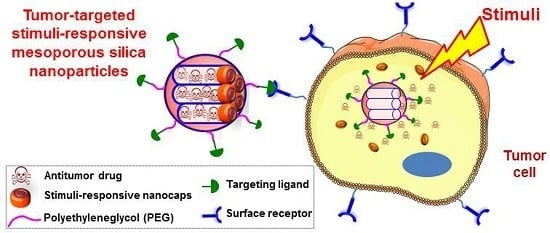
Smart Mesoporous Nanomaterials for Antitumor Therapy
Abstract
:1. Introduction

2. Selective Targeting
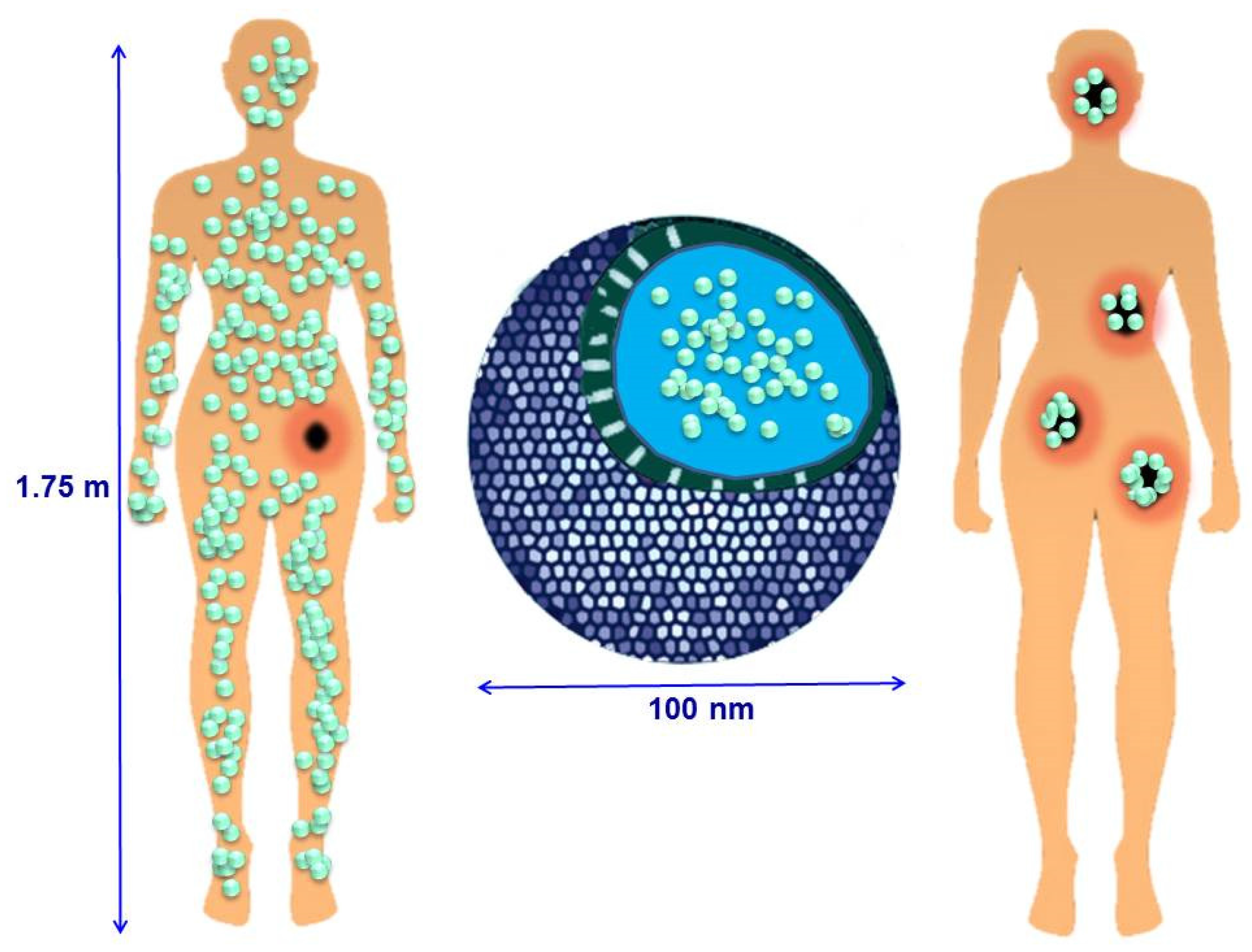
2.1. Passive Targeting
- (i)
- Particle size is considered one of the most important features of NPs. MSNs must be at least 50 nm in diameter to keep their inherent mesoporosity and avoid renal clearance, but have to be smaller than 300 nm to diffuse through the tumor interstitium in sufficient amounts to achieve therapeutic effect [11,64].
- (ii)
- Particle shape has gained considerable attention since it was found that non-spherical NPs could reduce phagocytosis by macrophages, thus exhibiting longer in vivo circulation times [65,66]. However, it is difficult to consider shape as a single variable because the fabrication techniques used to produce NPs with different shapes using biocompatible materials are limited [67]. Thus, it is difficult to exclude the relationship of various chemical, electrostatic and morphological factors by control experiments. In fact, there are only few reports regarding the effect of particle shape on in vitro and in vivo behavior, [68,69] even some of them with contradicting results. All these aspects make the influence of the shape of MSNs on cell internalization and cellular fate an unsolved question.
- (iii)
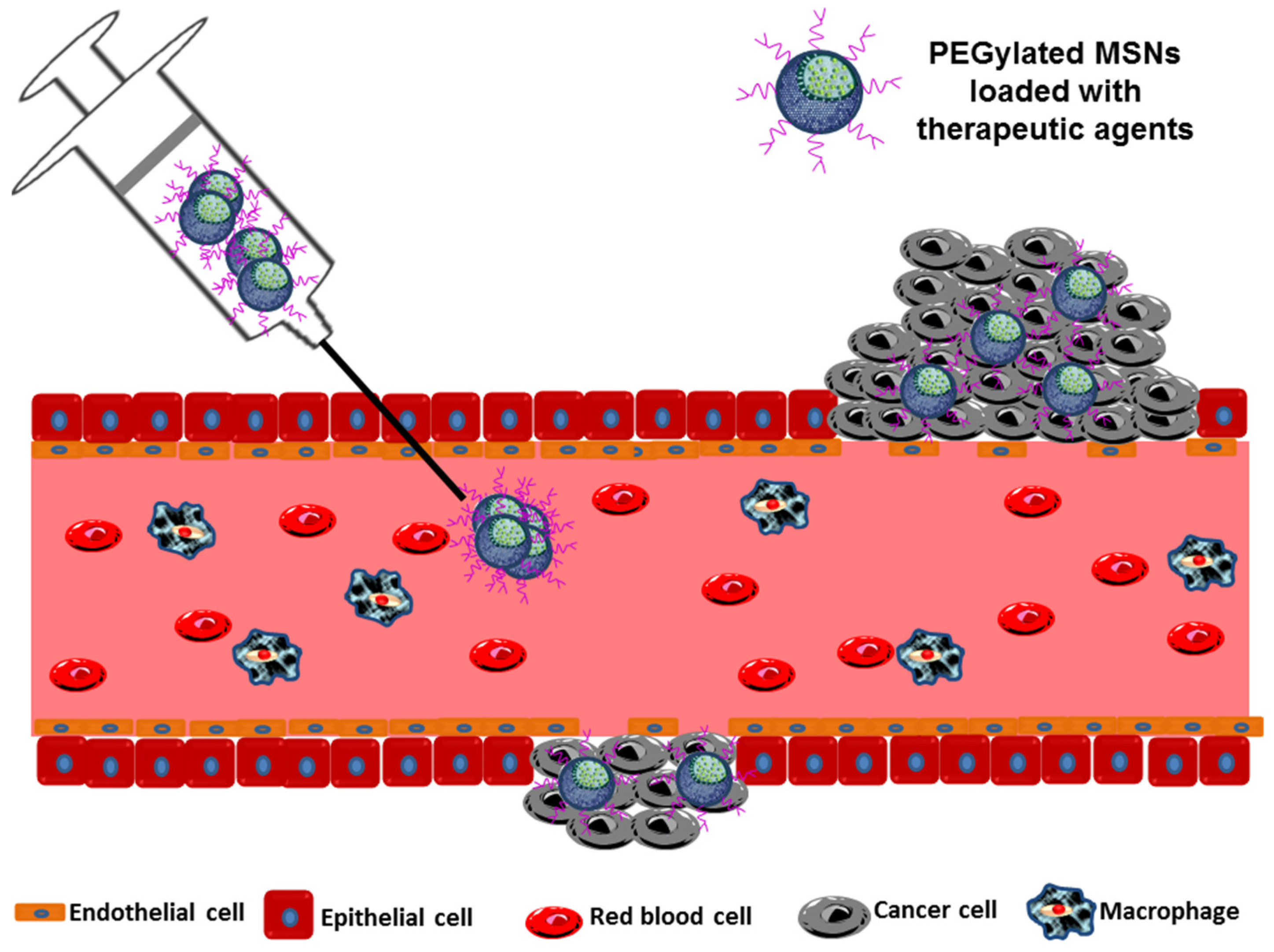
- Surface properties are considered, together with the most important aspects that influence the EPR effect as it is the nature of the surface the first aspect that the MSNs “show” to the cells. Surface modification is one of the fundamental approaches used to increase the time MSNs remain in circulation to ensure tumor accumulation. The aim is to make MSNs “invisible” for the RES avoiding a rapid clearance. Surface functionalization with hydrophilic polymers is one of the most used strategies, especially with PEG (polyethylene glycol) and their derivatives (Figure 3) [70,71,72,73]. PEG not only reduces RES uptake but also improves the stability of the MSNs in biological fluids [71].
