Gold Nanotheranostics: Proof-of-Concept or Clinical Tool?
Abstract
:1. Nanotheranostics: Concepts and Strategies at a Glance
2. Focus on Gold
2.1. Gold Nanoparticles at a Glance
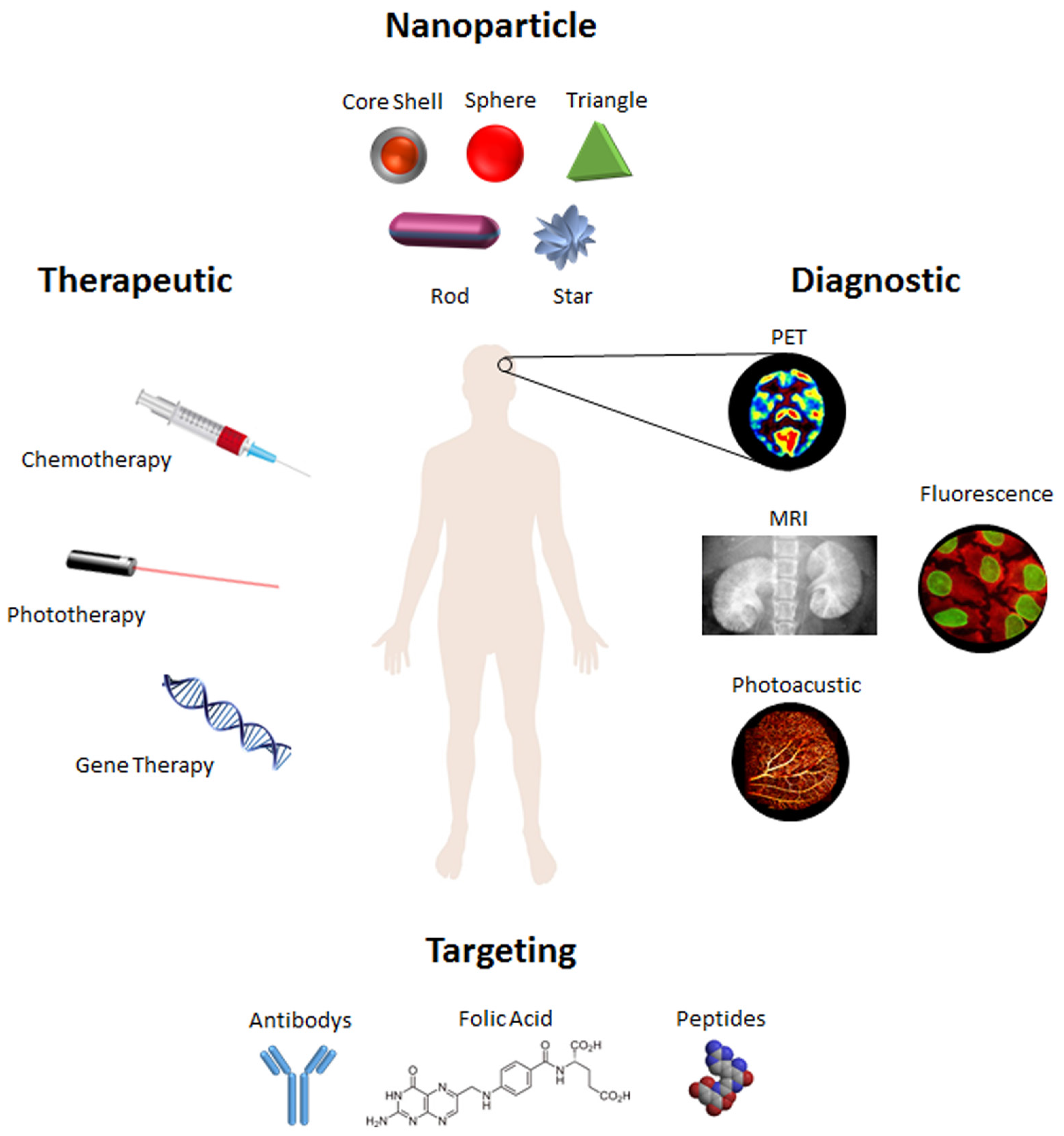
2.2. Targeting and Delivery
2.3. Therapeutic Agents
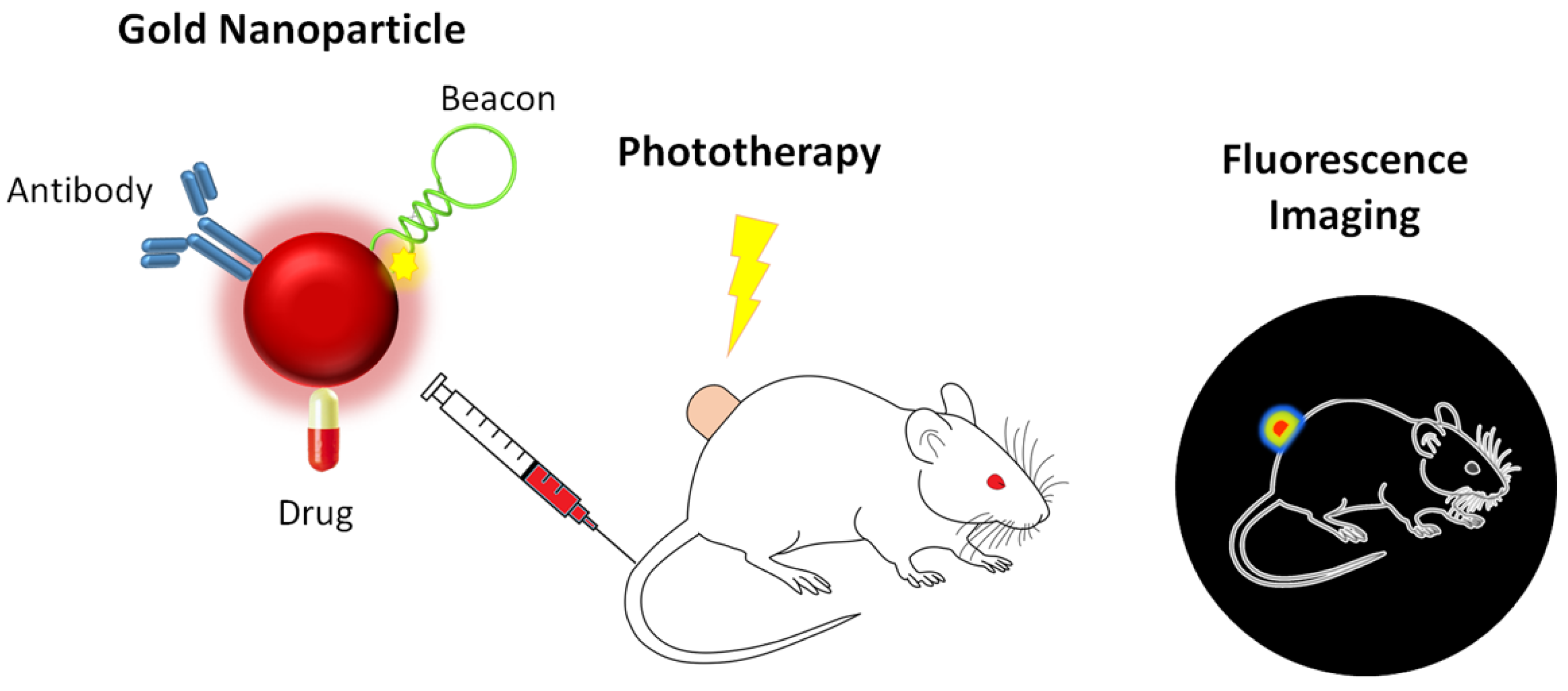
2.4. Phototherapy
2.5. Multimodal Imaging
3. From Research Lab to the Clinic
Commercial Impact
| Particle | Targeting | Therapeutic | Phototherapy | Imaging | Refs |
|---|---|---|---|---|---|
| PRE-CLINICAL STUDIES | |||||
| Silica-Gold Shells (150 nm) | - | - | Photothermal | MR | [150] |
| Gold Hollow Spheres (40 nm) | Melanocortin Type-1 Receptor | - | Photothermal | PET | [29] |
| Gold Spheres (60 nm) | EGFR | - | PNB | Scattering | [66] |
| Gold Hollow Spheres (40–50 nm) | Folate Receptor | Irinotecan + siRNA NF-κB p65 subunit | - | PET | [78] |
| Gold Cages (48 nm) | - | Photothermal | PET | [83] | |
| Gold Clusters (1 nm) | Folate Receptor | Doxorubicin | - | Fluorescence | [91] |
| Gold Hollow Spheres (37 nm) | Ephrin Type-B Receptor 4 | Doxorubicin | Photothermal | SPECT | [57] |
| Gold Stars (25 nm) | RGD | Doxorubicin | Photothermal | Fluorescence | [54] |
| Iron Oxide-Gold Spheres (6–18 nm) | A33 Antigen | - | Photothermal | MR | [151] |
| Gold Spheres (33 nm) | - | Tumor Necrosis Factor α | Photothermal | Photoacoustic | [130] |
| Gold Spheres (90 nm) | EGFR | Cetuximab | - | Raman Scattering | [64] |
| Gold Spheres (60 nm) | EGFR | Doxorubicin | PNB | Photoacoustic | [67] |
| Gold Spheres (5 nm) | EGFR | PC 4 | Photodynamic | Fluorescence | [65] |
| Gold-Cage (40–50 nm) + Silica Sphere Shell (50 nm) | - | Camptothecin | Photothermal | Fluorescence | [95] |
| PLGA-Gold Shell | Folate Receptor | Doxorubicin | Photodynamic; | Fluorescence | [96] |
| Gold Spheres (14 nm) | - | - | Photothermal | X-ray Computed Tomography | [152] |
| PLGA-Iron Oxide-Gold Shells (374 nm) | - | - | Photothermal | US; MR | [34] |
| Gold Spheres (3.3 nm) | Folate Receptor | α-Tocopheryl Succinate | - | X-ray Computed Tomography | [153] |
| Iron Oxide + Gold Clusters (150 nm) | Magnetic | Doxorubicin | Photothermal | MR | [154] |
| PLGA-Gold Shell (115 nm) | - | Doxorubicin | Photothermal | MR | [92] |
| Gold Rods + Liposome Hybrid | - | siRNA PLK1 | - | Multispectral Optoacoustic Tomography | [108] |
| Gold Bellflowers (180 nm) | - | - | Photothermal | Photoacoustic; US | [35] |
| Gold-Silica Rattles (150 nm) | - | Doxorubicin | Photothermal | Fluorescence; MR; Photoacoustic | [137] |
| Gold (20 nm) Gelatin shell (150 nm) | RGD | Doxorubicin | - | Fluorescence | [55] |
| Gold Stars (70 nm) | - | - | Photothermal | Thermal | [155] |
| Gold Spheres (12 nm); Gold Stars (30 nm; 60 nm) | - | - | Photothermal | SERS; X-ray CT; Two Photon Luminescence | [135] |
| Gold Rods (10:37 nm) | Folate Receptor | - | Photoacoustic | Photoacoustic | [77] |
| Gold Spheres (15 nm) | Scavenger Receptor (TAM) | siRNA Vascular endothelial growth factor | - | Fluorescence | [56] |
| Gold Rods (22:47 nm) | - | Doxorubicin + siRNA K-Ras | Photothermal | Fluorescence | [110] |
| Gold Spheres (15 nm) | - | Antisense K-Ras | - | Fluorescence | [98] |
| Gold Spheres (14 nm) | - | U5′-fluorouracile + siRNA MRP1 | - | Fluorescence | [94] |
| PLGA-Gold-Iron-Gold | RGD; Magnetic | Methotrexate | Photothermal | NIR; MR | [10] |
| Gold Spheres (30 nm) | Sonoporation | Levosimendan | - | US | [52] |
| Gold Rods | S. aureus: protein A; lipoprotein | - | Photothermal | Photoacoustic | [69] |
| Gold-Silver Core Shell (20 nm) | Anti-MRSA antibody | - | - | X-ray Computed Tomography | [70] |
| Gold Rods (10:33 nm) | Folate Receptor | - | Photothermal | SPECT; X-ray CT | [131] |
| Gadolinium-Gold (2–2.5 nm) | - | Healthy Pancreatic Islet Cells | - | MR; US; Computed Tomography | [138] |
| CLINICAL STUDIES | |||||
| Gold Spheres (20 nm) | - | - | - | - | [139] |
| Gold Spheres (27 nm) | - | Tumor Necrosis Factor α | - | - | [140] |
| Silica-Gold Shell (60–15 nm; 70–40 nm) | - | - | Photothermal | US | [141] |
4. Final Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Drug targeting to tumors: Principles, pitfalls and (pre-) clinical progress. J. Controll. Release 2012, 161, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Marques, M.; Coito, L.; Pombeiro, A.J.; Baptista, P.V.; Fernandes, A.R. Organometallic compounds in cancer therapy: Past lessons and future directions. Anti-Cancer Agents Med. Chem. 2014, 14, 1199–1212. [Google Scholar] [CrossRef]
- Vinhas, R.; Cordeiro, M.; Carlos, F.; Mendo, S.; Fernandes, A.; Figueiredo, S.; Baptista, P. Gold nanoparticle-based theranostics: Disease diagnostics and treatment using a single nanomaterial. Nanobiosensors Dis. Diagn. 2015, 4, 11–23. [Google Scholar]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mishra, P.K.; Talegaonkar, S.; Vaidya, B. Metal nanoparticles: A theranostic nanotool against cancer. Drug Discov. Today 2015, 20, 1143–1194. [Google Scholar] [CrossRef] [PubMed]
