Morphological, Chemical Surface, and Diffusive Transport Characterizations of a Nanoporous Alumina Membrane
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphological and Chemical Surface Characterization
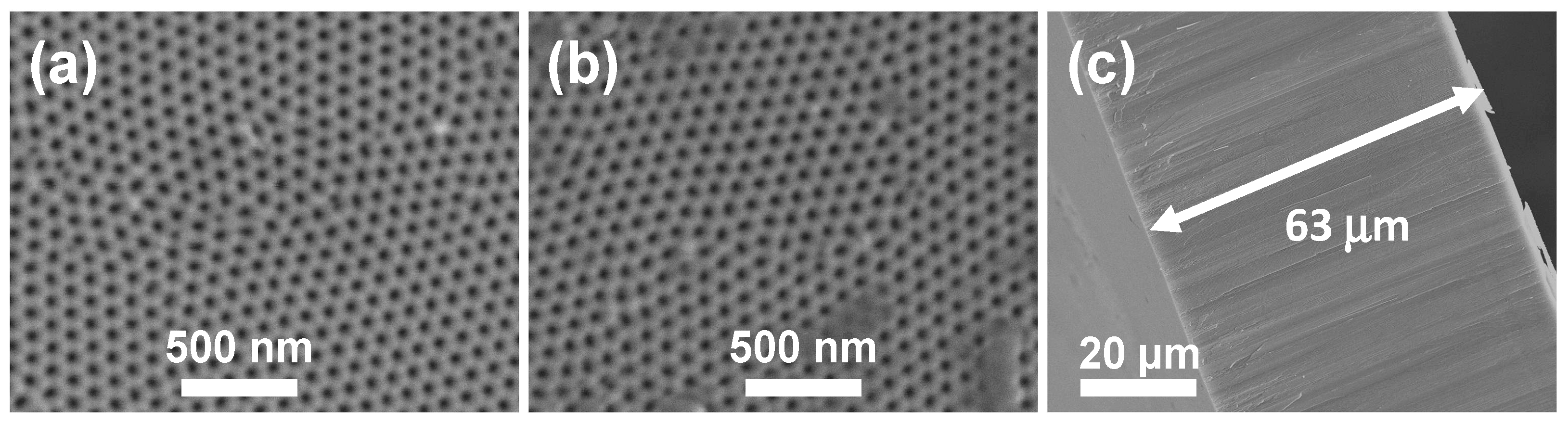
| Membrane | C 1s (%) | O 1s (%) | Al 2p (%) | P 2s (%) | (O/Al) |
|---|---|---|---|---|---|
| Al-Ox | 23.9 | 48.7 | 25.4 | 2.0 | 1.9 |
2.2. Diffusive Transport Analysis

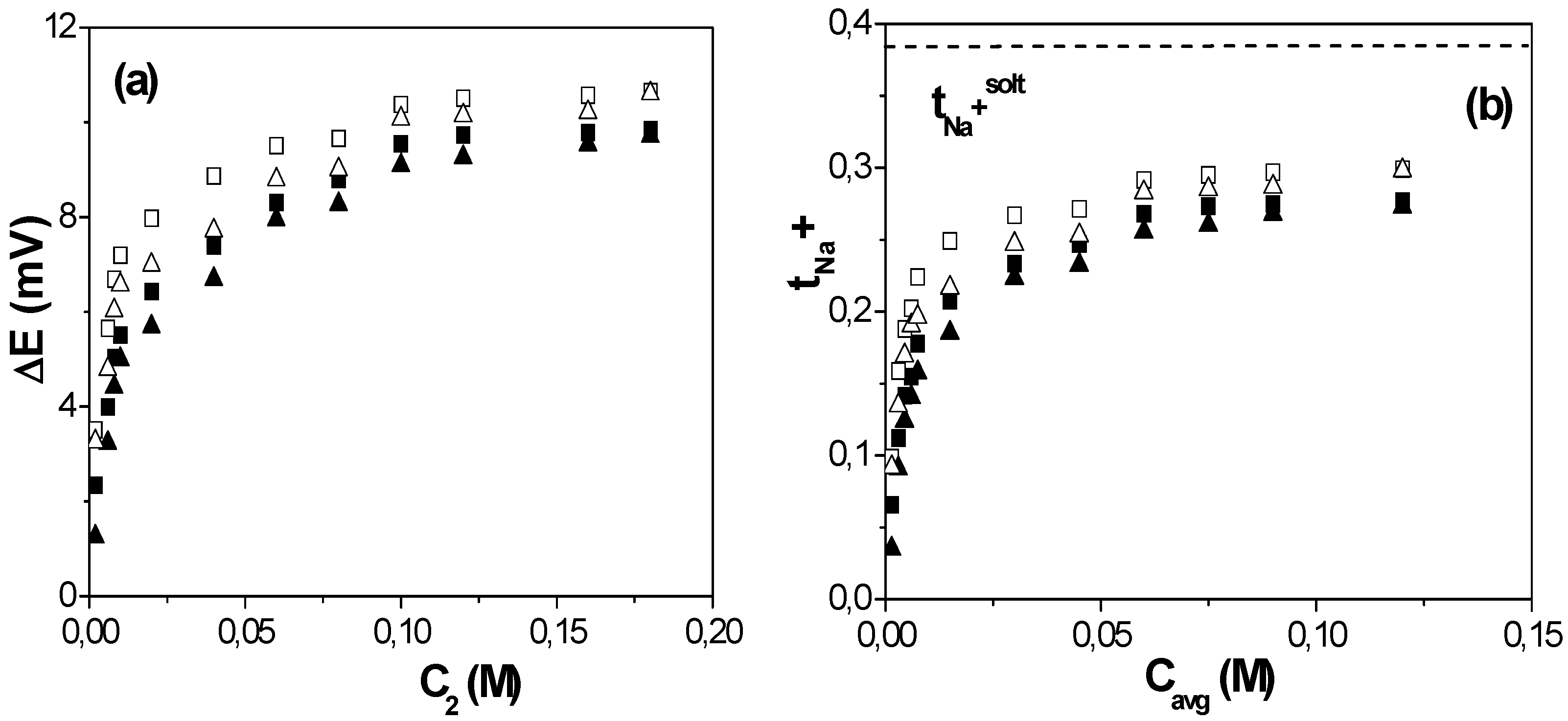
3. Experimental Section
3.1. Synthesis of the Nanoporous Alumina Membrane
3.2. Morphological Characterizations
3.3. XPS Measurements
3.4. Salt Diffusion and Concentration Potential Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eftekhari, A. Nanostructured Materials in Electrochemistry; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Mo, Y.; Fei, T. Nanoporous membrane for biosensing applications. Nano Life 2012, 2. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Zhang, Y.; Yu, J.-J.; Mak, A.F.-T.; Li, Y.; Yang, M. A. microfluidic chip with poly(ethylene glycol) hydrogel microarray on nanoporous alumina membrane for cell patterning and drug testing. Sens. Actuators B 2010, 143, 776–783. [Google Scholar] [CrossRef]
- Kennard, R.; DeSisto, W.J.; Mason, M.D. Molecular diffusivity measurements through an alumina membrane using time-resolved fluorescence imaging. Appl. Phys. 2010, 97, 213701–213705. [Google Scholar] [CrossRef]
- Karnik, R.; Fang, R.; Yue, M.; Li, D.; Yang, P.; Majumdar, A. Electrostatic control of ions and molecules in nanofluidic transistors. Nano Lett. 2005, 5, 943–948. [Google Scholar] [CrossRef] [PubMed]
- Penumetcha, S.S.; Kona, R.; Hardin, J.L.; Molder, A.L.; Steinle, E.D. Monitoring transport across modified nanoporous alumina membranes. Sensors 2007, 7, 2942–2952. [Google Scholar] [CrossRef]
- Holbein, J.; Steinhart, M.; Schiene-Fisher, C.; Benda, A.; Hof, M.; Hübner, C.G. Confinaded diffusion in ordered nanoporous alumina membranes. Small 2007, 3, 390–385. [Google Scholar]
- Jiang, X.; Mishra, N.; Turner, J.N.; Spencer, M.G. Diffusivity of sub-1,000 Da molecules in 40 nm silicon-based alumina membranes. Microfluid. Nanofluid. 2008, 5, 695–701. [Google Scholar] [CrossRef]