2.2. Active Targeting

| Targeting Cell Membrane Receptors | ||||
|---|---|---|---|---|
| Receptor a | Targeting Ligand b | Conjugation Strategy c | Target Cell Line d | Ref. |
| TfR | Tf | CS-1 | PANC-1, BT-549 | [74] |
| TfR | Tf | CS-2 | HeLa | [75] |
| TfR | Tf | CS-2 | HT1080 | [76] |
| EGFR | EGF | CS-3 | HuH-7 | [77] |
| FAR (FR-α) | FA | CS-2 | Hela, PANC, U2Os, MDA-MB-231, SK-BR-3, MiaPaca-2 | [78,79,80,81,82,83,84,85] |
| FR-α | Methotrexate | CS-2 | HeLa | [86] |
| Sigma receptor | Anisamide | CS-2 | ASPC-1 | [85] |
| Importing α and β receptors | TAT peptides | CS-2 | Hela; MCF-7/ADR | [87,88,89] |
| IL-13Rα2 | IL-13 peptide | CS-3 | U251 | [90] |
| Targeting Tumor Vasculature Receptors | ||||
| Receptor | Targeting Ligand | Conjugation Strategy | Target Cell Line | Ref. |
| HER2 | Anti-herceptin | CS-2 | SK-BR3 | [91] |
| HER2/neu | Anti-HER2/neu | CS-3 | BT474 | [92] |
| ErbB2 | Anti-ErbB2 | CS-4 | MCF-7 | [93] |
| Mesothelin | Anti-ME1 | CS-2 | MM | [94] |
| CD105/endoglin | Anti-TRC105 | CS-3 | HUVECs | [95] |
| NET | MABG | CS-2 | NB1691-luc | [96] |
| ανβ3-integrins | c(RGDyK) | CS-3 | U87-MG | [97] |
| ανβ3-integrins | cRGD | CS-5 | MDA-MB 435 | [74] |
| ανβ3-integrins | K7RGD, c-RGDFK | CS-2 | HeLa | [98] |
| ανβ3-integrins | K8(RGD)2 | CS-4 | U87-MG | [99] |
| ανβ3-integrins | N3GPLGRGRGDK-Ad | CS-6 | SCC-7, HT-29 | [100] |
| ανβ3-integrins | N3RGDFFFFC | CS-5 | U87-MG | [101] |
| ανβ3-integrins | Thiolated-RGD | CS-3 | A375, HepG2, MCF-7, Neuro-2a | [102] |
| (VCAM-1)R | Anti-(VCAM-1) | CS-2 | HUVEC-CS | [103] |
| VEGFR | VEGF | CS-3 | U87-MG | [104] |
- -
- Transferrin receptor (TfR): Tf is a membrane glycoprotein that operates together with its receptor, TfR, to assist the uptake of iron by the cell. The TfR may be overexpressed by up to 100-fold on tumor cells, making it an attractive alternative for targeted delivery of drugs by grafting Tf to MSNs.
- -
- Folic acid receptor (FAR): FAR is one of the most widely studied molecules for targeting MSNs to cancer cells, since FAR is up-regulated in several types of human cancers, including ovarian, endometrial, colorectal, breast, lung, renal carcinoma, brain metastases derived from epithelial cancer and neuroendocrine carcinoma [106].
- -
- Epidermal growth factor receptor (EGFR): is a receptor tyrosine kinase that belongs to the ErbB family, which is extremely activated in many epithelial tumors. The receptor’s aberrant abnormal activation found in cancer can obey different mechanisms, including receptor overexpression, mutation, ligand-dependent receptor dimerization, and ligand-independent activation is a receptor tyrosine kinase of the ErbB family that is abnormally activated in many epithelial tumors. Several mechanisms lead to the receptor’s aberrant activation that is observed in cancer, including receptor overexpression, mutation, ligand-dependent receptor dimerization, and ligand-independent activation. Thus, targeting of NPs to EGFR by grafting EGF or anti-EGFR agents is a good alternative for cancer treatment [107].
- -
- Antigens: Abnormal expression of certain antigens in the surface of tumor cells is the fundamental of antibody (Ab)-based cancer therapies [108]. The presence of cell surface antigens expressed by human cancers has provided a wide range of targets that are either overexpressed, mutated or selectively expressed in comparison definition of cell surface antigens that are expressed by human cancers has revealed a broad array of targets that are overexpressed, mutated or selectively expressed compared with normal tissues. This strategy can be used to target NPs to cancer cells. Thus, different Abs have been grafted to MSNs to design effective tumor-targeted nanodevices.
- (i)
- Extravasation of NPs from the blood vessels is not required.
- (ii)
- Tumor blood vessels usually overexpress certain receptors which are easily accessible to NPs.
- (iii)
- Endothelial cells that compose the tumor vessels are less susceptible to suffer mutations than tumor cells, which reduces the risk of multidrug resistance. This fact obeys the more stable environment of endothelial cells compared to that of tumor cells housed inside the solid tumor mass, which are exposed to hard conditions (low pH values, low O2 pressure, etc.).
- (iv)
- Endothelial cell markers are common in different tumors.
- -
- Vascular endothelial growth factor receptor (VEGFR): VEGF is a signal protein produced by cells to stimulate vasculogenesis and angiogenesis. The endothelial cells surrounding tumor cells overexpress VEGFRs. Thus, it is possible to target NPs to tumor blood vessels by grafting VEGF.
- -
- Vascular cell adhesion molecule-1 receptor ((VCAM-1)R): VCAM is a protein that mediates cell-to-cell adhesion. (VCAM-1)Rs are only expressed on the surface of tumor blood vessels and inflammation. The attachment of Ab specifically designed to bind to this molecule to NPs could be a good targeting strategy.
- -
- Matrix metalloproteinases (MMPs): are enzymes responsible of remodeling the extracellular matrix. These processes are necessary for a vast and varied array of physiological events (wound repair, organismal growth and development, and mediation of immune responses). MMPs degrade all kinds of extracellular matrix (ECM) proteins, playing a key role in angiogenesis and metastasis. Since MMPs are overexpressed in the extracellular environment of certain kinds of tumors, they can be used as a kind of tumor localization signal in cancer therapy [109,110]. Thus, some Ab able to selectively bind MMPs have been conjugated to different NPs, especially antibodies that recognize the membrane type-1 metallo-proteinase which is present on endothelial tumor cells of a large number of malignancies.
- -
- αβ-integrins: are endothelial cell receptors for ECM proteins which are highly overexpressed in neovascular endothelial tumor cells but is scarcely present in healthy cells. Oligopeptides harboring the RGD sequence (Arg-Gly-Asp) bind selectively to this receptor.