- Kojima, R.; Aubel, D.; Fussenegger, M. Novel theranostic agents for next-generation personalized medicine: Small molecules, nanoparticles, and engineered mammalian cells. Curr. Opin. Chem. Biol. 2015, 28, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Lee, S.; Son, S.; Kim, S.H.; Leary, J.F.; Choi, K.; Kwon, I.C. Theranostic nanoparticles for future personalized medicine. J. Controll. Release 2014, 190, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.M.; Baptista, P.V. Anti-cancer precision theranostics: A focus on multifunctional gold nanoparticles. Expert Rev. Mol. Diagn. 2014, 14, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Dreifuss, T.; Betzer, O.; Shilo, M.; Popovtzer, A.; Motiei, M.; Popovtzer, R. A challenge for theranostics: Is the optimal particle for therapy also optimal for diagnostics? Nanoscale 2015, 7, 15175–15184. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, S.M.; Park, K.H.; Mun, C.H.; Park, Y.B.; Yoo, K.H. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials 2015, 61, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, P.; Patravale, V. The upcoming field of theranostic nanomedicine: An overview. J. Biomed. Nanotechnol. 2012, 8, 859–882. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.A.; Maheshwari, R.G.; Tekade, M.; Tekade, R.K. Nanomaterial based approaches for the diagnosis and therapy of cardiovascular diseases. Curr. Pharm. Des. 2015, 21, 4465–4478. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Mei, L.; Feng, S.S. Nanotheranostics: Advanced nanomedicine for the integration of diagnosis and therapy. Nanomedicine 2014, 9, 1277–1280. [Google Scholar] [CrossRef] [PubMed]
- Frank, D.; Tyagi, C.; Tomar, L.; Choonara, Y.E.; du Toit, L.C.; Kumar, P.; Penny, C.; Pillay, V. Overview of the role of nanotechnological innovations in the detection and treatment of solid tumors. Int. J. Nanomed. 2014, 9, 589–613. [Google Scholar]
- Mohan, P.; Rapoport, N. Doxorubicin as a molecular nanotheranostic agent: Effect of doxorubicin encapsulation in micelles or nanoemulsions on the ultrasound-mediated intracellular delivery and nuclear trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Fang, B.A.; Zhang, P.; Hu, Z.; Gu, S.; Weng, K.C.; Gray, J.W.; Chen, F.F. Nanoparticle-mediated systemic delivery of siRNA for treatment of cancers and viral infections. Theranostics 2014, 4, 872–892. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Doria, G.; Baptista, P. Noble metal nanoparticles applications in cancer. J. Drug Deliv. 2012, 751075. [Google Scholar] [CrossRef] [PubMed]
- Veigas, B.; Fernandes, A.R.; Baptista, P.V. AuNPs for identification of molecular signatures of resistance. Front. Microbiol. 2014, 5, 455. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.M.; Baptista, P.V. The chemistry and biology of gold nanoparticle-mediated photothermal therapy: Promises and challenges. Nano Life 2013, 3, 1330001. [Google Scholar] [CrossRef]
- Baptista, P.V. Could gold nanoprobes be an important tool in cancer diagnostics? Expert Rev. Mol. Diagn. 2012, 12, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Badwaik, V.D.; Dakshinamurthy, R. Biological applications of gold nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 344–362. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Dias, J.T.; Grazu, V.; Moros, M.; Baptista, P.V.; de la Fuente, J.M. Revisiting 30 years of biofunctionalization and surface chemistry of inorganic nanoparticles for nanomedicine. Front. Chem. 2014, 2, 48. [Google Scholar] [CrossRef] [PubMed]
- Gerber, A.; Bundschuh, M.; Klingelhofer, D.; Groneberg, D.A. Gold nanoparticles: Recent aspects for human toxicology. J. Occupat. Med. Toxicol. 2013, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Ambrosone, A.; Hernandez, Y.; Tian, F.; McCully, M.; Berry, C.C.; Baptista, P.V.; Tortiglione, C.; de la Fuente, J.M. 15 years on siRNA delivery: Beyond the state-of-the-art on inorganic nanoparticles for RNAi therapeutics. Nano Today 2015. [Google Scholar] [CrossRef]
- Grzelczak, M.; Perez-Juste, J.; Mulvaney, P.; Liz-Marzan, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, G.; Nogami, M.; Matsuda, A. Shape-controlled metal nanoparticles and their assemblies with optical functionalities. J. Nanomater. 2013, 2013, 1–17. [Google Scholar] [CrossRef]
- Sajanlal, P.R.; Sreeprasad, T.S.; Samal, A.K.; Pradeep, T. Anisotropic nanomaterials: Structure, growth, assembly, and functions. Nano Rev. 2011, 2. [Google Scholar] [CrossRef]
