at a rotation speed of 2100 rpm with the kinematic viscosity (ν) and
listed in
Table 1.
Table 2 shows the rate constant for producing O
2•−,
kf, and the PC-electrolyte decomposition rate constant,
k, on the GC and MnO
2/C-GC electrodes. The value of
k (1.5 s
−1) on the GC electrode is close to that of a previously reported measurement (
k = 1.3 s
−1) [
37]. Obviously, the
k value on the MnO
2/C-GC electrode of 2.6 s
−1 is larger than that on the GC electrode. This result shows that MnO
2/C is more active for the first step of the ORR (larger rate constant;
kf), producing a higher concentration of O
2•− and leading to faster PC-electrolyte decomposition due to the attack by a large amount of O
2•−. Therefore, it is important to choose an appropriate electrolyte to avoid decomposition by O
2•− attack for highly active catalyst applications on the cathode materials in Li-O
2 batteries. More detailed RRDE experiments and analysis will be carried out to estimate the decomposition rates of various electrolytes with different active catalysts.
Table 2.
The rate constant for producing O2•−, kf, and the PC-electrolyte decomposition rate constant, k, on the GC and MnO2/C-GC electrodes.
Table 2.
The rate constant for producing O2•−, kf, and the PC-electrolyte decomposition rate constant, k, on the GC and MnO2/C-GC electrodes.
| Disk Material/Electrolyte | kf (cm·s−1) | k (s−1) | Reference |
|---|
| GC/0.1 M TBAPF6, PC | 1.9 × 10−2 | 1.5 | This work |
| MnO2/C-GC/0.1 M TBAPF6, PC | 4.3 × 10−2 | 2.6 | This work |
| GC/0.2M TBATFSI, PC | | 1.3 | [37] |
Figure 8.
(a) RRDE profiles of MnO2/C recorded at 50 mV·s−1 in an O2-saturated 0.1 M TBAPF6/PC solution, at rotation rates between 300 and 2100 rpm with continuous holding of the Pt ring at 2.85 VLi; (b) evolution of the absolute ratio between the ring and disk current (Nk) and the electrode rotation speed (ω).
Figure 8.
(a) RRDE profiles of MnO2/C recorded at 50 mV·s−1 in an O2-saturated 0.1 M TBAPF6/PC solution, at rotation rates between 300 and 2100 rpm with continuous holding of the Pt ring at 2.85 VLi; (b) evolution of the absolute ratio between the ring and disk current (Nk) and the electrode rotation speed (ω).