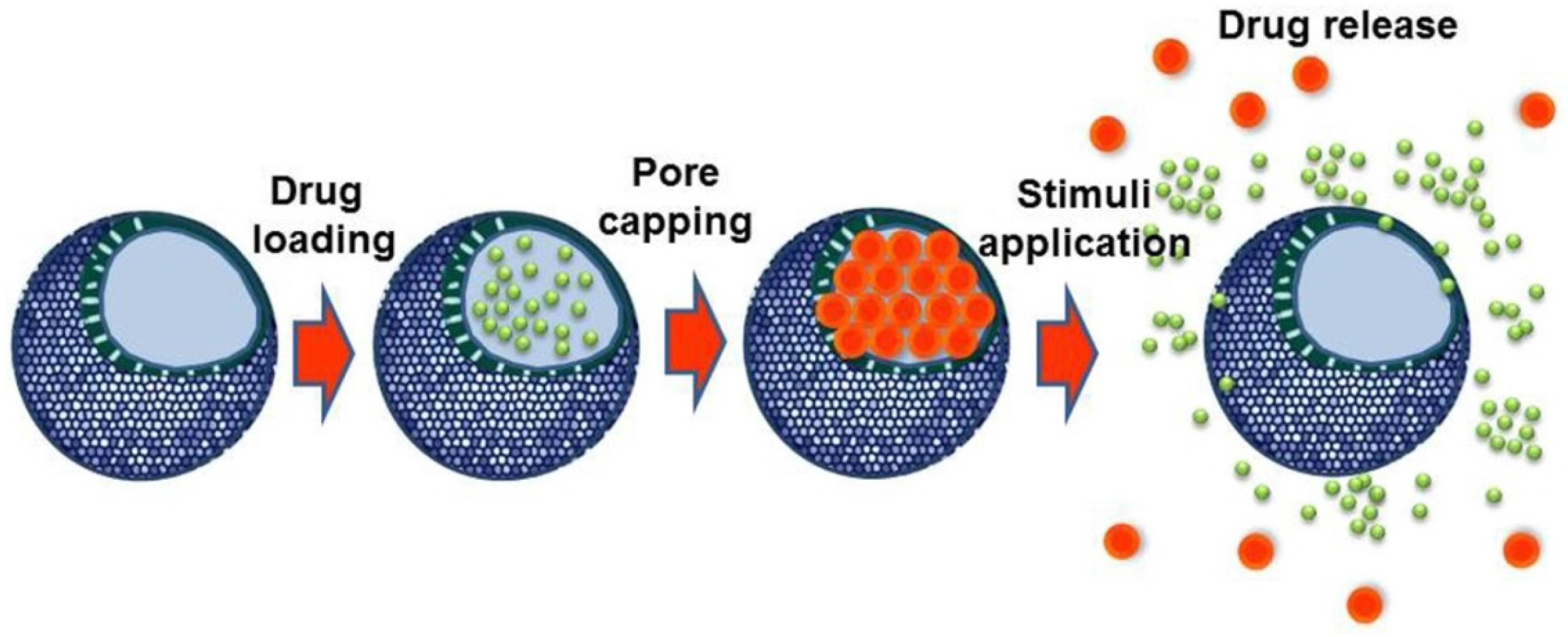
3. Stimuli-Responsive Mesoporous Silica Nanoparticles
| Stimuli | Responsive Linker | Capping Agent | Ref. | |
|---|---|---|---|---|
| External | Temperature | Octadecyl (C18) chains | Paraffins | [112] |
| Temperature | PNIPAm | PNIPAm | [113] | |
| Temperature | DNA strands | Biotin | [114] | |
| Temperature | Coiled-coil peptide motifs | Coiled-coil peptide motifs | [115] | |
| Electric field | 4(3-cyanophenyl)butylene dipolar molecule | - | [116] | |
| Magnetic field | Hybridization of 2 ssDNA | γ-Fe2O3 NPs | [117] | |
| Magnetic field | Alkylamonium chains (NH3+–(CH2)–NH2+–R) | CB[6] | [118] | |
| Magnetic field | PEI/PNIPAM polymer | PEI/PNIPAM chains + catalase | [119] | |
| Magnetic field | Azo bonds (–N=N–) | PEG | [120] | |
| Light | 4-[4-(1-(Fmoc)methyl)-2-methoxy-5-nitrophenoxy]butanoic acid photolinker | Protein shell (avidin-estreptavidin-biotin-transferrin) | [76] | |
| Light | DNA aptamer | DNA aptamer | [121] | |
| Light | Azobenzene/coumarin dimer | Coumarin dimer | [122] | |
| Light | Azobenzene derivatives | β-CDs | [123] | |
| Internal | pH | Acetal linker | Au NPs | [124] |
| pH | Boronate ester | Fe3O4 NPs | [125] | |
| pH | Ferrocenyl moieties | β-CD-modified CeO2 NPs | [126] | |
| pH | PAH-PSS PEM | PAH-PSS PEM | [127] | |
| pH | Aromatic amines | CDs | [128] | |
| pH | Benzoic-imine bonds | Polypseudorotaxanes | [129] | |
| pH | CaP soluble at acid pH | CaP coating | [130] | |
| Redox potential | –S–S– | ssDNA | [131] | |
| Redox potential | –S–S– | PEG | [132] | |
| Redox potential | –S–S– | CdS NPs | [133] | |
| Redox potential | –S–S– | PPI dendrimer | [134] | |
| Enzymes | MMP-degradable gelatin | Gelatin coating | ||
| Enzymes | β-galactosidase-cleavable oligosaccharide | β-galacto-oligosaccharide | [135] | |
| Enzymes | MMP9-sensitive peptide sequence (RSWMGLP) | Avidin | [136] | |
| Enzymes | Protease-sensitive peptide sequences (CGPQGIWGQGCR) | PNIPAm-PEGDA shell | [137] | |
| Enzymes | α-amylase and lipase cleavable stalks | CDs | [138] | |
| Enzymes | HRP-polymer nanocapsule | - | [139] | |
| Enzymes | Phosphate-phosphate APasa -hydrolizable bonds | ATP | [140] | |
| Small molecules | Ionizable benzimidazole group | CD-modified glucose oxidase | [141] | |
| Small molecules | pAb | pAb | [142] | |
| Small molecules | ATP aptamer | ATP aptamer | [143] |
3.1. External Stimuli
3.1.1. Temperature
3.1.2. Electric Field
3.1.3. Magnetic Field
3.1.4. Light
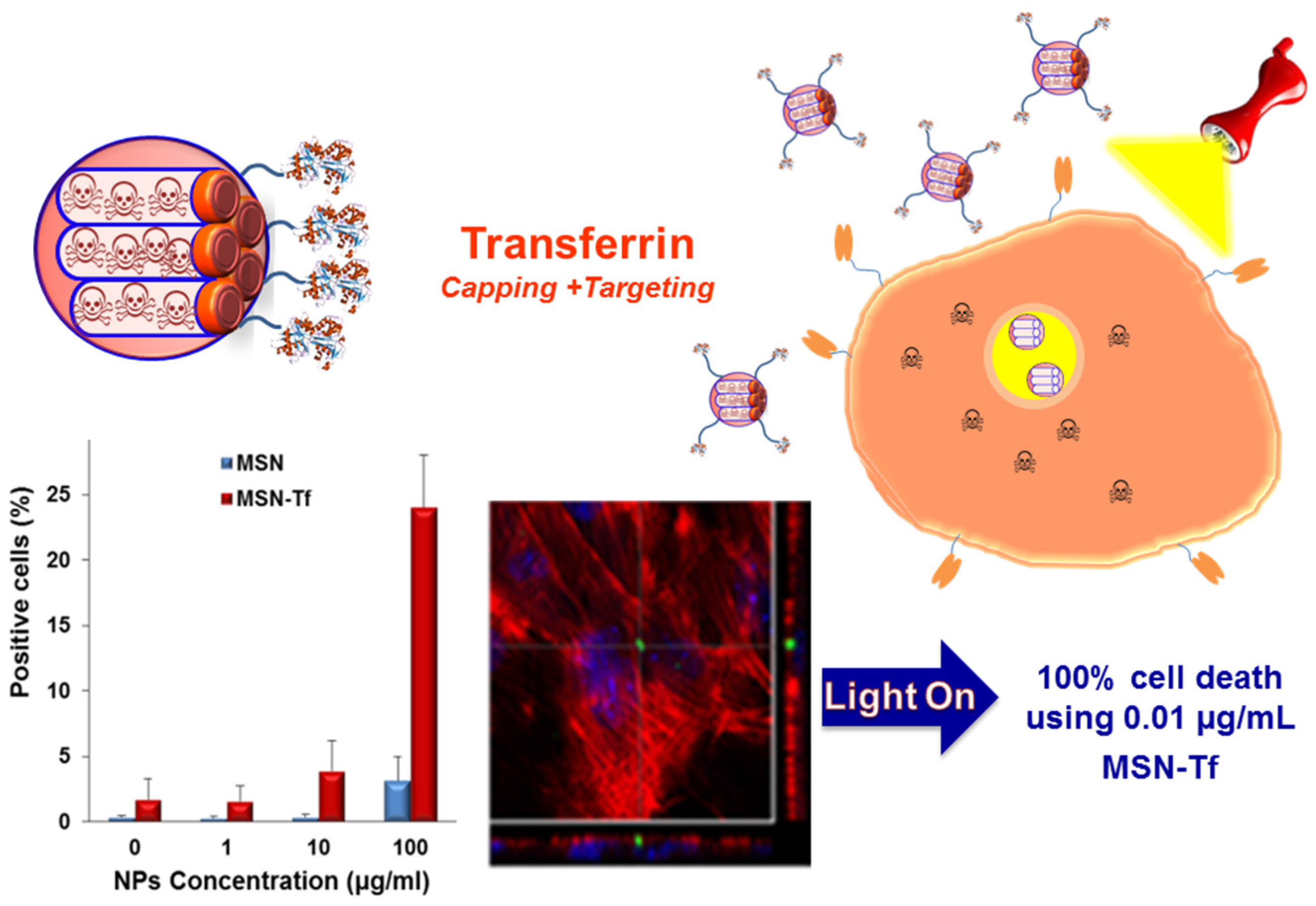
3.2. Internal Stimuli
3.2.1. pH
3.2.2. Redox Potential
3.2.3. Enzymes
3.2.4. Small Molecules
3.3. Multi-Stimuli Responsive Mesoporous Silica Nanoparticles
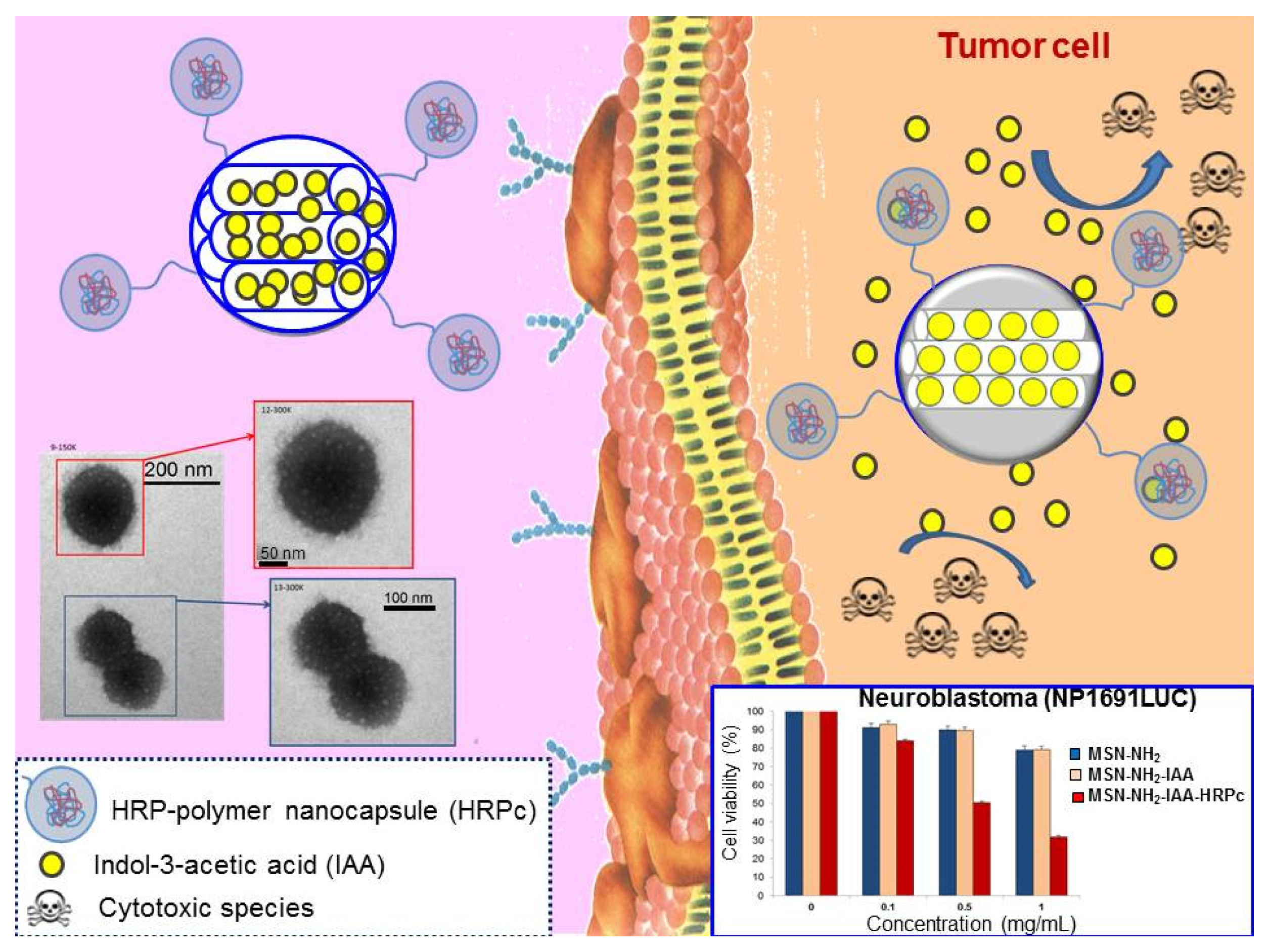
4. Safety, Tissue Accumulation and Elimination of Mesoporous Silica Nanoparticles
5. Current Challenges of Mesoporous Silica Nanoparticles
6. State of the Art
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic-inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Arcos, D. Nanoceramics in Clinical Use: From Materials to Applications, 2nd ed.; Royal Society of Chemistry: Cambridge, UK, 2015. [Google Scholar]
- Lanone, S.; Boczkowski, J. Biomedical applications and potential health risks of nanomaterials: Molecular mechanisms. Curr. Mol. Med. 2006, 6, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.S.; Rutka, J.T.; Chan, W.C.W. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Fernandez, A.; Manchanda, R.; McGoron, A. Theranostic applications of nanomaterials in cancer: Drug delivery, image-guided therapy, and multifunctional platforms. Appl. Biochem. Biotechnol. 2011, 165, 1628–1651. [Google Scholar] [CrossRef] [PubMed]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.A.; O’Malley, W.; Kubeil, M.; Graham, B.; Stephan, H.; Spiccia, L. Nanomaterials: Applications in cancer imaging and therapy. Adv. Mater. 2011, 23, H18–H40. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Liu, G.; Eden, H.S.; Ai, H.; Chen, X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc. Chem. Res. 2011, 44, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Ruiz-Hernandez, E. Bioceramics: From bone regeneration to cancer nanomedicine. Adv. Mater. 2011, 23, 5177–5218. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A. Ceramic Nanoparticles for Cancer Treatment. In Bio-Ceramics with Clinical Applications; Vallet-Regí, M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 421–455. [Google Scholar]
- International Agency for Research on Cancer. World Cancer Report; WHO: Geneva, Switzerland, 2014. [Google Scholar]
- Patil, Y.B.; Swaminathan, S.K.; Sadhukha, T.; Ma, L.; Panyam, J. The use of nanoparticle-mediated targeted gene silencing and drug delivery to overcome tumor drug resistance. Biomaterials 2010, 31, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Misra, R.; Acharya, S.; Sahoo, S.K. Cancer nanotechnology: Application of nanotechnology in cancer therapy. Drug Discov. Today 2010, 15, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, L.; Chen, Z.; Shin, D.M. Application of nanotechnology in cancer therapy and imaging. CA Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Rosenholm, J.; Areva, S.; Linden, M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro- and mesoporous silica matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
- Scheinberg, D.A.; Villa, C.H.; Escorcia, F.E.; McDevitt, M.R. Conscripts of the infinite armada: Systemic cancer therapy using nanomaterials. Nat. Rev. Clin. Oncol. 2010, 7, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Prasuhn, D.E.; Stewart, M.H.; Jennings, T.L.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. The controlled display of biomolecules on nanoparticles: A challenge suited to bioorthogonal chemistry. Bioconjug. Chem. 2011, 22, 825–858. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Ramila, A.; del Real, R.P.; Perez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Quan, Z.; Lu, L.; Huang, S.; Lin, J. Luminescence functionalization of mesoporous silica with different morphologies and applications as drug delivery systems. Biomaterials 2008, 29, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Vivero-Escoto, J.L.; Wu, C.-W.; Lin, V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008, 60, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Quan, Z.; Hou, Z.; Li, C.; Kang, X.; Cheng, Z.; Lin, J. A magnetic, luminescent and mesoporous core-shell structured composite material as drug carrier. Biomaterials 2009, 30, 4786–4795. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Colilla, M.; Vallet-Regí, M. Drug delivery from ordered mesoporous matrices. Expert Opin. Drug Deliv. 2009, 6, 1383–1400. [Google Scholar] [CrossRef] [PubMed]
- Gai, S.; Yang, P.; Li, C.; Wang, W.; Dai, Y.; Niu, N.; Lin, J. Synthesis of magnetic, up-conversion luminescent, and mesoporous core-shell-structured nanocomposites as drug carriers. Adv. Funct. Mater. 2010, 20, 1166–1172. [Google Scholar] [CrossRef]
- Colilla, M.; González, B.; Vallet-Regí, M. Mesoporous silica nanoparticles for the design of smart delivery nanodevices. Biomater. Sci. 2013, 1, 114–134. [Google Scholar] [CrossRef]
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef] [PubMed]
