High Rate Performance Nanocomposite Electrode of Mesoporous Manganese Dioxide/Silver Nanowires in KI Electrolytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of the Amount of Acid on the Structure of MnO2
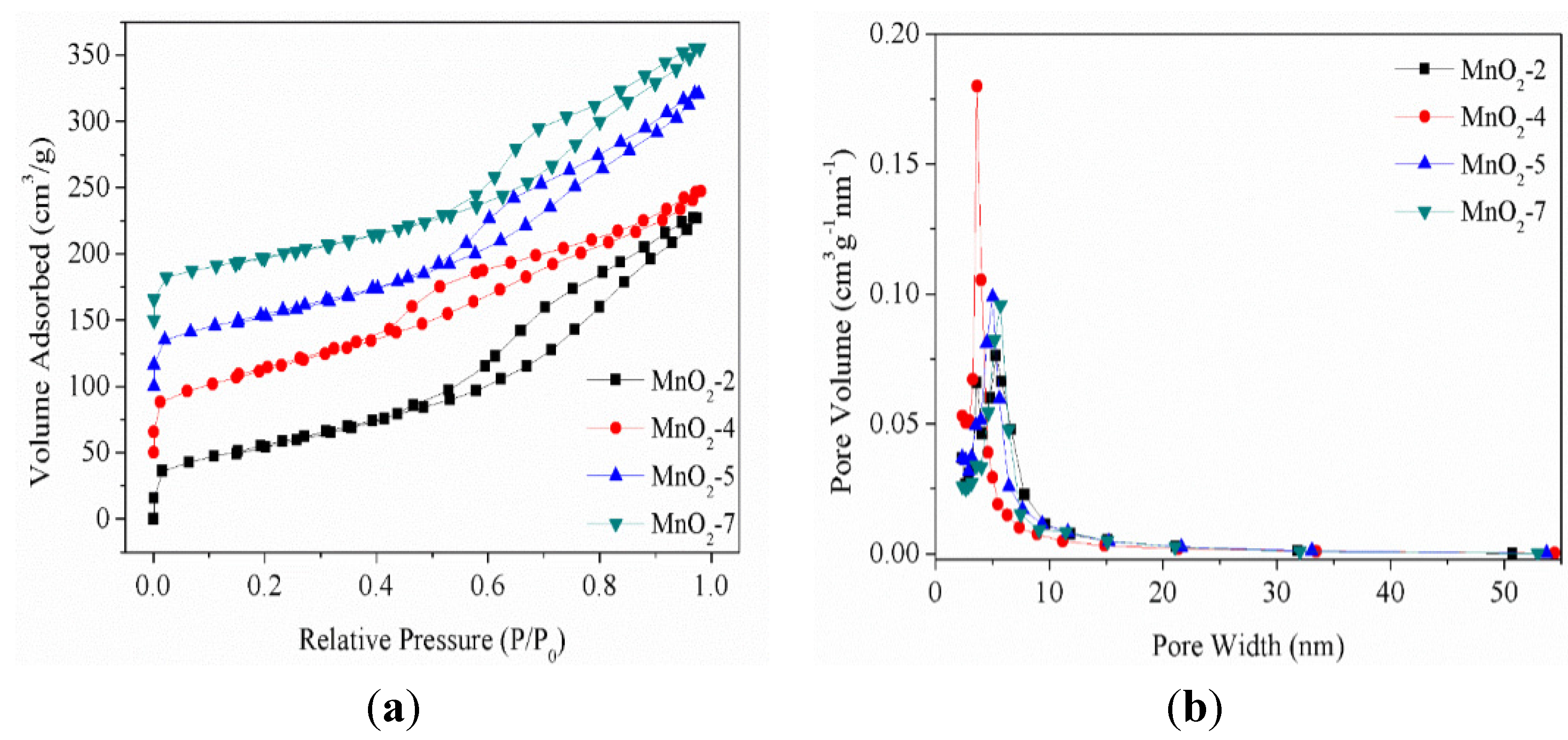
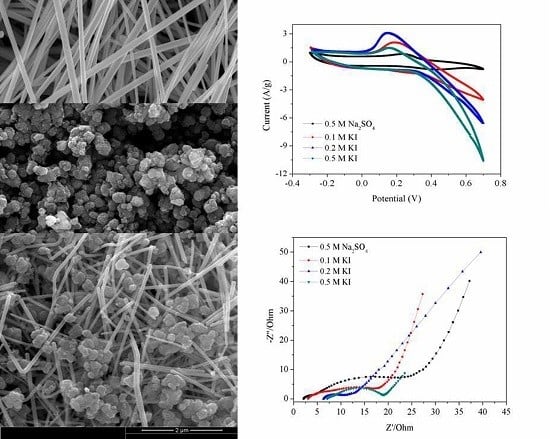
2.2. Microstructure Characterization


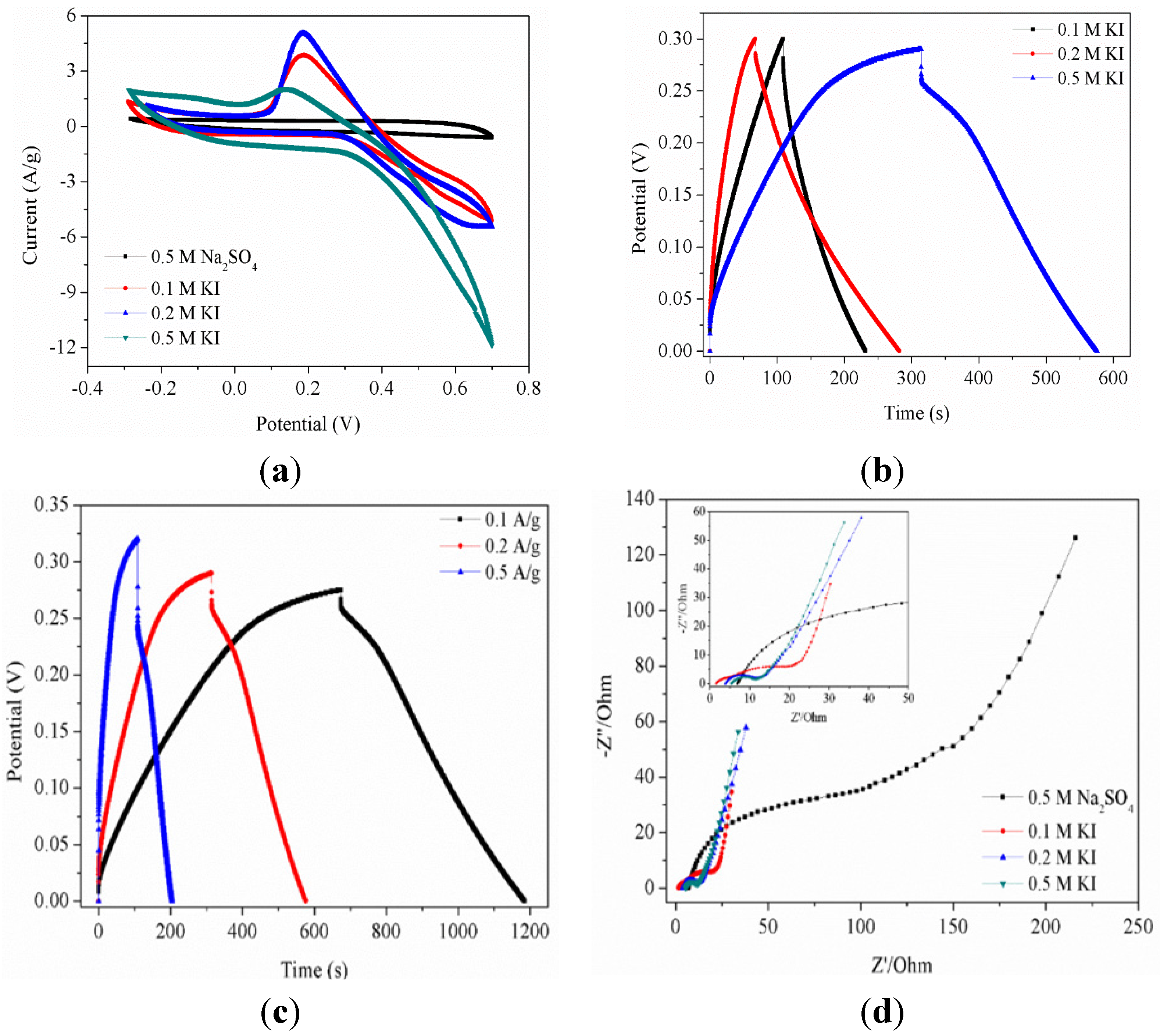
2.3. Electrochemical Characterizations
| Sample | ER (Ω·cm) | EC (S/cm) |
|---|---|---|
| MnO2-4 | 2.54 × 106 | 3.94 × 10−7 |
| Ag | 1.73 × 10−2 | 5.78 × 10 |
| MnO2:Ag = 1:0.25 | 4.33 × 104 | 2.31 × 10−5 |
| MnO2:Ag = 1:0.5 | 1.10 × 102 | 9.10 × 10−3 |
| MnO2:Ag = 1:1 | 8.37 | 1.19 × 10−1 |
| Samples | Cs (F·g−1) | Electrolyte | Test Condition | References |
|---|---|---|---|---|
| Ambigel MnO2 | 130 | 2 M NaCl | 5 mV·s−1 | [29] |
| α-MnO2 nanorod | 152 | 1 M Na2SO4 | 5 mV·s−1 | [30] |
| Birnessite hollow MnO2 | 169 | 1 M Na2SO4 | 0.25 A·g−1 | [31] |
| MnO2 spherical particle | 170.8 | 0.5 M K2SO4 | 0.5 A·g−1 | [32] |
| MnO2 nanowire | 176 | 1 M Na2SO4 | 5 mV·s−1 | [33] |
| MnO2 nano hollow sphere | 178 | 0.5 M K2SO4 | 0.5 A·g−1 | [34] |
| Porous MnO2 nanoparticle | 178.9 | 1 M Na2SO4 | 1 mV·s−1 | [35] |
| MnO2 particle | 180 | 0.5 M KOH | 1 mV·s−1 | [36] |
| Porous nano-MnO2 | 198.1 | 1 M Na2SO4 | 0.28 A·g−1 | [37] |
| MnO2 nanoparticle | 200 | 0.2 M K2SO4 | 5 mV·s−1 | [38] |
| MnO2 nanowisker | 200 | 1 M Na2SO4 | 2 mV·s−1 | [39] |
| Amorphous MnO2·nH2O | 200 | 2 M KCl | 5 mV·s−1 | [40] |
| MnO2 nanosheet array | 201 | 1 M Na2SO4 | 1 A·g−11 | [41] |
| Birnessite MnO2 nanosphere | 210 | 1 M Na2SO4 | 1 A·g−1 | [42] |
| Coral-like MnO2 | 221 | 1 M Na2SO4 | 0.5 A·g−1 | [43] |
| MnO2 thin sheet | 230 | 0.5 M Na2SO4 | 20 mV·s−1 | [44] |
| γ-MnO2 film | 240 | 0.1 M Na2SO4 | 1 mA·cm−2 | [45] |
| Lamellar MnO2 | 242.1 | 2 M NH4(SO4)2 | 2 mA·cm−2 | [46] |
| α-MnO2 nanorod | 245 | 1 M KOH | 1 A g−1 | [47] |
| Amorphous MnO2 particle | 251 | 1 M Na2SO4 | 2 mV·s−1 | [48] |
| Mesoporous MnO2 | 200.3 | 0.5 M Na2SO4 | 0.1 A·g−1 | This work |
| Mesoporous MnO2 | 205.2 | 0.5 M KI | 0.1 A·g−1 | This work |
| Mesoporous MnO2 | 200.6 | 0.5 M KI | 0.2 A·g−1 | This work |
| Mesoporous MnO2 | 197.1 | 0.5 M KI | 0.5A·g−1 | This work |
| Ag/Mesoporous MnO2 | 187.2 | 0.5 M Na2SO4 | 0.1 A·g−1 | This work |
| Ag/Mesoporous MnO2 | 208.1 | 0.2 M KI | 0.1 A·g−1 | This work |
| Ag/Mesoporous MnO2 | 200.5 | 0.2 M KI | 0.2 A·g−1 | This work |
| Ag/Mesoporous MnO2 | 191.7 | 0.2 M KI | 0.5 A·g−1 | This work |

