Coupling of Nanocrystalline Anatase TiO2 to Porous Nanosized LaFeO3 for Efficient Visible-Light Photocatalytic Degradation of Pollutants
Abstract
:1. Introduction
2. Results and Discussion
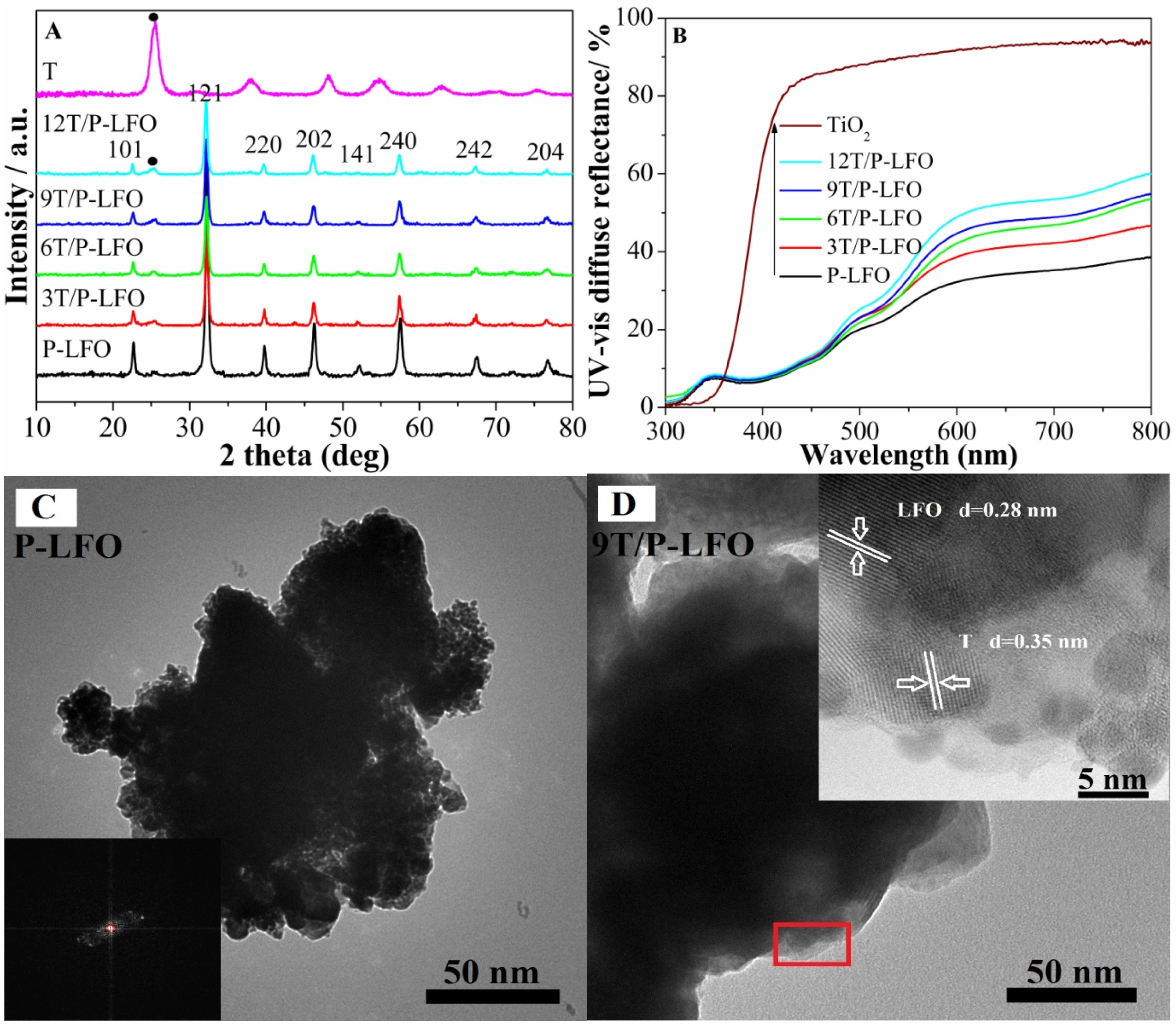
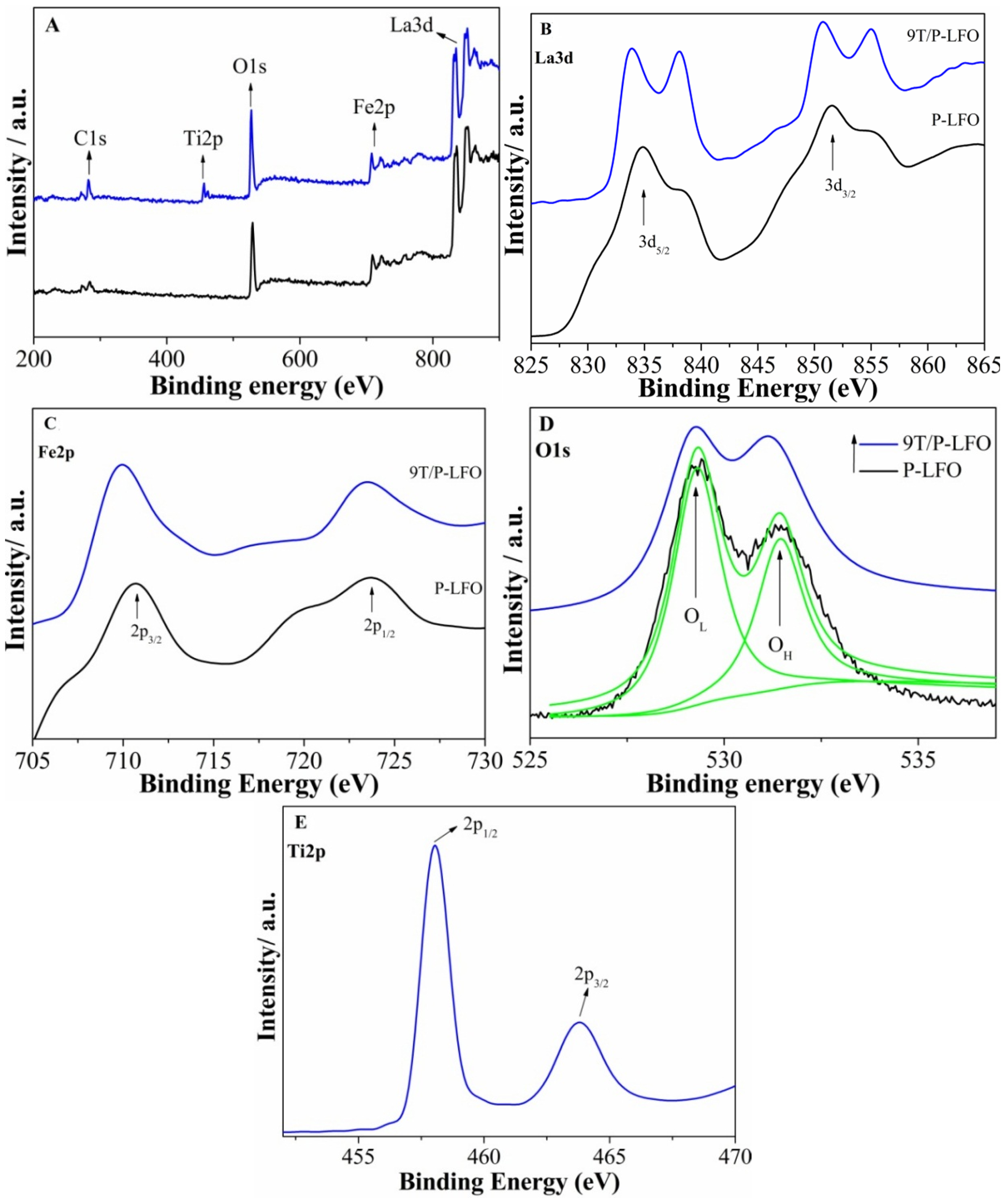
2.1. Structural Characterization and Surface Composition


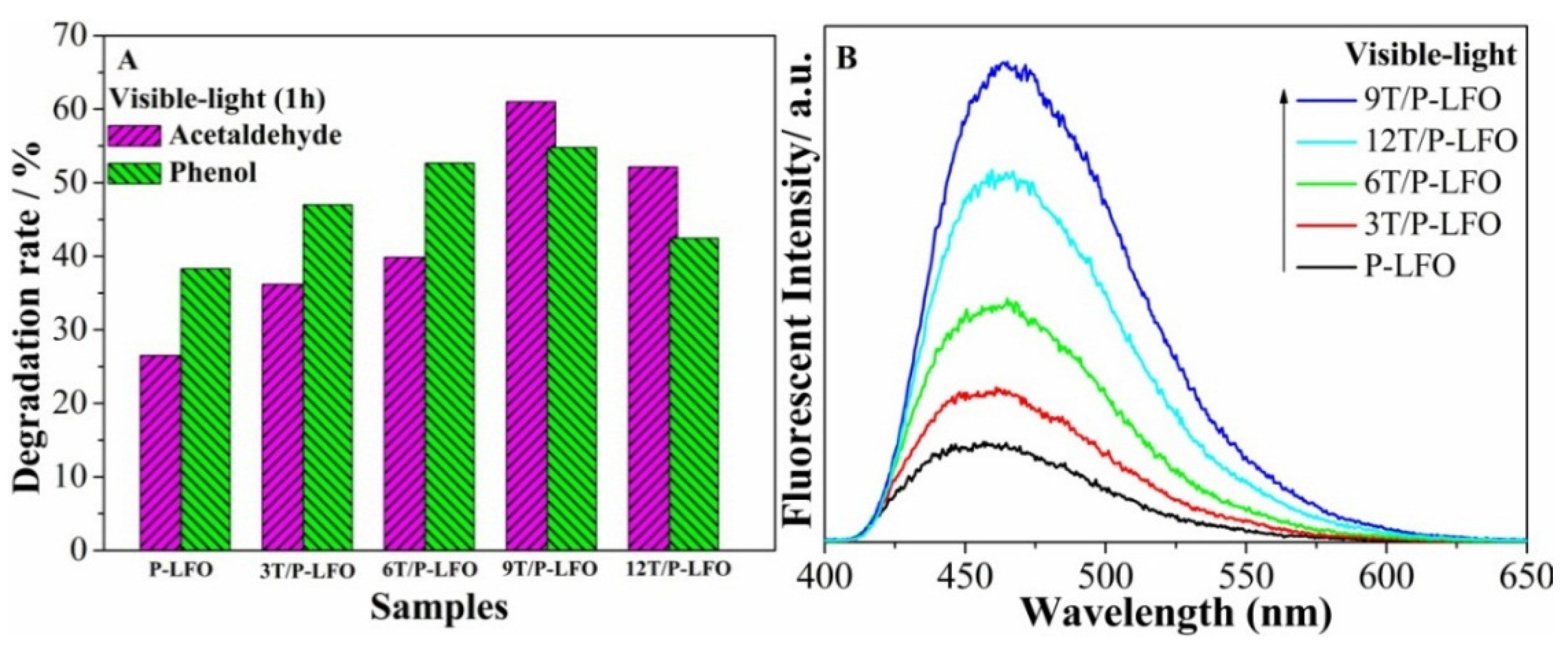
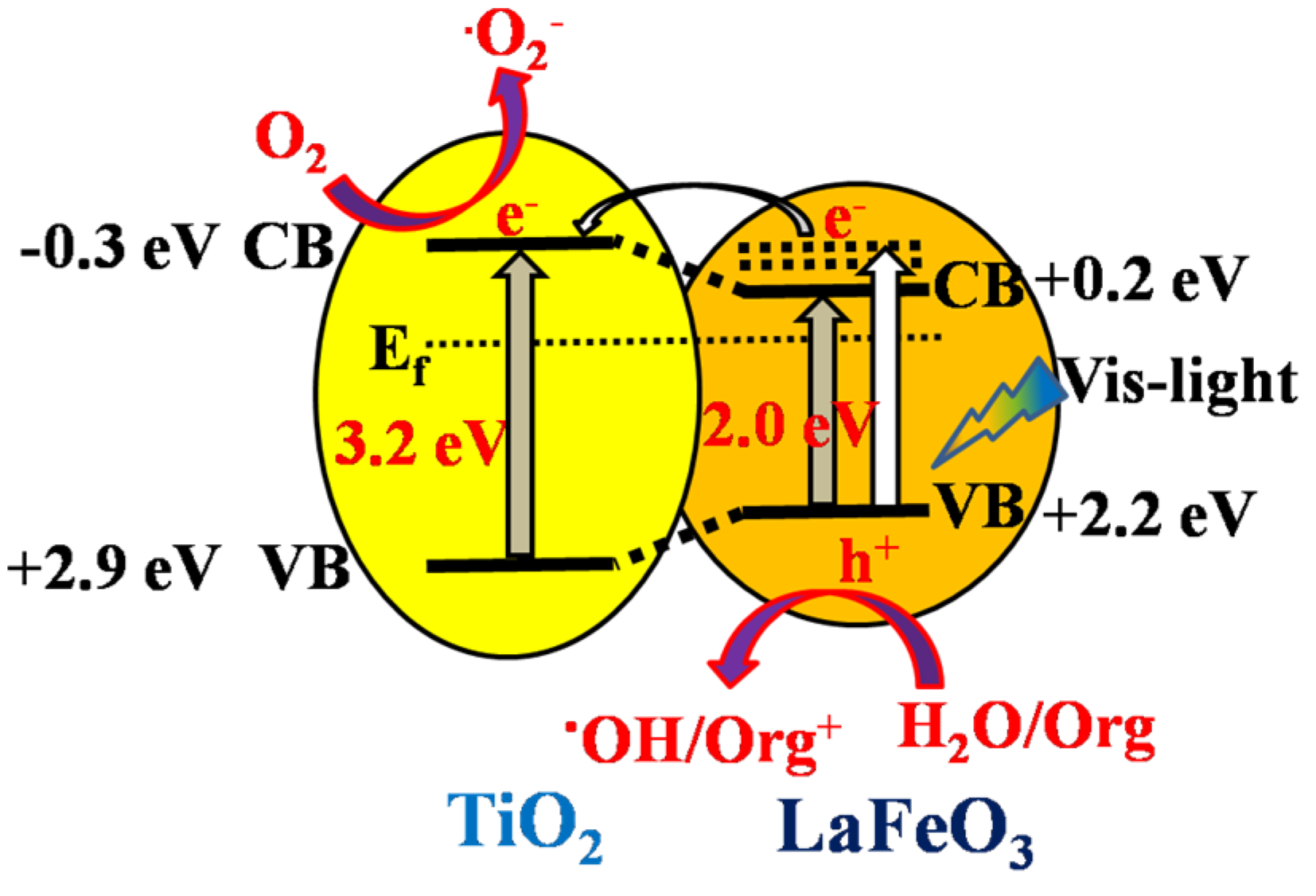
2.2. Photogenerated Charge Properties

2.3. Visible-Light Photoactivities
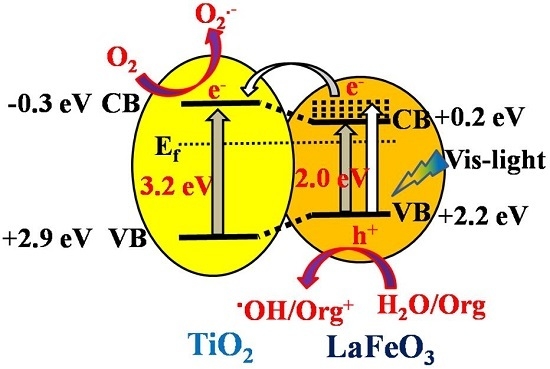
2.4. Discussion
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Synthesis of Porous LaFeO3
3.3. Fabrication of Nanocomposite Materials
3.4. Evaluation of Photocatalytic Activity for Pollutant Degradation
3.5. Measurement of the Produced Hydroxyl Radical (•OH) Amount
3.6. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, Z.X.; Shen, Y.; Yang, C.; Lei, Y.C.; Guan, Y.; Lin, Y.; Liu, D.; Nan, C.W. Significant Enhancement in the Visible Light Photocatalytic Properties of BiFeO3-Graphene Nanohybrids. J. Mater. Chem. A 2013, 1, 823–829. [Google Scholar] [CrossRef]