- Argyo, C.; Weiss, V.; Braeuchle, C.; Bein, T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem. Mater. 2014, 26, 435–451. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Colilla, M.; Vallet-Regí, M. Advances in mesoporous silica nanoparticles for targeted stimuli-responsive drug delivery. Expert Opin. Drug Deliv. 2014, 12, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Colilla, M.; Baeza, A.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery and Controlled Release Applications. In The Sol-Gel Handbook; Levy, D., Zayat, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Volume 3, pp. 1309–1344. [Google Scholar]
- Colilla, M.; Vallet-Regí, M. Responsive Mesoporous Silica Nanoparticles for Targeted Drug Delivery. In Chemoresponsive Materials: Stimulation by Chemical and Biological Signals; Schneider, H.J., Ed.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 136–166. [Google Scholar]
- Vallet-Regí, M.; Manzano, M.; Colilla, M. Biomedical Applications of Mesoporous Ceramics: Drug Delivery, Smart Materials and Bone Tissue Engineering; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012. [Google Scholar]
- Martínez, Á.; Fuentes-Paniagua, E.; Baeza, A.; Sánchez-Nieves, J.; Cicuéndez, M.; Gómez, R.; de la Mata, F.J.; González, B.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with carbosilane dendrons as new non-viral oligonucleotide delivery carriers. Chemistry 2015, 21, 15651–15666. [Google Scholar]
- Colilla, M.; Vallet-Regí, M. Smart Drug Delivery from Silica Nanoparticles. In Smart Materials for Drug Delivery; Alvarez-Lorenzo, C., Concheiro, A., Eds.; Royal Society of Chemistry: Cambridge, UK, 2013; Volume 2, pp. 63–89. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Colilla, M. Silica-based Ceramics: Mesoporous Silica. In Bio-Ceramics with Clinical Applications; Vallet-Regí, M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2014; pp. 109–151. [Google Scholar]
- Muñoz, B.; Rámila, A.; Pérez-Pariente, J.; Díaz, I.; Vallet-Regí, M. MCM-41 Organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froeba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Trewyn, B.G.; Slowing, I.I.; Giri, S.; Chen, H.-T.; Lin, V.S.Y. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol-gel process and applications in controlled release. Acc. Chem. Res. 2007, 40, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Colilla, M.; González, B. Medical applications of organic-inorganic hybrid materials within the field of silica-based bioceramics. Chem. Soc. Rev. 2011, 40, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Grün, M.; Lauer, I.; Unger, K.K. The synthesis of micrometer- and submicrometer-size spheres of ordered mesoporous oxide MCM-41. Adv. Mater. 1997, 9, 254–257. [Google Scholar] [CrossRef]
- Lu, Y.F.; Fan, H.Y.; Stump, A.; Ward, T.L.; Rieker, T.; Brinker, C.J. Aerosol-assisted self-assembly of mesostructured spherical nanoparticles. Nature 1999, 398, 223–226. [Google Scholar]
- Brinker, C.J.; Lu, Y.F.; Sellinger, A.; Fan, H.Y. Evaporation-induced self-assembly: Nanostructures made easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Arcos, D.; López-Noriega, A.; Ruiz-Hernández, E. Ordered mesoporous microspheres for bone grafting and drug delivery. Chem. Mater. 2009, 21, 1000–1009. [Google Scholar] [CrossRef]
- Colilla, M.; Manzano, M.; Izquierdo-Barba, I.; Vallet-Regí, M.; Boissiére, C.; Sanchez, C. Advanced drug delivery vectors with tailored surface properties made of mesoporous binary oxides submicronic spheres. Chem. Mater. 2010, 22, 1821–1830. [Google Scholar] [CrossRef]
- Boissiére, C.; Grosso, D.; Chaumonnot, A.; Nicole, L.; Sanchez, C. Aerosol route to functional nanostructured inorganic and hybrid porous materials. Adv. Mater. 2011, 23, 599–623. [Google Scholar] [CrossRef] [PubMed]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Hoffmann, F.; Fröba, M. Vitalising porous inorganic silica networks with organic functions—PMOs and related hybrid materials. Chem. Soc. Rev. 2011, 40, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Angelos, S.; Choi, E.F.; Zink, J.I. Mesostructured multifunctional nanoparticles for imaging and drug delivery. J. Mater. Chem. 2009, 19, 6251–6257. [Google Scholar] [CrossRef]
- Lin, Y.S.; Tsai, C.P.; Huang, H.Y.; Kuo, C.T.; Hung, Y.; Huang, D.M.; Chen, Y.C.; Mou, C.Y. Well-ordered mesoporous silica nanoparticles as cell markers. Chem. Mater. 2005, 17, 4570–4573. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Sherman, S.; Xia, T.; Kovochich, M.; Nel, A.E.; Zink, J.I.; Tamanoi, F. Mesoporous silica nanoparticles for cancer therapy: Energy-dependent cellular uptake and delivery of paclitaxel to cancer cells. Nanobiotechnology 2007, 3, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.-K.; Kim, T.; Paik, S.; Haam, S.; Huh, Y.-M.; Lee, K. Nanomaterials for Theranostics: Recent Advances and Future Challenges. Chem. Rev. 2015, 115, 327–394. [Google Scholar] [CrossRef] [PubMed]
- Langer, R. Drug delivery and targeting. Nature 1998, 392, 5–10. [Google Scholar] [PubMed]
- Farokhzad, O.C.; Langer, R. Impact of nanotechnology on drug delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Preat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Controll. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, N.; Wu, J.; Xu, X.; Kamaly, N.; Farokhzad, O.C. Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 2014, 66, 2–25. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy. Mechanism of tumoritropic accumulation of proteins and the antitumor agen smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Davis, M.E.; Chen, Z.; Shin, D.M. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat. Rev. Drug Discov. 2008, 7, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Dalhaimer, P.; Cai, S.; Tsai, R.; Tewari, M.; Minko, T.; Discher, D.E. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat. Nanotechnol. 2007, 2, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Champion, J.A.; Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl. Acad. Sci. USA 2006, 103, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S. In Drug Delivery, Shape Does Matter. Pharm. Res. 2009, 26, 232–234. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Teng, X.; Chen, D.; Tang, F.; He, J. The effect of the shape of mesoporous silica nanoparticles on cellular uptake and cell function. Biomaterials 2010, 31, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, L.; Liu, T.; Hao, N.; Liu, H.; Chen, D.; Tang, F. The shape effect of mesoporous silica nanoparticles on biodistribution, clearance, and biocompatibility in vivo. ACS Nano 2011, 5, 5390–5399. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xue, M.; Xia, T.; Ji, Z.; Tarn, D.Y.; Zink, J.I.; Nel, A.E. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano 2011, 5, 4131–4144. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.; Argyo, C.; Bein, T. Impact of different PEGylation patterns on the long-term bio-stability of colloidal mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 8693–8699. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated Inorganic Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef] [PubMed]
- Cauda, V.; Schlossbauer, A.; Bein, T. Bio-degradation study of colloidal mesoporous silica nanoparticles: Effect of surface functionalization with organo-silanes and poly(ethylene glycol). Microporous Mesoporous Mater. 2010, 132, 60–71. [Google Scholar] [CrossRef]