- Romero, V.; Vega, V.; García, J.; Prida, V.M.; Hernando, B.; Benavente, J. Ionic transport across tailored nanoporous alumina membranes. J. Colloid Interface Sci. 2012, 376, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Fukuda, K. Ordered Metal Nanohole Arrays Made by a Two-Step Replication of Honeycomb Structures of Anodic Alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Itoh, N.; Kato, K.; Tsuji, T.; Hongo, M. Preparation of a Tubular Anodic Aluminum Oxide Membrane. J. Membr. Sci. 1996, 117, 189–196. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. Anodic Oxide Films on Aluminum. Chem. Rev. 1969, 69, 365–405. [Google Scholar] [CrossRef]
- Lee, S.W.; Shang, H.; Haasch, R.T.; Petrova, V.; Lee, G.U. Transport and functional behaviour of poly(ethylene glycol)-modified nanoporous alumina membranes. Nanotechnology 2005, 16, 1335–1340. [Google Scholar] [CrossRef]
- Romero, V.; Vega, V.; García, J.; Zierold, R.; Nielsch, K.; Prida, V.M.; Hernando, B.; Benavente, J. Changes in morphology and ionic transport induced by ALD SiO2 coating of nanoporous alumina membranes. ACS Appl. Mater. Interfaces 2013, 5, 3556–3564. [Google Scholar] [CrossRef] [PubMed]
- Albu, S.P.; Ghicov, A.; Macak, J.M.; Hahn, R.; Schmuki, P. Self-organized, free-standing TiO2 nanotube membranes for flow-through photocatalytic application. Nanoletters 2007, 7, 1286–1289. [Google Scholar] [CrossRef] [PubMed]
- Romero, V.; Vázquez, M.I.; Cañete, S.; Vega, V.; García, J.; Prida, V.M.; Hernando, B.; Benavente, J. Frictional and electrical effects envolved in the diffusive transport through a nanoporous alumina membrane. J. Phys. Chem. C 2013, 117, 25513–25518. [Google Scholar] [CrossRef]
- Bluhm, E.A.; Bauer, E.; Chamberlin, R.M.; Abney, K.D.; Young, J.S.; Jarvinen, D. Surface effects on cation transport across porous alumina membranes. Langmuir 1998, 15, 8668–8672. [Google Scholar] [CrossRef]
- Kipke, S.; Schmid, G. Nanoporous Alumina Membranes as Diffusion Controlling Systems. Adv. Funct. Mater. 2004, 14, 1184–1188. [Google Scholar] [CrossRef]
- Benavente, J.; Hernandez, A.; Jonsson, G. Proper and absorbed charges on the surface of the polysilfone support of a composite membrane from electrokinetic phenomena. J. Membr. Sci. 1993, 80, 285–296. [Google Scholar] [CrossRef]
- Filippov, A.N.; Starov, V.M.; Kononenko, N.A.; Berezina, N.P. Asymmetry of Diffusion Permeability of Bilayer Membranes. Adv. Colloids Interface Sci. 2008, 139, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Ariza, M.J.; Benavente, J.; Rodríguez-Castellón, E. Handbook of Membrane Research: Properties, Performance and Applications; Nova Science Publishers, Inc.: New York, NY, USA, 2009; pp. 257–290. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Gilbert, D.L.; Okano, T.; Miyata, T.; Kim, S.W. Macromolecular Diffusion through Collagen Membranes. Int. J. Pharm. 1988, 47, 79–88. [Google Scholar] [CrossRef]