- Lu, W.; Xiong, C.; Zhang, G.; Huang, Q.; Zhang, R.; Zhang, J.Z.; Li, C. Targeted photothermal ablation of murine melanomas with melanocyte-stimulating hormone analog-conjugated hollow gold nanospheres. Clin. Cancer Res. 2009, 15, 876–886. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Zhang, G.; Li, C. Exceptionally high payload of doxorubicin in hollow gold nanospheres for near-infrared light-triggered drug release. ACS Nano 2010, 4, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.; Lin, A.; Hirsch, L.; Lee, M.H.; Barton, J.; Halas, N.; West, J.; Drezek, R. Nanoshell-enabled photonics-based imaging and therapy of cancer. Technol. Cancer Res. Treat. 2004, 3, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Zhao, J.; Wallace, M.; Melancon, M.P. Cancer theranostics with gold nanoshells. Nanomedicine 2014, 9, 2041–2057. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Wang, J.; Tong, S.; Jin, Y.; Wang, S.; Qu, E.; Bao, G.; Dai, Z. Gold nanoshelled liquid perfluorocarbon magnetic nanocapsules: A nanotheranostic platform for bimodal ultrasound/magnetic resonance imaging guided photothermal tumor ablation. Theranostics 2013, 4, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Rong, P.; Lin, J.; Li, W.; Yan, X.; Zhang, M.G.; Nie, L.; Niu, G.; Lu, J.; Wang, W.; et al. Triphase interface synthesis of plasmonic gold bellflowers as near-infrared light mediated acoustic and thermal theranostics. J. Am. Chem. Soc. 2014, 136, 8307–8313. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Parak, W.J.; Rejman, J.; Manshian, B. (Intra)cellular stability of inorganic nanoparticles: Effects on cytotoxicity, particle functionality, and biomedical applications. Chem. Rev. 2015, 115, 2109–2135. [Google Scholar] [CrossRef] [PubMed]
- Nazarenus, M.; Zhang, Q.; Soliman, M.G.; del Pino, P.; Pelaz, B.; Carregal-Romero, S.; Rejman, J.; Rothen-Rutishauser, B.; Clift, M.J.; Zellner, R.; et al. In vitro interaction of colloidal nanoparticles with mammalian cells: What have we learned thus far? Beilstein J. Nanotechnol. 2014, 5, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Mironava, T.; Hadjiargyrou, M.; Simon, M.; Jurukovski, V.; Rafailovich, M.H. Gold nanoparticles cellular toxicity and recovery: Effect of size, concentration and exposure time. Nanotoxicology 2010, 4, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wu, H.Y.; Wu, D.; Wang, Y.Y.; Chang, J.H.; Zhai, Z.B.; Meng, A.M.; Liu, P.X.; Zhang, L.A.; Fan, F.Y. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int. J. Nanomed. 2010, 5, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Favi, P.M.; Gao, M.; Johana Sepulveda Arango, L.; Ospina, S.P.; Morales, M.; Pavon, J.J.; Webster, T.J. Shape and surface effects on the cytotoxicity of nanoparticles: Gold nanospheres versus gold nanostars. J. Biomed. Mater. Res. A 2015, 103, 3449–3462. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Djaker, N.; Boca-Farcau, S.; Salerno, M.; Charnaux, N.; Astilean, S.; Hlawaty, H.; de la Chapelle, M.L. Comparative toxicity evaluation of flower-shaped and spherical gold nanoparticles on human endothelial cells. Nanotechnology 2015, 26, 055101. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Zhang, S.; Zhang, B.; Zhang, C.; Fang, C.Y.; Rehor, I.; Cigler, P.; Chang, H.C.; Lin, G.; Liu, R.; et al. Unambiguous observation of shape effects on cellular fate of nanoparticles. Sci. Rep. 2014, 4, 4495. [Google Scholar] [CrossRef] [PubMed]
- Yildirimer, L.; Thanh, N.T.; Loizidou, M.; Seifalian, A.M. Toxicology and clinical potential of nanoparticles. Nano Today 2011, 6, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.E.; Parak, W.J.; Chan, W.C.; Xia, T.; Hersam, M.C.; Brinker, C.J.; Zink, J.I.; Pinkerton, K.E.; Baer, D.R.; Weiss, P.S. Where are we heading in nanotechnology environmental health and safety and materials characterization? ACS Nano 2015, 9, 5627–5630. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Maldiney, T.; Richard, C.; Seguin, J.; Wattier, N.; Bessodes, M.; Scherman, D. Effect of core diameter, surface coating, and PEG chain length on the biodistribution of persistent luminescence nanoparticles in mice. ACS Nano 2011, 5, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Feng, S.S. Effects of PEG tethering chain length of vitamin E TPGS with a herceptin-functionalized nanoparticle formulation for targeted delivery of anticancer drugs. Biomaterials 2014, 35, 3340–3347. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Austin, L.A.; Mackey, M.A.; El-Sayed, M.A. Size matters: Gold nanoparticles in targeted cancer drug delivery. Ther. Deliv. 2012, 3, 457–478. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target? Theranostics 2013, 4, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Controll. Release 2012, 164, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Spivak, M.Y.; Bubnov, R.V.; Yemets, I.M.; Lazarenko, L.M.; Tymoshok, N.O.; Ulberg, Z.R. Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: A theranostic potential for PPP cardiology. EPMA J. 2013, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Kattumuri, V.; Shukla, R.; Zambre, A.; Katti, K.; Upendran, A.; Kulkarni, R.R.; Kan, P.; Fent, G.M.; Casteel, S.W.; et al. Bombesin functionalized gold nanoparticles show in vitro and in vivo cancer receptor specificity. Proc. Natl. Acad. Sci. USA 2010, 107, 8760–8765. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, X.; Dai, S.; Ma, Y.; Cui, S.; Achilefu, S.; Gu, Y. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics 2013, 3, 633–649. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; He, Q.; Gao, H. Matrix metalloproteinase triggered size-shrinkable gelatin-gold fabricated nanoparticles for tumor microenvironment sensitive penetration and diagnosis of glioma. Nanoscale 2015, 7, 9487–9496. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Bao, C.; Tan, Y.; Cui, D.; Edelman, E.R.; Azevedo, H.S.; Byrne, H.J.; Artzi, N.; Tian, F. Dual targeted immunotherapy via in vivo delivery of biohybrid RNAi-peptide nanoparticles to tumor-associated macrophages and cancer cells. Adv. Funct. Mater. 2015, 25, 4183–4194. [Google Scholar] [CrossRef]
- You, J.; Zhang, R.; Xiong, C.; Zhong, M.; Melancon, M.; Gupta, S.; Nick, A.M.; Sood, A.K.; Li, C. Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target ephb4 receptors in tumors. Cancer Res. 2012, 72, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.H.; Alabi, C.A.; Webster, P.; Davis, M.E. Mechanism of active targeting in solid tumors with transferrin-containing gold nanoparticles. Proc. Natl. Acad. Sci. USA 2010, 107, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Pan, D.W.; Davis, M.E. Lack of in vivo antibody dependent cellular cytotoxicity with antibody containing gold nanoparticles. Bioconj. Chem. 2015, 26, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Qiu, M.; Wu, Q.; Tian, Y.; Zhang, Y.; Gu, N.; Li, S.; Xu, L.; Yin, R. Enhanced cytotoxic activity of cetuximab in EGFR-positive lung cancer by conjugating with gold nanoparticles. Sci. Rep. 2014, 4, 7490. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C. EGF mutant receptor VIII as a molecular target in cancer therapy. Endocr. Relat. Cancer 2001, 8, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Karmani, L.; Labar, D.; Valembois, V.; Bouchat, V.; Nagaswaran, P.G.; Bol, A.; Gillart, J.; Leveque, P.; Bouzin, C.; Bonifazi, D.; et al. Antibody-functionalized nanoparticles for imaging cancer: Influence of conjugation to gold nanoparticles on the biodistribution of 89zr-labeled cetuximab in mice. Contrast Media Mol. Imaging 2013, 8, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kao, H.W.; Lin, Y.Y.; Chen, C.C.; Chi, K.H.; Tien, D.C.; Hsia, C.C.; Lin, W.J.; Chen, F.D.; Lin, M.H.; Wang, H.E. Biological characterization of cetuximab-conjugated gold nanoparticles in a tumor animal model. Nanotechnology 2014, 25, 295102. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Bao, C.; Cui, D.; Baptista, P.V.; Tian, F. Antibody-drug gold nanoantennas with raman spectroscopic fingerprints for in vivo tumour theranostics. J. Controll. Release 2014, 183, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Cheng, Y.; Broome, A.M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-targeted gold nanoparticles for photodynamic therapy of brain cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.S.; Delk, N.A.; Lukianova-Hleb, E.Y.; Hafner, J.H.; Farach-Carson, M.C.; Lapotko, D.O. The in vivo performance of plasmonic nanobubbles as cell theranostic agents in zebrafish hosting prostate cancer xenografts. Biomaterials 2010, 31, 7567–7574. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.Y.; Ren, X.; Sawant, R.R.; Wu, X.; Torchilin, V.P.; Lapotko, D.O. On-demand intracellular amplification of chemoradiation with cancer-specific plasmonic nanobubbles. Nat. Med. 2014, 20, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Van de Broek, B.; Devoogdt, N.; D’Hollander, A.; Gijs, H.L.; Jans, K.; Lagae, L.; Muyldermans, S.; Maes, G.; Borghs, G. Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. ACS Nano 2011, 5, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Galanzha, E.I.; Shashkov, E.; Sarimollaoglu, M.; Beenken, K.E.; Basnakian, A.G.; Shirtliff, M.E.; Kim, J.W.; Smeltzer, M.S.; Zharov, V.P. In vivo magnetic enrichment, photoacoustic diagnosis, and photothermal purging of infected blood using multifunctional gold and magnetic nanoparticles. PLoS ONE 2012, 7, e45557. [Google Scholar] [CrossRef] [PubMed]
- Huo, D.; Ding, J.; Cui, Y.X.; Xia, L.Y.; Li, H.; He, J.; Zhou, Z.Y.; Wang, H.W.; Hu, Y. X-ray CT and pneumonia inhibition properties of gold-silver nanoparticles for targeting MRSA induced pneumonia. Biomaterials 2014, 35, 7032–7041. [Google Scholar] [CrossRef] [PubMed]