| Sample | Mesoporous MnO2 | |||
|---|---|---|---|---|
| Experimental Conditions | 0.1 M KI | 0.2 M KI | 0.5 M KI | 0.5 M Na2SO4 |
| 0.1 A/g | 106.6 | 229.2 | 205.5 | 200.3 |
| 0.2 A/g | 89.3 | 152.5 | 200.6 | 85.72 |
| 0.5 A/g | 57.1 | 152.9 | 197.1 | 49.12 |
| Sample | Mesoporous MnO2/Silver Nanowires | |||
|---|---|---|---|---|
| Experimental Conditions | 0.1 M KI | 0.2 M KI | 0.5 M KI | 0.5 M Na2SO4 |
| 0.1 A/g | 152.1 | 208.1 | 158.1 | 187.2 |
| 0.2 A/g | 130.8 | 200.5 | 133.7 | 150.2 |
| 0.5 A/g | 117.2 | 191.7 | 115.8 | 113.1 |
3. Experimental Section
3.1. Preparation of Mesoporous MnO2
3.2. Preparation of Silver Nanowires
3.3. Preparation of Mesoporous Manganese Dioxide-Based Composite Materials
3.4. Material Characterization
3.5. Preparation of Electrodes and Electrochemical Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bhattacharjya, D.; Kim, M.-S.; Bae, T.-S.; Yu, J.-S. High performance supercapacitor prepared from hollow mesoporous carbon capsules with hierarchical nanoarchitecture. J. Power Sources 2013, 244, 799–805. [Google Scholar] [CrossRef]
- Tsay, K.-C.; Zhang, L.; Zhang, J. Effects of electrode layer composition/thickness and electrolyte concentration on both specific capacitance and energy density of supercapacitor. Electrochim. Acta 2012, 60, 428–436. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Pusawale, S.N.; Jamadade, V.S.; Patil, U.M.; Lokhande, C.D. Microwave assisted chemical bath deposited polyaniline films for supercapacitor application. J. Alloys Compd. 2011, 509, 5064–5069. [Google Scholar] [CrossRef]
- Khosrozadeh, A.; Xing, M.; Wang, Q. A high-capacitance solid-state supercapacitor based on free-standing film of polyaniline and carbon particles. Appl. Energy 2015, 153, 87–93. [Google Scholar] [CrossRef]
- Karandikar, P.B.; Talange, D.B.; Mhaskar, U.P.; Bansal, R. Development, modeling and characterization of aqueous metal oxide based supercapacitor. Energy 2012, 40, 131–138. [Google Scholar] [CrossRef]
- Jiang, J.; Kucernak, A. Electrochemical supercapacitor material based on manganese oxide: Preparation and characterization. Electrochim. Acta 2002, 47, 2381–2386. [Google Scholar] [CrossRef]