- Xie, M.Z.; Fu, X.D.; Jing, L.Q.; Luan, P.; Feng, Y.J.; Fu, H.G. Long-Lived, Visible-Light-Excited Charge Carriers of TiO2/BiVO4 Nanocomposites and Their Unexpected Photoactivity for Water Splitting. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Fujii, T.; Matsusue, I.; Nakatsuka, D.; Nakanishi, M.; Takada, J. Synthesis and anomalous magnetic properties of LaFeO3 nanoparticles by hot soap method. Mater. Chem. Phys. 2011, 129, 805–809. [Google Scholar] [CrossRef]
- Zhu, J.J.; Li, H.L.; Zhong, L.Y.; Xiao, P.; Xu, X.L.; Yang, X.G.; Zhao, Z.; Li, J.L. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Kanade, K.G.; Baek, J.O.; Kong, K.J.; Kale, B.B.; Lee, S.M.; Moon, S.J.; Lee, C.W.; Yoon, S.H. A new layer perovskites Pb2Ga2Nb2O10 and RbPb2Nb2O7: An efficient visible light driven photocatalysts to hydrogen generation. Int. J. Hydrog. Energy 2008, 33, 6904–6912. [Google Scholar] [CrossRef]
- Hirohisa, T.; Makoto, M. Advances in designing perovskite catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar]
- Abazari, R.; Sanati, S. Perovskite LaFeO3 nanoparticles synthesized by the reverse microemulsion nanoreactors in the presence of aerosol-OT: Morphology, crystal structure, and their optical properties. Superlattices Microst. 2013, 64, 148–157. [Google Scholar] [CrossRef]
- May, K.J.; Fenning, D.P.; Ming, T.; Hong, W.T.; Lee, D.Y.; Stoerzinger, K.A.; Biegalski, M.D.; Kolpak, A.M.; Yang, S.H. Thickness-Dependent Photoelectrochemical Water Splitting on Ultrathin LaFeO3 Films Grown on Nb:SrTiO3. J. Phys. Chem. Lett. 2015, 6, 977–985. [Google Scholar] [CrossRef]