- Kalli, K.R.; Oberg, A.L.; Keeney, G.L.; Christianson, T.J.; Low, P.S.; Knutson, K.L.; Hartmann, L.C. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol. Oncol. 2008, 108, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Gu, Y.-J.; Cheng, S.H.; Wong, W.-T. Surface functionalized gold nanoparticles for drug delivery. J. Biomed. Nanotechnol. 2013, 9, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, B.; Mohammadnia-Afrouzi, M.; Bakhshaei, P.; Yazdani, Y.; Ghalamfarsa, G.; Yousefi, M.; Sadreddini, S.; Jadidi-Niaragh, F.; Hojjat-Farsangi, M. Folate-conjugated nanoparticles as a potent therapeutic approach in targeted cancer therapy. Tumour Biol. 2015, 8, 5727–5742. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Fu, F.; Xiong, Z.; Shen, M.; Shi, X. Dendrimer-entrapped gold nanoparticles modified with RGD peptide and alpha-tocopheryl succinate enable targeted theranostics of cancer cells. Colloids Surf. B 2015, 133, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Alatorre-Meda, M.; Iglesias, P.; Villar-Alvarez, E.M.; Barbosa, S.; Costoya, J.A.; Taboada, P.; Mosquera, V. Fluorescent drug-loaded, polymeric-based, branched gold nanoshells for localized multimodal therapy and imaging of tumoral cells. ACS Nano 2014, 8, 2725–2738. [Google Scholar] [CrossRef] [PubMed]
- Topete, A.; Alatorre-Meda, M.; Villar-Alvarez, E.M.; Carregal-Romero, S.; Barbosa, S.; Parak, W.J.; Taboada, P.; Mosquera, V. Polymeric-gold nanohybrids for combined imaging and cancer therapy. Adv. Healthc. Mater. 2014, 3, 1309–1325. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wen, L.; Yang, S.; Xiang, L.; Chen, Q.; Xing, D. Imaging-guided high-efficient photoacoustic tumor therapy with targeting gold nanorods. Nanomedicine 2015, 11, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Zhang, G.; Zhang, R.; Flores, L.G., 2nd; Huang, Q.; Gelovani, J.G.; Li, C. Tumor site-specific silencing of nf-kappab p65 by targeted hollow gold nanosphere-mediated photothermal transfection. Cancer Res. 2010, 70, 3177–3188. [Google Scholar] [CrossRef] [PubMed]
- Chiche, J.; Brahimi-Horn, M.C.; Pouyssegur, J. Tumour hypoxia induces a metabolic shift causing acidosis: A common feature in cancer. J. Cell. Mol. Med. 2010, 14, 771–794. [Google Scholar] [CrossRef] [PubMed]
- Arachchige, M.C.; Reshetnyak, Y.K.; Andreev, O.A. Advanced targeted nanomedicine. J. Biotechnol. 2015, 202, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Antosh, M.P.; Wijesinghe, D.D.; Shrestha, S.; Lanou, R.; Huang, Y.H.; Hasselbacher, T.; Fox, D.; Neretti, N.; Sun, S.; Katenka, N.; et al. Enhancement of radiation effect on cancer cells by gold-pHLIP. Proc. Natl. Acad. Sci. USA 2015, 112, 5372–5376. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Glaus, C.; Laforest, R.; Zhang, Q.; Yang, M.; Gidding, M.; Welch, M.J.; Xia, Y. Gold nanocages as photothermal transducers for cancer treatment. Small 2010, 6, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, M.S.; Cheng, Y.; Chen, J.; Cobley, C.M.; Zhang, Q.; Rycenga, M.; Xie, J.; Kim, C.; Song, K.H.; Schwartz, A.G.; et al. Gold nanocages covered by smart polymers for controlled release with near-infrared light. Nat. Mater. 2009, 8, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Cobley, C.M.; Au, L.; Chen, J.; Xia, Y. Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery. Expert Opin. Drug Deliv. 2010, 7, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Urban, C.; Urban, A.S.; Charron, H.; Joshi, A. Externally modulated theranostic nanoparticles. Transl. Cancer Res. 2013, 2, 292–308. [Google Scholar] [PubMed]
- Li, W.; Cai, X.; Kim, C.; Sun, G.; Zhang, Y.; Deng, R.; Yang, M.; Chen, J.; Achilefu, S.; Wang, L.V.; et al. Gold nanocages covered with thermally-responsive polymers for controlled release by high-intensity focused ultrasound. Nanoscale 2011, 3, 1724–1730. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chen, K.J.; Noh, S.H.; Garcia, M.A.; Wang, H.; Lin, W.Y.; Jeong, H.; Kong, B.J.; Stout, D.B.; Cheon, J.; et al. On-demand drug release system for in vivo cancer treatment through self-assembled magnetic nanoparticles. Angew. Chem. 2013, 52, 4384–4388. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.P.; Rees, S.; Kalindjian, S.B.; Philpott, K.L. Principles of early drug discovery. Br. J. Pharmacol. 2011, 162, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Teodoro, J.G.; Nadeau, J.L. Intratumoral gold-doxorubicin is effective in treating melanoma in mice. Nanomedicine 2015, 11, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal doxorubicin: Review of animal and human studies. Clin. Pharm. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, S.; Li, B.; Ren, X.; Li, S.; Mahounga, D.M.; Cui, S.; Gu, Y.; Achilefu, S. Folate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapy. Nanoscale 2012, 4, 6050–6064. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Liang, X.; Li, X.; Lin, L.; Yang, Y.; Yue, X.; Dai, Z. Mn-porphyrin conjugated au nanoshells encapsulating doxorubicin for potential magnetic resonance imaging and light triggered synergistic therapy of cancer. Theranostics 2014, 4, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Dhar, S.; Daniel, W.L.; Giljohann, D.A.; Mirkin, C.A.; Lippard, S.J. Polyvalent oligonucleotide gold nanoparticle conjugates as delivery vehicles for platinum(IV) warheads. J. Am. Chem. Soc. 2009, 131, 14652–14653. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Oliva, N.; Artzi, N. Implantable hydrogel embedded dark-gold nanoswitch as a theranostic probe to sense and overcome cancer multidrug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, E1278–E1287. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Liu, Z.; Dong, K.; Ju, E.; Ren, J.; Du, Y.; Li, Z.; Qu, X. A smart “sense-act-treat” system: Combining a ratiometric pH sensor with a near infrared therapeutic gold nanocage. Adv. Mater. 2014, 26, 6635–6641. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S.; Wolfgang, M.; Hwang, J.; Ryan, J.; Eliasof, S. Preclinical to clinical development of the novel camptothecin nanopharmaceutical CRLX101. J. Controll. Release 2011, 153, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, B.; van Veggel, F.C.J.M.; Tomanek, B. Applications of nanoparticles for MRI cancer diagnosis and therapy. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Bao, C.; Conde, J.; Curtin, J.; Artzi, N.; Tian, F.; Cui, D. Bioresponsive antisense DNA gold nanobeacons as a hybrid in vivo theranostics platform for the inhibition of cancer cells and metastasis. Sci. Rep. 2015, 5, 12297. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, Q.; Braun, G.B.; Pallaoro, A.; Morales, D.P.; Zasadzinski, J.; Clegg, D.O.; Reich, N.O. Light-activated RNA interference in human embryonic stem cells. Biomaterials 2015, 63, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, L.; He, Y.; Shen, Y.; Li, Y. Enhanced shRNA delivery and ABCG2 silencing by charge-reversible layered nanocarriers. Small 2015, 11, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.J.; Tzeng, S.Y.; Green, J.J. Degradable polymer-coated gold nanoparticles for co-delivery of DNA and siRNA. Acta Biomater. 2015, 11, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Takemoto, H.; Yi, Y.; Zheng, M.; Maeda, Y.; Chaya, H.; Hayashi, K.; Mi, P.; Pittella, F.; Christie, R.J.; et al. Precise engineering of siRNA delivery vehicles to tumors using polyion complexes and gold nanoparticles. ACS Nano 2014, 8, 8979–8991. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Rosa, J.; de la Fuente, J.M.; Baptista, P.V. Gold-nanobeacons for simultaneous gene specific silencing and intracellular tracking of the silencing events. Biomaterials 2013, 34, 2516–2523. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Jiang, Z.; Saha, K.; Kim, C.S.; Kim, S.T.; Landis, R.F.; Rotello, V.M. Gold nanoparticles for nucleic acid delivery. Mol. Ther. 2014, 22, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Cao-Milan, R.; Liz-Marzan, L.M. Gold nanoparticle conjugates: Recent advances toward clinical applications. Expert Opin. Drug Deliv. 2014, 11, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Webb, J.A.; Bardhan, R. Emerging advances in nanomedicine with engineered gold nanostructures. Nanoscale 2014, 6, 2502–2530. [Google Scholar] [CrossRef] [PubMed]
- Bonoiu, A.C.; Bergey, E.J.; Ding, H.; Hu, R.; Kumar, R.; Yong, K.T.; Prasad, P.N.; Mahajan, S.; Picchione, K.E.; Bhattacharjee, A.; et al. Gold nanorod-siRNA induces efficient in vivo gene silencing in the rat hippocampus. Nanomedicine 2011, 6, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Taruttis, A.; Lozano, N.; Nunes, A.; Jasim, D.A.; Beziere, N.; Herzog, E.; Kostarelos, K.; Ntziachristos, V. Sirna liposome-gold nanorod vectors for multispectral optoacoustic tomography theranostics. Nanoscale 2014, 6, 13451–13456. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kim, H.C.; Mu, C.; Gentile, E.; Mai, J.; Wolfram, J.; Ji, L.N.; Ferrari, M.; Mao, Z.W.; Shen, H. Multifunctional gold nanorods for siRNA gene silencing and photothermal therapy. Adv. Healthc. Mater. 2014, 3, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yang, C.; Wang, Q.; Zeng, S.; Hu, R.; Lin, G.; Tian, J.; Hu, S.; Lan, R.F.; Yoon, H.S.; et al. A light-driven therapy of pancreatic adenocarcinoma using gold nanorods-based nanocarriers for co-delivery of doxorubicin and siRNA. Theranostics 2015, 5, 818–833. [Google Scholar] [CrossRef] [PubMed]