- Yan, X.; Tong, X.; Wang, J.; Gong, C.; Zhang, M.; Liang, L. Synthesis of mesoporous NiO nanoflake array and its enhanced electrochemical performance for supercapacitor application. J. Alloys Compd. 2014, 593, 184–189. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Kurungot, S. Electrochemically grown nanoporous MnO2 nanowalls on a porous carbon substrate with enhanced capacitance through faster ionic and electrical mobility. Chem. Commun. 2014, 50, 7188–7190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ren, L.; Deng, L.; Wang, J.; Kang, L.; Liu, Z.-H. Graphene-MnO2 nanocomposite for high-performance asymmetrical electrochemical capacitor. Mater. Res. Bull. 2014, 49, 577–583. [Google Scholar] [CrossRef]
- Liu, T.; Shao, G.; Ji, M.; Wang, G. Synthesis of MnO2-graphene composites with enhanced supercapacitive performance via pulse electrodeposition under supergravity field. J. Solid State Chem. 2014, 215, 160–166. [Google Scholar] [CrossRef]
- Li, Z.; Wang, J.; Liu, S.; Liu, X.; Yang, S. Synthesis of hydrothermally reduced graphene/MnO2 composites and their electrochemical properties as supercapacitors. J. Power Sources 2011, 196, 8160–8165. [Google Scholar] [CrossRef]
- Wang, Q.; Plylahan, N.; Shelke, M.V.; Devarapalli, R.R.; Li, M.; Subramanian, P.; Djenizian, T.; Boukherroub, R.; Szunerits, S. Nanodiamond particles/reduced graphene oxide composites as efficient supercapacitor electrodes. Carbon 2014, 68, 175–184. [Google Scholar] [CrossRef]
- Chang, J.-K.; Lin, C.-T.; Tsai, W.-T. Manganese oxide/carbon composite electrodes for electrochemical capacitors. Electrochem. Commun. 2004, 6, 666–671. [Google Scholar] [CrossRef]
- Zhang, L.L.; Wei, T.; Wang, W.; Zhao, X.S. Manganese oxide–carbon composite as supercapacitor electrode materials. Micropor. Mesopor. Mat. 2009, 123, 260–267. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, D.; Zhang, T.; Chen, H.; Zhao, H.; Wang, Y.; Sun, Z.; Tang, S.; Fang, X.; Cao, X. Capacitive properties of PANI/MnO2 synthesized via simultaneous-oxidation route. J. Alloys Compd. 2012, 532, 1–9. [Google Scholar] [CrossRef]
- Kharade, P.M.; Chavan, S.G.; Salunkhe, D.J.; Joshi, P.B.; Mane, S.M.; Kulkarni, S.B. Synthesis and characterization of PANI/MnO2 bi-layered electrode and its electrochemical supercapacitor properties. Mater. Res. Bull. 2014, 52, 37–41. [Google Scholar] [CrossRef]
- Yu, L.; Gan, M.; Ma, L.; Huang, H.; Hu, H.; Li, Y.; Tu, Y.; Ge, C.; Yang, F.; Yan, J. Facile synthesis of MnO2/polyaniline nanorod arrays based on graphene and its electrochemical performance. Synth. Met. 2014, 198, 167–174. [Google Scholar] [CrossRef]
- Yao, W.; Zhou, H.; Lu, Y. Synthesis and property of novel MnO2@polypyrrole coaxial nanotubes as electrode material for supercapacitors. J. Power Sources 2013, 241, 359–366. [Google Scholar] [CrossRef]