- Jing, L.Q.; Qu, Y.C.; Su, H.J.; Yao, C.H.; Fu, H.G. Synthesis of High-Activity TiO2-Based Photocatalysts by Compounding a Small Amount of Porous Nanosized LaFeO3 and the Activity-Enhanced Mechanisms. J. Phys. Chem. C 2011, 115, 12375–12380. [Google Scholar] [CrossRef]
- Farhadi, S.; Siadatnasab, F. Perovskite-type LaFeO3 nanoparticles prepared by thermal decomposition of the La[Fe(CN)6]·5H2O complex: A new reusable catalyst for rapid and efficient reduction of aromatic nitro compounds to arylamines with propan-2-ol under microwave irradiation. J. Mol. Catal. A 2011, 339, 108–116. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.G.; Wang, T.; Li, P.; Liu, L.Q.; Chang, K.; Liu, G.G.; Ye, J.H. A highly durable p-LaFeO3/n-Fe2O3 photocell for effective water splitting under visible light. Chem. Commun. 2015, 51, 3630–3633. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Xie, Y.; Jiang, L.; Wang, J.; Tian, G.; Ren, Z.; Tian, C.; Fu, H. A novel phase-mixed MgTiO3–MgTi2O5 heterogeneous nanorod for high efficiency photocatalytic hydrogen production. Chem. Commun. 2013, 49, 8510–8512. [Google Scholar] [CrossRef]