- Lakshminarayanaiah, N. Transport Phenomena in Membranes; Academic Press: New York, NY, USA, 1969. [Google Scholar]
- Romero, V.; Vega, V.; García, J.; Prida, V.M.; Hernando, B.; Benavente, J. Effect of Porosity and Concentration Polarization on Electrolyte Diffusive Transport Parameters through Ceramic Membranes with Similar Nanopore Size. Nanomaterials 2014, 4, 700–711. [Google Scholar] [CrossRef]
- Robinson, R.A.; Stokes, R.H. Electrolyte Solutions; Butterworths Scientific Publication: London, UK, 1959; pp. 284–335. [Google Scholar]
- Helfferich, F. Ion Exchange Membranes; Mcgraw-Hill: New York, NY, USA, 1962; pp. 340–420. [Google Scholar]
- Porras, B.; Romero, V.; Benavente, J. Effect of acid/basic solutions contact on ion transport numbers and conductivity for an anion-exchange membrane. Desal. Water Treat. 2014, 57, 127–134. [Google Scholar] [CrossRef]
- Prida, V.; Vega, V.; García, J.; Iglesias, L.; Hernando, B.; Minguez-Bacho, I. Electrochemical Methods for Template-Assisted Synthesis of Nanostructured Materials. In Magnetic Micro and Nanowires; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Minneapolis, MN, USA, 1992. [Google Scholar]
- Romero, V.; Vázquez, M.I.; Benavente, J. Study of ionic and diffusive transport through a regenerated cellulose nanoporous membrane. J. Membr. Sci. 2013, 433, 152–159. [Google Scholar] [CrossRef]
- Pelaez, L; Vázquez, M.I.; Benavente, J. Interfacial and fouling effects on diffusional permeability across a composite ceramic membrane. Ceramics Int. 2010, 36, 797–801. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez, M.I.; Romero, V.; Vega, V.; García, J.; Prida, V.M.; Hernando, B.; Benavente, J. Morphological, Chemical Surface, and Diffusive Transport Characterizations of a Nanoporous Alumina Membrane. Nanomaterials 2015, 5, 2192-2202. https://doi.org/10.3390/nano5042192
Vázquez MI, Romero V, Vega V, García J, Prida VM, Hernando B, Benavente J. Morphological, Chemical Surface, and Diffusive Transport Characterizations of a Nanoporous Alumina Membrane. Nanomaterials. 2015; 5(4):2192-2202. https://doi.org/10.3390/nano5042192
Chicago/Turabian StyleVázquez, María I., Virgina Romero, Victor Vega, Javier García, Victor M. Prida, Blanca Hernando, and Juana Benavente. 2015. "Morphological, Chemical Surface, and Diffusive Transport Characterizations of a Nanoporous Alumina Membrane" Nanomaterials 5, no. 4: 2192-2202. https://doi.org/10.3390/nano5042192