- Wang, J.-G.; Yang, Y.; Huang, Z.-H.; Kang, F. MnO2/polypyrrole nanotubular composites: Reactive template synthesis, characterization and application as superior electrode materials for high-performance supercapacitors. Electrochim. Acta 2014, 130, 642–649. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhou, H.; Long, X.; Guo, E.; Wang, X. Electrodeposition of hierarchical manganese oxide on metal nanoparticles decorated nanoporous gold with enhanced supercapacitor performance. J. Alloys Compd. 2015, 632, 376–385. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, E.; Luo, Z.; Hu, T.; Liu, T.; Li, Z.; Zhao, Q. A hydroquinone redox electrolyte for polyaniline/SnO2 supercapacitors. Electrochim. Acta 2014, 118, 106–111. [Google Scholar] [CrossRef]
- Roldán, S.; Granda, M.; Menéndez, R.; Santamaría, R.; Blanco, C. Supercapacitor modified with methylene blue as redox active electrolyte. Electrochim. Acta 2012, 83, 241–246. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Lu, A.-H.; Li, W.-C. Mesoporous manganese dioxide prepared under acidic conditions as high performance electrode material for hybrid supercapacitors. Microporous Mesoporous Mater. 2012, 153, 247–253. [Google Scholar] [CrossRef]
- Zhang, Z.; Zheng, Y.; He, P.; Sun, Z. High yield preparation of silver nanowires by CuCl2-mediated polyol method and application in semitransparent conduction electrode. Phys. E 2011, 44, 535–540. [Google Scholar] [CrossRef]
- Amirjani, A.; Marashi, P.; Fatmehsari, D.H. Effect of AgNO3 addition rate on aspect ratio of CuCl2–mediated synthesized silver nanowires using response surface methodology. Colloid Surface A 2014, 444, 33–39. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, S.; Li, F.; Liu, K.; Liu, L. Hybrids of silver nanowires and silica nanoparticles as morphology controlled conductive filler applied in flexible conductive nanocomposites. Compos. A 2015, 73, 195–203. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol-gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Tang, N.; Tian, X.; Yang, C.; Pi, Z. Facile synthesis of α-MnO2 nanostructures for supercapacitors. Mater. Res. Bull. 2009, 44, 2062–2067. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Chen, Y.; Ma, Y. Self-template route to MnO2 hollow structures for supercapacitors. Mater. Lett. 2010, 64, 1480–1482. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wang, C. Influence of surfactant on the capacitive performance of manganese dioxide prepared at different temperatures. Energy Convers. Manag. 2013, 74, 286–292. [Google Scholar] [CrossRef]