- Varaprasad, K.; Ramam, K.; Reddy, G.S.M.; Sadiku, R. Development and characterization of nano-multifunctional materials for advanced applications. RSC Adv. 2014, 4, 60363–60370. [Google Scholar] [CrossRef]
- Luan, P.; Xie, M.Z.; Fu, X.; Qu, Y.; Sun, X.; Jing, L. Improved photoactivity of TiO2–Fe2O3 nanocomposites for visible-light water splitting after phosphate bridging and its mechanism. Phys. Chem. Chem. Phys. 2015, 17, 5043. [Google Scholar] [CrossRef]
- Xu, J.J.; Wang, Z.L.; Xu, D.; Meng, F.Z.; Zhang, X.B. 3D ordered macroporous LaFeO3 as efficient electrocatalyst for Li–O2 batteries with enhanced rate capability and cyclic performance. Energy Environ. Sci. 2014, 7, 2213–2219. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.P.; Li, X.W.; Lu, G.Y.; You, L.; Liang, X.; Liu, F.; Zhang, T.; Du, Y. Highly sensitive humidity sensor based on high surface area mesophorous LaFeO3 prepared by a nanocasting route. Sens. Actuators B 2013, 181, 802–809. [Google Scholar] [CrossRef]
- Wang, P.; Zhai, Y.M.; Wang, D.J.; Dong, S.J. Synthesis of reduced graphene oxide-anatase TiO2 nanocomposite and its improved photo-induced charge transfer properties. Nanoscale 2011, 3, 1640–1645. [Google Scholar] [CrossRef]