- Ferris, D.P.; Lu, J.; Gothard, C.; Yanes, R.; Thomas, C.R.; Olsen, J.-C.; Stoddart, J.F.; Tamanoi, F.; Zink, J.I. Synthesis of biomolecule-modified mesoporous silica nanoparticles for targeted hydrophobic drug delivery to cancer cells. Small 2011, 7, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Zhong, Y.; Cui, Y. pH-controllable drug carrier with SERS activity for targeting cancer cells. Biosens. Bioelectron. 2014, 57, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Carmona, M.; Baeza, A.; Rodríguez-Milla, M.A.; García-Castro, J.; Vallet-Regí, M. Mesoporous silica nanoparticles grafted with a light-responsive protein shell for highly cytotoxic antitumoral therapy. J. Mater. Chem. B 2015, 3, 5746–5752. [Google Scholar] [CrossRef]
- Mickler, F.M.; Moeckl, L.; Ruthardt, N.; Ogris, M.; Wagner, E.; Braeuchle, C. Tuning nanoparticle uptake: Live-cell imaging reveals two distinct endocytosis mechanisms mediated by natural and artificial EGFR targeting ligand. Nano Lett. 2012, 12, 3417–3423. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Meinander, A.; Peuhu, E.; Niemi, R.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Targeting of porous hybrid silica nanoparticles to Cancer Cells. ACS Nano 2009, 3, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liong, M.; Li, Z.X.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, Z.; Zink, J.I.; Tamanoi, F. In vivo tumor suppression efficacy of mesoporous silica nanoparticles-based drug-delivery system: Enhanced efficacy by folate modification. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S.; Wu, L.-C.; Lu, S.-Y.; Chang, L.-L.; Teng, I.T.; Yang, C.-M.; Ho, J.-A.A. Biofunctionalized phospholipid-capped mesoporous silica nanoshuttles for targeted drug delivery: Improved water suspensibility and decreased nonspecific protein binding. ACS Nano 2010, 4, 4371–4379. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.; Trewyn, B.G.; Lin, V.S.Y. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticles on the endocytosis by human cancer cells. J. Am. Chem. Soc. 2006, 128, 14792–14793. [Google Scholar] [CrossRef] [PubMed]
- Porta, F.; Lamers, G.E.M.; Morrhayim, J.; Chatzopoulou, A.; Schaaf, M.; den Dulk, H.; Backendorf, C.; Zink, J.I.; Kros, A. Folic acid-modified mesoporous silica nanoparticles for cellular and nuclear targeted drug delivery. Adv. Healthc. Mater. 2013, 2, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Taylor-Pashow, K.M.L.; Huxford, R.C.; Della Rocca, J.; Okoruwa, C.; An, H.; Lin, W.; Lin, W. Multifunctional mesoporous silica nanospheres with cleavable Gd(III) chelates as MRI contrast agents: Synthesis, characterization, target-specificity, and renal clearance. Small 2011, 7, 3519–3528. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Peuhu, E.; Bate-Eya, L.T.; Eriksson, J.E.; Sahlgren, C.; Linden, M. Cancer-cell-specific induction of apoptosis using mesoporous silica nanoparticles as drug-delivery vectors. Small 2010, 6, 1234–1241. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-targeted drug delivery of TAT peptide-conjugated monodisperse mesoporous silica nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Dong, K.; Huang, S.; Ju, E.; Liu, Z.; Yin, M.; Ren, J.; Qu, X. A smart nanoassembly for multistage targeted drug delivery and magnetic resonance imaging. Adv. Funct. Mater. 2014, 24, 3612–3620. [Google Scholar] [CrossRef]
- Pan, L.; Liu, J.; He, Q.; Wang, L.; Shi, J. Overcoming multidrug resistance of cancer cells by direct intranuclear drug delivery using TAT-conjugated mesoporous silica nanoparticles. Biomaterials 2013, 34, 2719–2730. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Zhao, J.; Liu, X.; Bu, J.; Yan, X.; Huang, R. Multifunctional mesoporous silica-coated graphene nanosheet used for chemo-photothermal synergistic targeted therapy of glioma. J. Am. Chem. Soc. 2013, 135, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Milgroom, A.; Intrator, M.; Madhavan, K.; Mazzaro, L.; Shandas, R.; Liu, B.; Park, D. Mesoporous silica nanoparticles as a breast-cancer targeting ultrasound contrast agent. Colloids Surfaces B 2014, 116, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-P.; Chen, C.-Y.; Hung, Y.; Chang, F.-H.; Mou, C.-Y. Monoclonal antibody-functionalized mesoporous silica nanoparticles (MSN) for selective targeting breast cancer cells. J. Mater. Chem. 2009, 19, 5737–5743. [Google Scholar] [CrossRef]
- Deng, Z.; Zhen, Z.; Hu, X.; Wu, S.; Xu, Z.; Chu, P.K. Hollow chitosan-silica nanospheres as pH-sensitive targeted delivery carriers in breast cancer therapy. Biomaterials 2011, 32, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Blumen, S.R.; MacPherson, M.B.; Steinbacher, J.L.; Mossman, B.T.; Landry, C.C. Enhanced uptake of porous silica microparticles by bifunctional surface modification with a targeting antibody and a biocompatible polymer. ACS Appl. Mater. Interfaces 2010, 2, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In vivo tumor targeting and image-guided drug delivery with antibody-conjugated, radio labeled mesoporous silica nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, G.; Baeza, A.; Melen, G.J.; Alfranca, A.; Ramirez, M.; Vallet-Regí, M. A new targeting agent for the selective drug delivery of nanocarriers for treating neuroblastoma. J. Mater. Chem. B 2015, 3, 4831–4842. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Lee, C.-H.; Chen, M.-C.; Souris, J.S.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Chen, C.-T.; Lo, L.-W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics-the trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149–6157. [Google Scholar] [CrossRef]
- Fang, I.J.; Slowing, I.I.; Wu, K.C.W.; Lin, V.S.Y.; Trewyn, B.G. Ligand conformation dictates membrane and endosomal trafficking of arginine-glycine-aspartate (RGD)-functionalized mesoporous silica nanoparticles. Chemistry 2012, 18, 7787–7792. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.-F.; Chen, W.-H.; Liu, Y.; Zhang, J.; Cheng, S.-X.; Zhuo, R.-X.; Zhang, X.-Z. Charge-reversal plug gate nanovalves on peptide-functionalized mesoporous silica nanoparticles for targeted drug delivery. J. Mater. Chem. B 2013, 1, 5723–5732. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Z.-F.; Wang, Y.; Chen, W.-H.; Luo, G.-F.; Cheng, S.-X.; Zhuo, R.-X.; Zhang, X.-Z. Multifunctional envelope-type mesoporous silica nanoparticles for tumor-triggered targeting drug delivery. J. Am. Chem. Soc. 2013, 135, 5068–5073. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Jia, H.-Z.; Zhang, J.; Liu, C.-W.; Zhuo, R.-X.; Zhang, X.-Z. A dual-responsive mesoporous silica nanoparticle for tumor-triggered targeting drug delivery. Small 2014, 10, 591–598. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, Y.; Zhu, H.; Pang, G.; Zheng, W.; Wong, Y.-S.; Chen, T. Cancer-targeted monodisperse mesoporous silica nanoparticles as carrier of ruthenium polypyridyl complexes to enhance theranostic effects. Adv. Funct. Mater. 2014, 24, 2754–2763. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, F.; Li, Y.; Xu, M.; Li, L.; Wu, C.; Miyoshi, H.; Liu, Y. VCAM-1-targeted core/shell nanoparticles for selective adhesion and delivery to endothelial cells with lipopolysaccharide-induced inflammation under shear flow and cellular magnetic resonance imaging in vitro. Int. J. Nanomed. 2013, 8, 1897–1906. [Google Scholar]