- Jiang, R.; Huang, T.; Liu, J.; Zhuang, J.; Yu, A. A novel method to prepare nanostructured manganese dioxide and its electrochemical properties as a supercapacitor electrode. Electrochim. Acta 2009, 54, 3047–3052. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Liu, C.; Jiang, H. Influence of surfactant CTAB on the electrochemical performance of manganese dioxide used as supercapacitor electrode material. J. Alloys Compd. 2012, 517, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Qi, L.; Lu, L.; Wang, H. Cotton-assisted preparation of mesoporous manganese oxide for supercapacitors. RSC Adv. 2012, 2, 6741–6743. [Google Scholar] [CrossRef]
- Rusi, S.R.M. Synthesis of MnO2 particles under slow cooling process and their capacitive performances. Mater. Lett. 2013, 108, 69–71. [Google Scholar] [CrossRef]
- Wang, H.-Q.; Yang, G.-F.; Li, Q.-Y.; Zhong, X.-X.; Wang, F.-P.; Li, Z.-S.; Li, Y.-H. Porous nano-MnO2: Large scale synthesis via a facile quick-redox procedure and application in a supercapacitor. New J. Chem. 2011, 35, 469–475. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Shen, C.; Wang, J.; Kang, F. Nanostructured manganese dioxides as active materials for micro-supercapacitors. Micro Nano Lett. 2012, 7, 744–748. [Google Scholar] [CrossRef]
- Subramanian, V.; Zhu, H.; Wei, B. Alcohol-assisted room temperature synthesis of different nanostructured manganese oxides and their pseudocapacitance properties in neutral electrolyte. Chem. Phys. Lett. 2008, 453, 242–249. [Google Scholar] [CrossRef]
- Lee, H.Y.; Goodenough, J.B. Supercapacitor Behavior with KCl Electrolyte. J. Solid State Chem. 1999, 144, 220–223. [Google Scholar] [CrossRef]
- Kundu, M.; Liu, L. Direct growth of mesoporous MnO2 nanosheet arrays on nickel foam current collectors for high-performance pseudocapacitors. J. Power Sources 2013, 243, 676–681. [Google Scholar] [CrossRef]
- Ming, B.; Li, J.; Kang, F.; Pang, G.; Zhang, Y.; Chen, L.; Xu, J.; Wang, X. Microwave-hydrothermal synthesis of birnessite-type MnO2 nanospheres as supercapacitor electrode materials. J. Power Sources 2012, 198, 428–431. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, P.; Xie, S.-S. ZnO-template-mediated synthesis of three-dimensional coral-like MnO2 nanostructure for supercapacitors. J. Power Sources 2013, 239, 393–398. [Google Scholar] [CrossRef]