- Su, H.J.; Jing, L.Q.; Shi, K.Y.; Yao, C.H.; Fu, H.G. Synthesis of large surface area LaFeO3 nanoparticles by SBA-16 template method as high active visible photocatalysts. J. Nanopart. Res. 2010, 12, 967–974. [Google Scholar] [CrossRef]
- Parida, K.M.; Reddy, K.H.; Martha, S.; Das, D.P.; Biswal, N. Fabrication of nanocrystalline LaFeO3: An efficient sole gel auto-combustion assisted visible light responsive photocatalyst for water decomposition. Int. J. Hydrog. Energy 2010, 35, 12161–12168. [Google Scholar] [CrossRef]
- Li, Z.L.; Wnetrzak, R.; Kwapinski, W.; Leahy, J.J. Synthesis and characterization of sulfated TiO2 nanorods and ZrO2/TiO2 nanocomposites for the esterification of bio-based organic acid. ACS Appl. Mater. Interfaces 2012, 4, 4499–4505. [Google Scholar] [CrossRef]
- Song, P.; Zhang, H.H.; Han, D.; Li, J.; Yang, Z.X.; Wang, Q. Preparation of biomorphic porous LaFeO3 by sorghum straw biotemplate method and its acetone sensing properties. Sens. Actuators B 2014, 196, 140–146. [Google Scholar] [CrossRef]
- Gao, K.; Li, S.D. Multi-modal TiO2–LaFeO3 composite films with high photocatalytic activity and hydrophilicity. Appl. Surf. Sci. 2012, 258, 6460–6464. [Google Scholar] [CrossRef]