- Goel, S.; Chen, F.; Hong, H.; Valdovinos, H.F.; Hernandez, R.; Shi, S.; Barnhart, T.E.; Cai, W. VEGF(121)-Conjugated Mesoporous Silica Nanoparticle: A Tumor Targeted Drug Delivery System. ACS Appl. Mater. Interfaces 2014, 6, 21677–21685. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Olenyuk, B.Z.; Okamoto, C.K.; Hamm-Alvarez, S.F. Targeting receptor-mediated endocytotic pathways with nanoparticles: Rationale and advances. Adv. Drug Deliv. Rev. 2013, 65, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Elnakat, H.; Ratnam, M. Distribution, functionality and gene regulation of folate receptor isoforms: Implications in targeted therapy. Adv. Drug Deliv. Rev. 2004, 56, 1067–1084. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, J.; Baselga, J. Epidermal growth factor receptor targeting in cancer. Semin. Oncol. 2006, 33, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.M.; Wolchok, J.D.; Old, L.J. Antibody therapy of cancer. Nat. Rev. Cancer 2012, 12, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Cathcart, J.; Pulkoski-Gross, A.; Cao, J. Targeting matrix metalloproteinases in cancer: Bringing new life to old ideas. Genes Dis. 2015, 2, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gialeli, C.; Theocharis, A.D.; Karamanos, N.K. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. FEBS J. 2011, 278, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Zeng, D.; Tian, Y.; Chen, F.; Feng, J.; Shi, J. Core/shell structured hollow mesoporous nanocapsules: A potential platform for simultaneous cell imaging and anticancer drug delivery. ACS Nano 2010, 4, 6001–6013. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Mondragon, L.; Ros-Lis, J.V.; Sancenon, F.; Dolores Marcos, M.; Martinez-Manez, R.; Soto, J.; Perez-Paya, E.; Amoros, P. Finely tuned temperature-controlled cargo release using paraffin-capped mesoporous silica nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 11172–11175. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.A.; Miletto, I.; Brunella, V.; Berlier, G.; Scalarone, D. Controlled post-synthesis grafting of thermoresponsive poly(N-isopropylacrylamide) on mesoporous silica nanoparticles. Polym. Adv. Technol. 2015, 26, 1070–1075. [Google Scholar] [CrossRef]
- Schlossbauer, A.; Warncke, S.; Gramlich, P.M.E.; Kecht, J.; Manetto, A.; Carell, T.; Bein, T. A programmable DNA-based molecular valve for colloidal mesoporous silica. Angew. Chem. Int. Ed. 2010, 49, 4734–4737. [Google Scholar] [CrossRef] [PubMed]
- Martelli, G.; Zope, H.R.; Capell, M.B.; Kros, A. Coiled-coil peptide motifs as thermoresponsive valves for mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 9932–9934. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, H.; Li, F.; Ruan, Q.; Wang, H.; Fujiwara, M.; Wang, L.; Lu, G.Q. Dipolar molecules as impellers achieving electric-field-stimulated release. J. Am. Chem. Soc. 2010, 132, 1450–1451. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, E.; Baeza, A.; Vallet-Regí, M. Smart drug delivery through DNA/magnetic nanoparticle gates. ACS Nano 2011, 5, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.R.; Ferris, D.P.; Lee, J.-H.; Choi, E.; Cho, M.H.; Kim, E.S.; Stoddart, J.F.; Shin, J.-S.; Cheon, J.; Zink, J.I. Non-invasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J. Am. Chem. Soc. 2010, 132, 10623–10625. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Guisasola, E.; Ruiz-Hernandez, E.; Vallet-Regí, M. Magnetically triggered multidrug release by hybrid mesoporous silica nanoparticles. Chem. Mater. 2012, 24, 517–524. [Google Scholar] [CrossRef]
- Saint-Cricq, P.; Deshayes, S.; Zink, J.I.; Kasko, A.M. Magnetic field activated drug delivery using thermodegradable azo-functionalised PEG-coated core-shell mesoporous silica nanoparticles. Nanoscale 2015, 7, 13168–13172. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, X.; Liu, Z.; Pu, F.; Ren, J.; Qu, X. Near-infrared light-triggered, targeted drug delivery to cancer cells by aptamer gated nanovehicles. Adv. Mater. 2012, 24, 2890–2895. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Fujiwara, M. Installing dynamic molecular photomechanics in mesopores: A multifunctional controlled-release nanosystem. Angew. Chem. Int. Ed. 2007, 46, 2241–2244. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Zhao, Y.-L.; Khashab, N.M.; Khatib, H.A.; Stoddart, J.F.; Zink, J.I. Light-operated mechanized nanoparticles. J. Am. Chem. Soc. 2009, 131, 1686–1688. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, Y.; Zhao, X.; Agarwal, A.; Mueller, L.J.; Feng, P. pH-responsive nanogated ensemble based on gold-capped mesoporous silica through an acid-labile acetal linker. J. Am. Chem. Soc. 2010, 132, 1500–1501. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Lu, X.; Yuan, Y.; Qian, J.; Zhou, H.; Lu, X.; Shi, J.; Liu, C. A magnetic, reversible pH-responsive nanogated ensemble based on Fe3O4 nanoparticles-capped mesoporous silica. Biomaterials 2011, 32, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Lin, Y.; Wang, J.; Wu, L.; Wei, W.; Ren, J.; Qu, X. Nanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host-guest interactions and switchable enzymatic activity and cellular effects of CeO2. Adv. Healthc. Mater. 2013, 2, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Zhou, X.; He, C.; Qiu, K.; Nie, W.; Chen, L.; Wang, H.; Mo, X.; Zhang, Y. Polyelectrolyte multilayer functionalized mesoporous silica nanoparticles for pH-responsive drug delivery: Layer thickness-dependent release profiles and biocompatibility. J. Mater. Chem. B 2013, 1, 5886–5898. [Google Scholar] [CrossRef]
- Meng, H.; Xue, M.; Xia, T.; Zhao, Y.-L.; Tamanoi, F.; Stoddart, J.F.; Zink, J.I.; Nel, A.E. Autonomous in vitro anticancer drug release from mesoporous silica nanoparticles by pH-sensitive nanovalves. J. Am. Chem. Soc. 2010, 132, 12690–12697. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yang, C.; Liu, X.; Ma, R.; Kong, D.; Shi, L. A multifunctional nanocarrier based on nanogated mesoporous silica for enhanced tumor-specific uptake and intracellular delivery. Macromol. Biosci. 2012, 12, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Rim, H.P.; Min, K.H.; Lee, H.J.; Jeong, S.Y.; Lee, S.C. pH-tunable calcium phosphate covered mesoporous silica nanocontainers for intracellular controlled release of guest drugs. Angew. Chem. Int. Ed. 2011, 50, 8853–8857. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nguyen, K.T.; Borah, P.; Ang, C.Y.; Zhao, Y. Functional silica nanoparticles for redox-triggered drug/ssDNA co-delivery. Adv. Healthc. Mater. 2012, 1, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Niemela, M.; Westermarck, J.; Rosenholm, J.M. Mesoporous silica nanoparticles with redox-responsive surface linkers for charge-reversible loading and release of short oligonucleotides. Dalton Trans. 2014, 43, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-Y.; Trewyn, B.G.; Jeftinija, D.M.; Jeftinija, K.; Xu, S.; Jeftinija, S.; Lin, V.S.Y. A mesoporous silica nanosphere-based carrier system with chemically removable CdS nanoparticle caps for stimuli-responsive controlled release of neurotransmitters and drug molecules. J. Am. Chem. Soc. 2003, 125, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Nadrah, P.; Porta, F.; Planinsek, O.; Kros, A.; Gaberscek, M. Poly(propylene imine) dendrimer caps on mesoporous silica nanoparticles for redox-responsive release: Smaller is better. Phys. Chem. Chem. Phys. 2013, 15, 10740–10748. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Mondragón, L.; Coll, C.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Pérez-Payá, E.; Amorós, P. Dual enzyme-triggered controlled release on capped nanometric silica mesoporous supports. ChemistryOpen 2012, 1, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.H.; Bölükbas, D.A.; Argyo, C.; Datz, S.; Lindner, M.; Eickelberg, O.; Königshoff, M.; Bein, T.; Meiners, S. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano 2015, 9, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Karambelkar, A.; Gu, L.; Lin, K.; Miller, J.S.; Chen, C.S.; Sailor, M.J.; Bhatia, S.N. Bioresponsive mesoporous silica nanoparticles for triggered drug release. J. Am. Chem. Soc. 2011, 133, 19582–19585. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Kim, H.; Kim, S.; Kim, C. Enzyme responsive nanocontainers with cyclodextrin gatekeepers and synergistic effects in release of guests. J. Am. Chem. Soc. 2009, 131, 16614–16615. [Google Scholar] [CrossRef] [PubMed]