- Babakhani, B.; Ivey, D.G. Effect of electrodeposition conditions on the electrochemical capacitive behavior of synthesized manganese oxide electrodes. J. Power Sources 2011, 196, 10762–10774. [Google Scholar] [CrossRef]
- Chou, S.; Cheng, F.; Chen, J. Electrodeposition synthesis and electrochemical properties of nanostructured γ-MnO2 films. J. Power Sources 2006, 162, 727–734. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Cheng, J.; Fan, Z.; Zhang, M. Preparation and electrochemical properties of lamellar MnO2 for supercapacitors. Mater. Res. Bull. 2010, 45, 210–215. [Google Scholar] [CrossRef]
- Song, Z.; Liu, W.; Zhao, M.; Zhang, Y.; Liu, G.; Yu, C.; Qiu, J. A facile template-free synthesis of α-MnO2 nanorods for supercapacitor. J. Alloys Compd. 2013, 560, 151–155. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Liu, E.-H.; Li, L.-M.; Huang, Z.-Z.; Shen, H.-J.; Xiang, X.-X. Nanostructured amorphous MnO2 prepared by reaction of KMnO4 with triethanolamine. J. Alloys Compd. 2010, 505, 555–559. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Melo, J.S. Redox additive/active electrolytes: A novel approach to enhance the performance of supercapacitors. J. Mater. Chem. A 2013, 1, 12386–12394. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Lee, Y.S.; Melo, J.S. Electric double layer capacitor and its improved specific capacitance using redox additive electrolyte. J. Mater. Chem. A 2013, 1, 1086–1095. [Google Scholar] [CrossRef]
- Lee, Y.W.; Do, K.; Lee, T.H.; Jeon, S.S.; Yoon, W.J.; Kim, C.; Ko, J.; Im, S.S. Iodine vapor doped polyaniline nanoparticles counter electrodes for dye-sensitized solar cells. Synth. Met. 2013, 174, 6–13. [Google Scholar] [CrossRef]
- Wan, C.; Yuan, L.; Ye, X.; Wu, F. Silver chloride micelle-induced tuning of pseudocapacitive manganese dioxide. Electrochim. Acta 2014, 147, 712–719. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, M.; Cheng, J.; Gong, Y.; Li, X.; Chi, B.; Pu, J.; Jian, L. Silver decorated beta-manganese oxide nanorods as an effective cathode electrocatalyst for rechargeable lithium-oxygen battery. J. Alloys Compd. 2015, 626, 173–179. [Google Scholar] [CrossRef]
- Wu, X.M.; He, Z.Q.; Chen, S.; Ma, M.Y.; Xiao, Z.B.; Liu, J.B. Silver-doped lithium manganese oxide thin films prepared by solution deposition. Mater. Lett. 2006, 60, 2497–2500. [Google Scholar] [CrossRef]