- Humayun, M.; Zada, A.; Li, Z.J.; Xie, M.Z.; Zhang, X.; Qu, Y.; Raziq, F.; Jing, L.Q. Enhanced visible-light activities of porous BiFeO3 by coupling with nanocrystalline TiO2 and mechanism. Appl. Catal. B 2016, 180, 219–226. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humayun, M.; Li, Z.; Sun, L.; Zhang, X.; Raziq, F.; Zada, A.; Qu, Y.; Jing, L. Coupling of Nanocrystalline Anatase TiO2 to Porous Nanosized LaFeO3 for Efficient Visible-Light Photocatalytic Degradation of Pollutants. Nanomaterials 2016, 6, 22. https://doi.org/10.3390/nano6010022
Humayun M, Li Z, Sun L, Zhang X, Raziq F, Zada A, Qu Y, Jing L. Coupling of Nanocrystalline Anatase TiO2 to Porous Nanosized LaFeO3 for Efficient Visible-Light Photocatalytic Degradation of Pollutants. Nanomaterials. 2016; 6(1):22. https://doi.org/10.3390/nano6010022
Chicago/Turabian StyleHumayun, Muhammad, Zhijun Li, Liqun Sun, Xuliang Zhang, Fazal Raziq, Amir Zada, Yang Qu, and Liqiang Jing. 2016. "Coupling of Nanocrystalline Anatase TiO2 to Porous Nanosized LaFeO3 for Efficient Visible-Light Photocatalytic Degradation of Pollutants" Nanomaterials 6, no. 1: 22. https://doi.org/10.3390/nano6010022
APA StyleHumayun, M., Li, Z., Sun, L., Zhang, X., Raziq, F., Zada, A., Qu, Y., & Jing, L. (2016). Coupling of Nanocrystalline Anatase TiO2 to Porous Nanosized LaFeO3 for Efficient Visible-Light Photocatalytic Degradation of Pollutants. Nanomaterials, 6(1), 22. https://doi.org/10.3390/nano6010022