- Baeza, A.; Guisasola, E.; Torres-Pardo, A.; González-Calbet, J.M.; Melen, G.J.; Ramirez, M.; Vallet-Regí, M. Hybrid enzyme-polymeric capsules/mesoporous silica nanodevice for in situ cytotoxic agent generation. Adv. Funct. Mater. 2014, 24, 4625–4633. [Google Scholar] [CrossRef]
- Mas, N.; Arcos, D.; Polo, L.; Aznar, E.; Sánchez-Salcedo, S.; Sancenón, F.; García, A.; Marcos, M.D.; Baeza, A.; Vallet-Regí, M.; Martínez-Máñez, R. Towards the development of smart 3D “gated scaffolds” for on-command delivery. Small 2014, 10, 4859–4864. [Google Scholar] [CrossRef] [PubMed]
- Aznar, E.; Villalonga, R.; Gimenez, C.; Sancenon, F.; Marcos, M.D.; Martinez-Mañez, R.; Diez, P.; Pingarron, J.M.; Amoros, P. Glucose-triggered release using enzyme-gated mesoporous silica nanoparticles. Chem. Commun. 2013, 49, 6391–6393. [Google Scholar] [CrossRef] [PubMed]
- Climent, E.; Bernardos, A.; Martínez-Máñez, R.; Maquieira, A.; Marcos, M.D.; Pastor-Navarro, N.; Puchades, R.; Sancenón, F.; Soto, J.; Amorós, P. Controlled delivery systems using antibody-capped mesoporous nanocontainers. J. Am. Chem. Soc. 2009, 131, 14075–14080. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhao, Y.; He, D.; Wang, K.; Xu, F.; Tang, J. ATP-responsive controlled release system using aptamer-functionalized mesoporous silica nanoparticles. Langmuir 2012, 28, 12909–12915. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Lorenzo, C.; Concheiro, A. From Drug Dosage Forms to Intelligent Drug-delivery Systems: A Change of Paradigm. In Smart Materials for Drug Delivery; Royal Society of Chemistry: London, UK, 2013; Volume 1, pp. 1–32. [Google Scholar]
- Gustafson, T.P.; Cao, Q.; Wang, S.; Berezin, M.Y. Design of irreversible optical nanothermometers for thermal ablations. Chem. Commun. 2013, 49, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Nuyken, O.; Dauth, J.; Pekruhn, W. Synthesis and application of thermosensitive microcapsules containing azo groups. Die Angew. Makromol. Chem. 1991, 187, 207–224. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef] [PubMed]
- Gilman, M.; Gilman, S.L. Electrotherapy and the human voice: A literature review of the historical origins and contemporary applications. J. Voice 2008, 22, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Gilula, M.F. Cranial electrotherapy stimulation and fibromyalgia. Expert Rev. Med. Devices 2007, 4, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ciria, H.C.; Quevedo, M.S.; Cabrales, L.B.; Bruzon, R.P.; Salas, M.F.; Pena, O.G.; González, T.R.; Lopez, D.S.; Flores, J.M. Antitumor effectiveness of different amounts of electrical charge in Ehrlich and fibrosarcoma Sa-37 tumors. BMC Cancer 2004, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santini, J.T.; Cima, M.J.; Langer, R. A controlled-release microchip. Nature 1999, 397, 335–338. [Google Scholar] [PubMed]
- Kwon, I.C.; Bae, Y.H.; Kim, S.W. Electrically erodible polymer gel for controlled release of drugs. Nature 1991, 354, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Neofytou, E.; Cahill, T.J., III; Beygui, R.E.; Zare, R.N. Drug release from electric-field-responsive nanoparticles. ACS Nano 2012, 6, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, E.; López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Terasaki, O.; Vallet-Regí, M. Aerosol-assisted synthesis of magnetic mesoporous silica spheres for drug targeting. Chem. Mater. 2007, 19, 3455–3463. [Google Scholar] [CrossRef]
- Van de Linde, S.; Sauer, M. How to switch a fluorophore: From undesired blinking to controlled photoswitching. Chem. Soc. Rev. 2014, 43, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Zhang, Y. Photocontrolled nanoparticle delivery systems for biomedical applications. Acc. Chem. Res. 2014, 47, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Gao, Z.; Bae, Y.H. Recent progress in tumor pH targeting nanotechnology. J. Controll. Release 2008, 132, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2010, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Cheng, S.-H.; Huang, I.P.; Souris, J.S.; Yang, C.-S.; Mou, C.-Y.; Lo, L.-W. Intracellular pH-responsive mesoporous silica nanoparticles for the controlled release of anticancer chemotherapeutics. Angew. Chem. Int. Ed. 2010, 49, 8214–8219. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Lee, D.J.; Lee, N.; Kim, B.H.; Choi, S.H.; Hyeon, T. Multifunctional mesoporous silica nanocomposite nanoparticles for pH controlled drug release and dual modal imaging. J. Mater. Chem. 2011, 21, 16869–16872. [Google Scholar] [CrossRef]
- Cheng, R.; Feng, F.; Meng, F.; Deng, C.; Feijen, J.; Zhong, Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J. Controll. Release 2011, 152, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Li, H.; Ilangovan, G.; Cardounel, A.J.; Zweier, J.L.; Yamada, K.; Krishna, M.C.; Mitchell, J.B. Noninvasive imaging of tumor redox status and its modification by tissue glutathione levels. Cancer Res. 2002, 62, 307–312. [Google Scholar] [PubMed]
- Yuan, L.; Chen, W.; Hu, J.; Zhang, J.Z.; Yang, D. Mechanistic study of the covalent loading of Paclitaxel via disulfide linkers for controlled drug release. Langmuir 2013, 29, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Mortera, R.; Vivero-Escoto, J.; Slowing, I.I.; Garrone, E.; Onida, B.; Lin, V.S.Y. Cell-induced intracellular controlled release of membrane impermeable cysteine from a mesoporous silica nanoparticle-based drug delivery system. Chem. Commun. 2009, 14, 3219–3221. [Google Scholar] [CrossRef] [PubMed]
- Bareford, L.A.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Lovric, J.; Warwicker, J. Subcellular pH and predicted pH-dependent features of proteins. Proteomics 2006, 6, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Geisow, M.J. Fluorescein conjugates as indicators of subcellular pH-A critical evaluacion. Exp. Cell Res. 1984, 150, 29–35. [Google Scholar] [CrossRef]
- De la Rica, R.; Aili, D.; Stevens, M.M. Enzyme-responsive nanoparticles for drug release and diagnostics. Adv. Drug Deliv. Rev. 2012, 64, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Fukami, T.; Yokoi, T. The Emerging role of human esterases. Drug Metabol. Pharm. 2012, 27, 466–477. [Google Scholar] [CrossRef]
- Runquist, E.A.; Havel, R.J. Acid-hydrolases in early and late endosome fractions from rat-liver. J. Biol. Chem. 1991, 266, 22557–22563. [Google Scholar] [PubMed]