- Gamby, J.; Taberna, P.L.; Simon, P.; Fauvarque, J.F.; Chesneau, M. Studies and characterisations of various activated carbons used for carbon/carbon supercapacitors. J. Power Sources 2001, 101, 109–116. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Yu, L.-L.; Zhao, J.-T. 3D network mesoporous beta-manganese dioxide: Template-free synthesis and supercapacitive performance. J. Power Sources 2014, 270, 411–417. [Google Scholar] [CrossRef]
- Zeng, Z.; Long, X.; Zhou, H.; Guo, E.; Wang, X.; Hu, Z. On-chip interdigitated supercapacitor based on nano-porous gold/manganese oxide nanowires hybrid electrode. Electrochim. Acta 2015, 163, 107–115. [Google Scholar] [CrossRef]
- Khomenko, V.; Raymundo-Piñero, E.; Béguin, F. Optimisation of an asymmetric manganese oxide/activated carbon capacitor working at 2 V in aqueous medium. J. Power Sources 2006, 153, 183–190. [Google Scholar] [CrossRef]
- Yousefi, T.; Davarkhah, R.; Golikand, A.N.; Mashhadizadeh, M.H. Synthesis, characterization, and supercapacitor studies of manganese (IV) oxide nanowires. Mater. Sci. Semicond. Process. 2013, 16, 868–876. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, C.; Min, S.; Qian, X. Facile Synthesis of Cu2O/RGO/Ni(OH)2 Nanocomposite and Its Double Synergistic Effect on Supercapacitor Performance. Electrochim. Acta 2015, 165, 314–322. [Google Scholar] [CrossRef]
- Gong, H.; Wang, X.; Zhang, S.; Li, L. Synergistic effect of rare-earth elements on the dielectric properties and reliability of batio3-based ceramics for multilayer ceramic capacitors. Mater. Res. Bull. 2016, 73, 233–239. [Google Scholar] [CrossRef]
- Korte, K.E.; Skrabalak, S.E.; Xia, Y. Rapid synthesis of silver nanowires through a CuCl- or CuCl2-mediated polyol process. J. Mater. Chem. 2008, 18, 437–441. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Cui, X.; Zu, L.; Hu, Z.; Gan, J.; Lian, H.; Liu, Y.; Xing, G. High Rate Performance Nanocomposite Electrode of Mesoporous Manganese Dioxide/Silver Nanowires in KI Electrolytes. Nanomaterials 2015, 5, 1638-1653. https://doi.org/10.3390/nano5041638
Jiang Y, Cui X, Zu L, Hu Z, Gan J, Lian H, Liu Y, Xing G. High Rate Performance Nanocomposite Electrode of Mesoporous Manganese Dioxide/Silver Nanowires in KI Electrolytes. Nanomaterials. 2015; 5(4):1638-1653. https://doi.org/10.3390/nano5041638
Chicago/Turabian StyleJiang, Yanhua, Xiuguo Cui, Lei Zu, Zhongkai Hu, Jing Gan, Huiqin Lian, Yang Liu, and Guangjian Xing. 2015. "High Rate Performance Nanocomposite Electrode of Mesoporous Manganese Dioxide/Silver Nanowires in KI Electrolytes" Nanomaterials 5, no. 4: 1638-1653. https://doi.org/10.3390/nano5041638