- Randeria, P.S.; Seeger, M.A.; Wang, X.Q.; Wilson, H.; Shipp, D.; Mirkin, C.A.; Paller, A.S. SiRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside gm3 synthase knockdown. Proc. Natl. Acad. Sci. USA 2015, 112, 5573–5578. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Ambrosone, A.; Sanz, V.; Hernandez, Y.; Marchesano, V.; Tian, F.; Child, H.; Berry, C.C.; Ibarra, M.R.; Baptista, P.V.; et al. Design of multifunctional gold nanoparticles for in vitro and in vivo gene silencing. ACS Nano 2012, 6, 8316–8324. [Google Scholar] [CrossRef] [PubMed]
- Rosa, J.; Conde, J.; de la Fuente, J.M.; Lima, J.C.; Baptista, P.V. Gold-nanobeacons for real-time monitoring of RNA synthesis. Biosensors Bioelectron. 2012, 36, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Rosa, J.; Baptista, P. Gold-nanobeacons as a theranostic system for the detection and inhibition of specific genes. Protocol Exchang. 2013. [Google Scholar] [CrossRef]
- Conde, J.; Larguinho, M.; Cordeiro, A.; Raposo, L.R.; Costa, P.M.; Santos, S.; Diniz, M.S.; Fernandes, A.R.; Baptista, P.V. Gold-nanobeacons for gene therapy: Evaluation of genotoxicity, cell toxicity and proteome profiling analysis. Nanotoxicology 2014, 8, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; de la Fuente, J.M.; Baptista, P.V. In vitro transcription and translation inhibition via DNA functionalized gold nanoparticles. Nanotechnology 2010, 21, 505101. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, M.; Chen, Q.; Guan, G.; Hu, W.; Zhao, X.; Qiao, M.; Hu, H.; Liang, Y.; Zhu, H.; et al. Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser. Int. J. Nanomed. 2015, 10, 4747–4761. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Xu, Z.; Zhou, F.; Yu, H.; Sun, Q.; Wang, D.; Tang, Z.; Yu, H.; Yin, Q.; Zhang, Z.; et al. Near infrared light-actuated gold nanorods with cisplatin-polypeptide wrapping for targeted therapy of triple negative breast cancer. Nanoscale 2015, 7, 14854–14864. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.Y.; Yang, X.Q.; An, J.; Zhang, L.; Zhao, K.; Qin, M.Y.; Fang, B.Y.; Li, C.; Xuan, Y.; Zhang, X.S.; et al. Multifunctional magnetic-hollow gold nanospheres for bimodal cancer cell imaging and photothermal therapy. Nanotechnology 2015, 26, 315701. [Google Scholar] [CrossRef] [PubMed]
- Lapotko, D. Plasmonic nanobubbles as tunable cellular probes for cancer theranostics. Cancers 2011, 3, 802–840. [Google Scholar] [CrossRef] [PubMed]
- Lukianova-Hleb, E.Y.; Ren, X.; Townley, D.; Wu, X.; Kupferman, M.E.; Lapotko, D.O. Plasmonic nanobubbles rapidly detect and destroy drug-resistant tumors. Theranostics 2012, 2, 976–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hsu, C.H.; Huang, C.C.; Chang, P.Y. Development of therapeutic Au-methylene blue nanoparticles for targeted photodynamic therapy of cervical cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 432–441. [Google Scholar] [CrossRef] [PubMed]
- Willmann, J.K.; van Bruggen, N.; Dinkelborg, L.M.; Gambhir, S.S. Molecular imaging in drug development. Nature Reviews. Drug Discov. 2008, 7, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Zhang, Y.; Sun, J.; Cai, W. Molecular imaging and therapy of cancer with radiolabeled nanoparticles. Nano Today 2009, 4, 399–413. [Google Scholar] [CrossRef] [PubMed]
- Lusic, H.; Grinstaff, M.W. X-ray-computed tomography contrast agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [PubMed]
- Hasebroock, K.M.; Serkova, N.J. Toxicity of MRI and CT contrast agents. Expert Opin. Drug Metabol. Toxicol. 2009, 5, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Griffin, R.J.; Galanzha, E.I.; Kim, J.W.; Koonce, N.; Webber, J.; Mustafa, T.; Biris, A.S.; Nedosekin, D.A.; Zharov, V.P. Photothermal nanodrugs: Potential of TNF-gold nanospheres for cancer theranostics. Sci. Rep. 2013, 3, 1293. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, S.; Kang, S.H.; Kim, J.K.; Kim, S.K.; Kim, I.H.; Choi, Y. Gold nanorods for target selective SPECT/CT imaging and photothermal therapy in vivo. Quant. Imaging Med. Surg. 2012, 2, 1–11. [Google Scholar] [PubMed]
- Kosaka, N.; Ogawa, M.; Choyke, P.L.; Kobayashi, H. Clinical implications of near-infrared fluorescence imaging in cancer. Future Oncol. 2009, 5, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Zhang, M. Cancer nanotheranostics: Improving imaging and therapy by targeted delivery across biological barriers. Adv. Mater. 2011, 23, H217–H247. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Cho, H.Y.; Kim, K.J.; Choi, J.W. In situ monitoring of doxorubicin release from biohybrid nanoparticles modified with antibody and cell-penetrating peptides in breast cancer cells using surface-enhanced raman spectroscopy. Biosensors Bioelectron. 