- Swarnakar, S.; Paul, S.; Singh, L.P.; Reiter, R.J. Matrix metalloproteinases in health and disease: Regulation by melatonin. J. Pineal Res. 2011, 50, 8–20. [Google Scholar] [CrossRef] [PubMed]
- De Melo, M.P.; de Lima, T.M.; Pithon-Curi, T.C.; Curi, R. The mechanism of indole acetic acid cytotoxicity. Toxicol. Lett. 2004, 148, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, X.; Zheng, C.; Wu, Z.; Li, C. A pH gated, clucose-sensitive nanoparticle based on worm-like mesoporous silica for controlled insulin release. J. Phys. Chem. B 2013, 117, 3852–3860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Meng, H.; Wang, N.; Donovan, M.J.; Fu, T.; You, M.; Chen, Z.; Zhang, X.; Tan, W. A Controlled-release nanocarrier with extracellular pH value driven tumor targeting and translocation for drug delivery. Angew. Chem. Int. Ed. 2013, 52, 7487–7491. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Geng, J.; Pu, F.; Yang, X.; Ren, J.; Qu, X. Polyvalent nucleic acid/mesoporous silica nanoparticle conjugates: Dual stimuli-responsive vehicles for intracellular drug delivery. Angew. Chem. Int. Ed. 2011, 50, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, P.; Dai, Y.; Ma, P.A.; Li, X.; Cheng, Z.; Hou, Z.; Kang, X.; Li, C.; Lin, J. Multifunctional up-converting nanocomposites with smart polymer brushes gated mesopores for cell imaging and thermo/pH dual-responsive drug controlled release. Adv. Funct. Mater. 2013, 23, 4067–4078. [Google Scholar] [CrossRef]
- Fang, W.; Yang, J.; Gong, J.; Zheng, N. Photo- and pH-Triggered Release of Anticancer Drugs from Mesoporous Silica-Coated Pd@Ag Nanoparticles. Adv. Funct. Mater. 2012, 22, 842–848. [Google Scholar] [CrossRef]
- Angelos, S.; Yang, Y.-W.; Khashab, N.M.; Stoddart, J.F.; Zink, J.I. Dual-controlled nanoparticles exhibiting AND logic. J. Am. Chem. Soc. 2009, 131, 11344–11346. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Chen, X.; He, C.; Chen, L.; Chen, X. Dual pH-responsive mesoporous silica nanoparticles for efficient combination of chemotherapy and photodynamic therapy. J. Mater. Chem. B 2015, 3, 4707–4714. [Google Scholar] [CrossRef]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-F.; Zhu, X.-M.; Wang, Y.-X.J.; Xuan, S.-H.; You, Q.; Chan, W.-H.; Wong, C.-H.; Wang, F.; Yu, J.C.; Cheng, C.H.K.; et al. Ultrasound, pH, and magnetically responsive crown-ether-coated core/shell nanoparticles as drug encapsulation and release systems. ACS Appl. Mater. Interfaces 2013, 5, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Shi, J. In vivo bio-Safety evaluations and diagnostic/therapeutic applications of chemically designed mesoporous silica nanoparticles. Adv. Mater. 2013, 25, 3144–3176. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-S.; Hurley, K.R.; Haynes, C.L. Critical considerations in the biomedical use of mesoporous silica nanoparticles. J. Phys. Chem. Lett. 2012, 3, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Rosenholm, J.M.; Mamaeva, V.; Sahlgren, C.; Linden, M. Nanoparticles in targeted cancer therapy: Mesoporous silica nanoparticles entering preclinical development stage. Nanomedicine 2012, 7, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Benezra, M.; Penate-Medina, O.; Zanzonico, P.B.; Schaer, D.; Ow, H.; Burns, A.; DeStanchina, E.; Longo, V.; Herz, E.; Iyer, S.; et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Investig. 2011, 121, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, H.; Godin, B. Biocompatibility assessment of Si-based nano- and micro-particles. Adv. Drug Deliv. Rev. 2012, 64, 1800–1819. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Wu, C.-W.; Vivero-Escoto, J.L.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Reducing Hemolytic Activity towards Mammalian Red Blood Cells. Small 2009, 5, 57–62. [Google Scholar] [CrossRef] [PubMed]
- He, Q.J.; Shi, J.L.; Chen, F.; Zhu, M.; Zhang, L.X. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials 2010, 31, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, Z.; Gao, F.; Li, Y.; Shi, J. In vivo biodistribution and urinary excretion of mesoporous silica nanoparticles: Effects of particle size and PEGylation. Small 2011, 7, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; Zhimin, T. Biocompatibility of Mesoporous Silica Nanoparticles. Chem. Res. Toxicol. 2012, 25, 2265–2284. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Greish, K.; McGill, L.D.; Ray, A.; Ghandehari, H. Influence of geometry, porosity, and surface characteristics of silica nanoparticles on acute toxicity: Their vasculature effect and tolerance threshold. ACS Nano 2012, 6, 2289–2301. [Google Scholar] [CrossRef] [PubMed]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.L.; Li, L.L.; Teng, X.; Huang, X.L.; Liu, H.Y.; Chen, D.; Ren, J.; He, J.Q.; Tang, F.Q. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomaterials 2011, 32, 1657–1668. [Google Scholar] [CrossRef] [PubMed]
- Fadeel, B.; García-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef] [PubMed]
- He, Q.J.; Zhang, J.M.; Shi, J.L.; Zhu, Z.Y.; Zhang, L.X.; Bu, W.B.; Guo, L.M.; Chen, Y. The effect of PEGylation of mesoporous silica nanoparticles on nonspecific binding of serum proteins and cellular responses. Biomaterials 2010, 31, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Lin, Y.-S.; Hung, Y.; Chou, Y.-H.; Hsu, Y.-H.; Chang, C.; Mou, C.-Y. Multifunctional mesoporous silica nanoparticles for intracellular labeling and animal magnetic resonance imaging studies. Chembiochem 2008, 9, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Cheng, S.-H.; Wang, Y.; Chen, Y.-C.; Chen, N.-T.; Souris, J.; Chen, C.-T.; Mou, C.-Y.; Yang, C.-S.; Lo, L.-W. Near-infrared mesoporous silica sanoparticles for optical imaging: Characterization and in vivo biodistribution. Adv. Funct. Mater. 2009, 19, 215–222. [Google Scholar] [CrossRef]
- Martin, K.R. The chemistry of silica and its potential health benefits. J. Nutr. Health Aging 2007, 11, 94–98. [Google Scholar] [PubMed]
- Mamaeva, V.; Rosenholm, J.M.; Bate-Eya, L.T.; Bergman, L.; Peuhu, E.; Duchanoy, A.; Fortelius, L.E.; Landor, S.; Toivola, D.M.; Linden, M.; et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of notch signaling in cancer. Mol. Ther. 2011, 19, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.W.; Bae, Y.H. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, A.S.; Allen, C. Block copolymer micelles for delivery of cancer therapy: Transport at the whole body, tissue and cellular levels. J. Controll. Release 2009, 138, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-H.; Mou, C.-Y.; Lin, H.-P. Synthesis of mesoporous silica nanoparticles. Chem. Soc. Rev. 2013, 42, 3862–3875. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Carmona, M.; Colilla, M.; Vallet-Regí, M. Smart Mesoporous Nanomaterials for Antitumor Therapy. Nanomaterials 2015, 5, 1906-1937. https://doi.org/10.3390/nano5041906
Martínez-Carmona M, Colilla M, Vallet-Regí M. Smart Mesoporous Nanomaterials for Antitumor Therapy. Nanomaterials. 2015; 5(4):1906-1937. https://doi.org/10.3390/nano5041906
Chicago/Turabian StyleMartínez-Carmona, Marina, Montserrat Colilla, and Maria Vallet-Regí. 2015. "Smart Mesoporous Nanomaterials for Antitumor Therapy" Nanomaterials 5, no. 4: 1906-1937. https://doi.org/10.3390/nano5041906