2015, 71, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hembury, M.; Chiappini, C.; Bertazzo, S.; Kalber, T.L.; Drisko, G.L.; Ogunlade, O.; Walker-Samuel, S.; Krishna, K.S.; Jumeaux, C.; Beard, P.; et al. Gold-silica quantum rattles for multimodal imaging and therapy. Proc. Natl. Acad. Sci. USA 2015, 112, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Arifin, D.R.; Long, C.M.; Gilad, A.A.; Alric, C.; Roux, S.; Tillement, O.; Link, T.W.; Arepally, A.; Bulte, J.W. Trimodal gadolinium-gold microcapsules containing pancreatic islet cells restore normoglycemia in diabetic mice and can be tracked by using US, CT, and positive-contrast MR imaging. Radiology 2011, 260, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.E.; Himmel, P.B. Management of rheumatoid arthritis: Rationale for the use of colloidal metallic gold. J. Nutr. Environ. Med. 1997, 7, 295–305. [Google Scholar]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R., Jr.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and pharmacokinetic studies of CYT-6091, a novel pegylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [PubMed]
- Kharlamov, A.N.; Tyurnina, A.E.; Veselova, V.S.; Kovtun, O.P.; Shur, V.Y.; Gabinsky, J.L. Silica-gold nanoparticles for atheroprotective management of plaques: Results of the NANOM-FIM trial. Nanoscale 2015, 7, 8003–8015. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research, Inc. Gold Nanoparticles Market Analysis by End-Use (Medical & Dentistry, Electronics, Catalysis) and Segment Forecasts to 2020; Grand View Research, Inc.: San Francisco, CA, USA, 2015. [Google Scholar]
- National Nanotechnology Initiative, Federal Budget 2016. Available online: http://www.nano.gov/about-nni/what/funding (accessed on 29 July 2015).
- Commission of the European Communities: Towards a European Strategy for Nanotechnology. Available online: http://ec.europa.eu/research/industrial_technologies/pdf/policy/nano_com_en.pdf (accessed on 29 July 2015).
- CRC Press. Nanotoxicology: Progress toward nanomedicine, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Bawa, R. FDA and Nanotech: Baby Steps Lead to Regulatory Uncertainty. In Bio-Nanotechnology: A Revolution in Food, Biomedical and Health Sciences; Bagchi, D., Ed.; Wiley-Blackwell: Chichester, UK, 2013; pp. 720–732. [Google Scholar]
- Horizon 2020, Leadership in Enabling and Industrial Technologies: Nanotechnologies, Advanced Materials, Biotechnology and Advanced Manufacturing and Processing Revised. Available online: http://ec.europa.eu/research/participants/data/ref/h2020/wp/2014_2015/main/h2020-wp1415-leit-nmp_en.pdf (accessed on 29 July 2015).
- Bosetti, R.; Ferrandina, G.; Marneffe, W.; Scambia, G.; Vereeck, L. Cost-effectiveness of gemcitabine versus pegylated liposomal doxorubicin for recurrent or progressive ovarian cancer: Comparing chemotherapy with nanotherapy. Nanomedicine 2014, 9, 2175–2186. [Google Scholar] [CrossRef] [PubMed]
- Nanomaterials in Theranostics: Global Markets; PR Newswire: New York, NY, USA, 2013.
- Diagaradjane, P.; Shetty, A.; Wang, J.C.; Elliott, A.M.; Schwartz, J.; Shentu, S.; Park, H.C.; Deorukhkar, A.; Stafford, R.J.; Cho, S.H.; et al. Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: Characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett. 2008, 8, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Kirui, D.K.; Khalidov, I.; Wang, Y.; Batt, C.A. Targeted near-IR hybrid magnetic nanoparticles for in vivo cancer therapy and imaging. Nanomedicine 2013, 9, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Zhong, Y.; Du, M.; Liu, Q.; Fan, Z.; Dai, F.; Zhang, X. Theranostic self-assembly structure of gold nanoparticles for NIR photothermal therapy and X-ray computed tomography imaging. Theranostics 2014, 4, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zheng, L.; Wen, S.; Tang, Y.; Shen, M.; Zhang, G.; Shi, X. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials 2014, 35, 7635–7646. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Qi, T.; Liao, J.; Chu, B.; Yang, Q.; Qu, Y.; Li, W.; Li, H.; Luo, F.; Qian, Z. Mesoporous magnetic gold “nanoclusters” as theranostic carrier for chemo-photothermal co-therapy of breast cancer. Theranostics 2014, 4, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Teng, Z.; Huang, P.; Liu, D.; Liu, Y.; Tian, Y.; Sun, J.; Li, Y.; Ju, H.; Chen, X.; et al. Reversibly extracellular pH controlled cellular uptake and photothermal therapy by pegylated mixed-charge gold nanostars. Small 2015, 11, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrosa, P.; Vinhas, R.; Fernandes, A.; Baptista, P.V. Gold Nanotheranostics: Proof-of-Concept or Clinical Tool? Nanomaterials 2015, 5, 1853-1879. https://doi.org/10.3390/nano5041853
Pedrosa P, Vinhas R, Fernandes A, Baptista PV. Gold Nanotheranostics: Proof-of-Concept or Clinical Tool? Nanomaterials. 2015; 5(4):1853-1879. https://doi.org/10.3390/nano5041853
Chicago/Turabian StylePedrosa, Pedro, Raquel Vinhas, Alexandra Fernandes, and Pedro V Baptista. 2015. "Gold Nanotheranostics: Proof-of-Concept or Clinical Tool?" Nanomaterials 5, no. 4: 1853-1879. https://doi.org/10.3390/nano5041853






