Synthesis of Mesoporous Metal Oxides by Structure Replication: Thermal Analysis of Metal Nitrates in Porous Carbon Matrices
Abstract
:1. Introduction
2. Results and Discussion
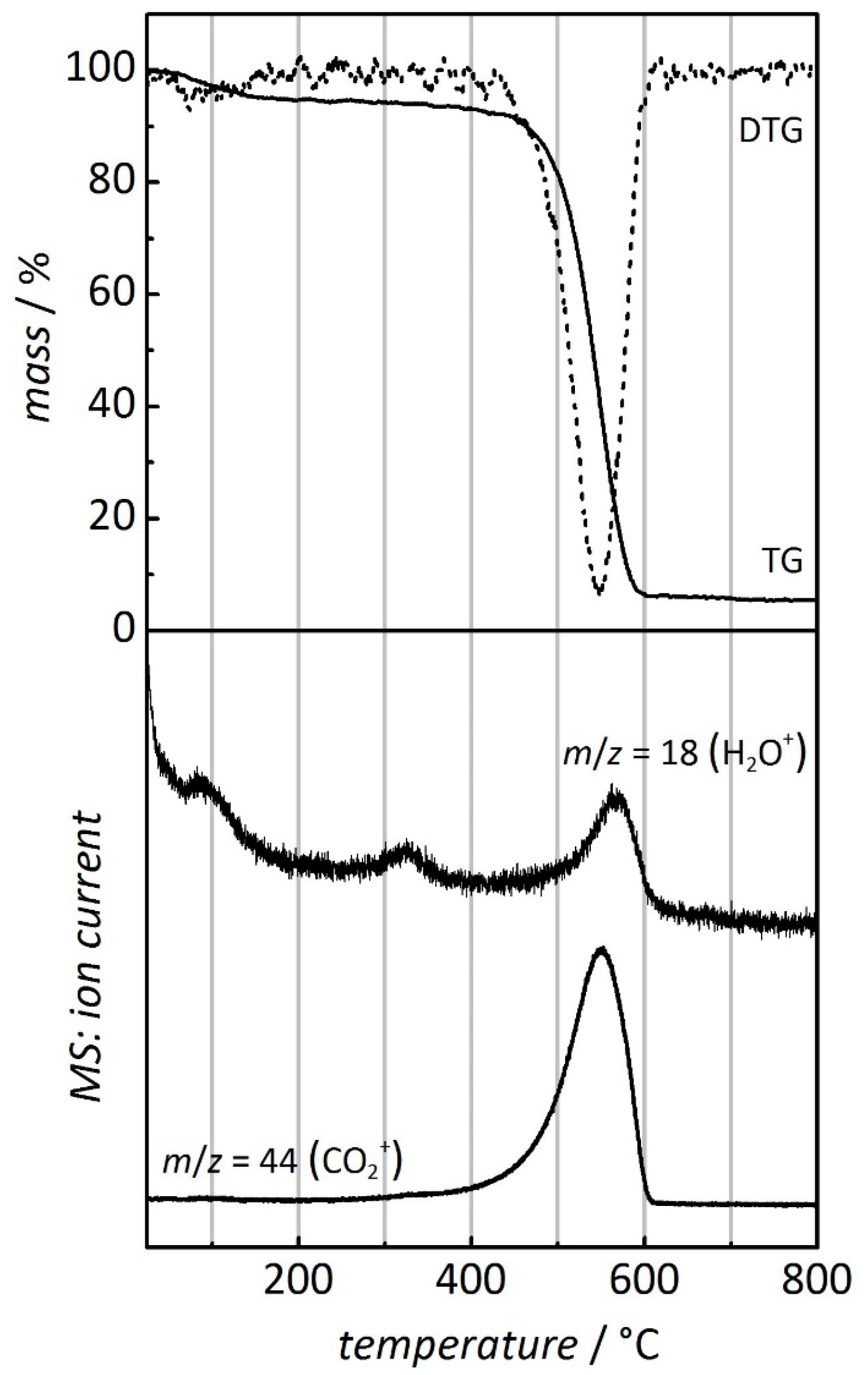
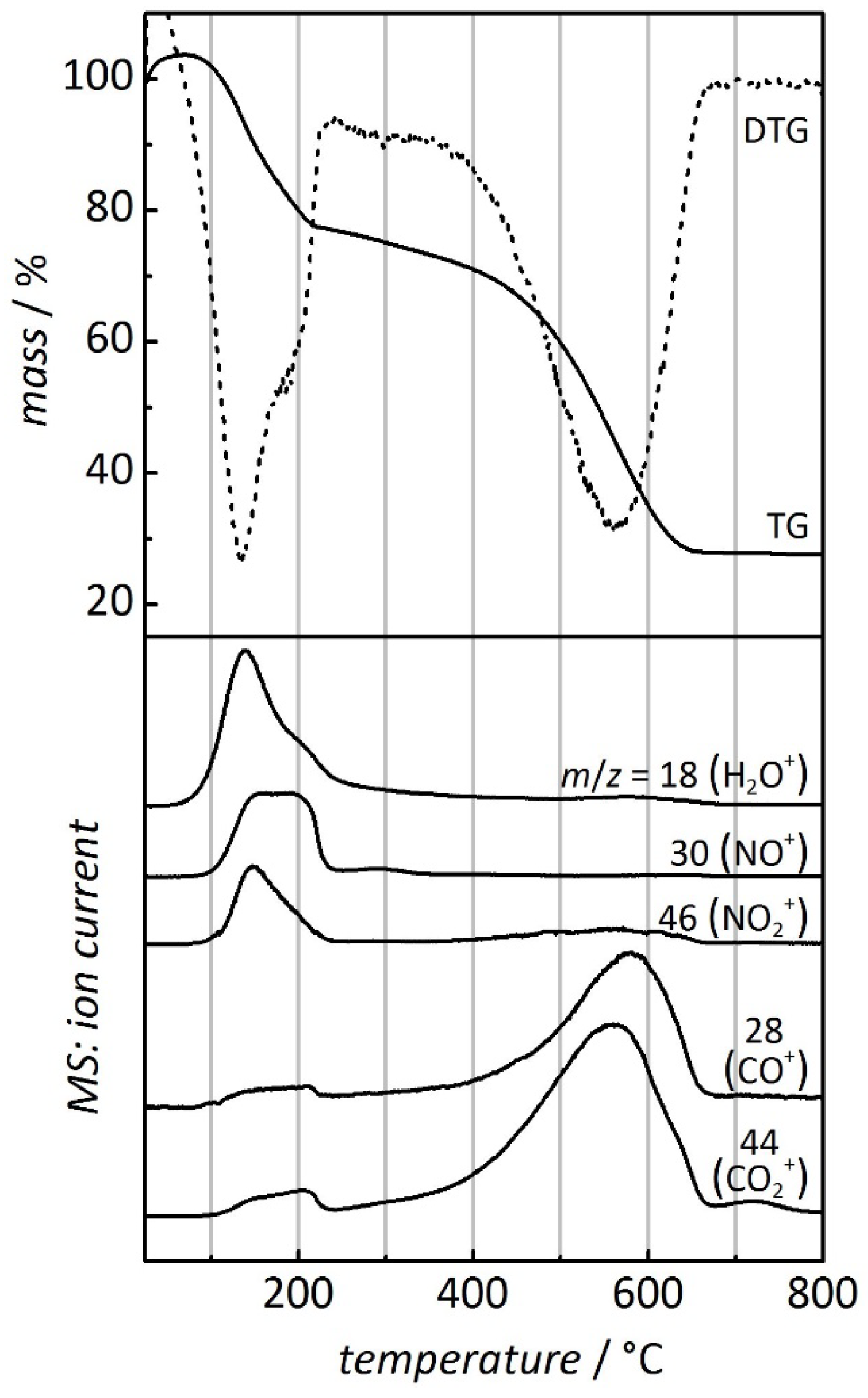
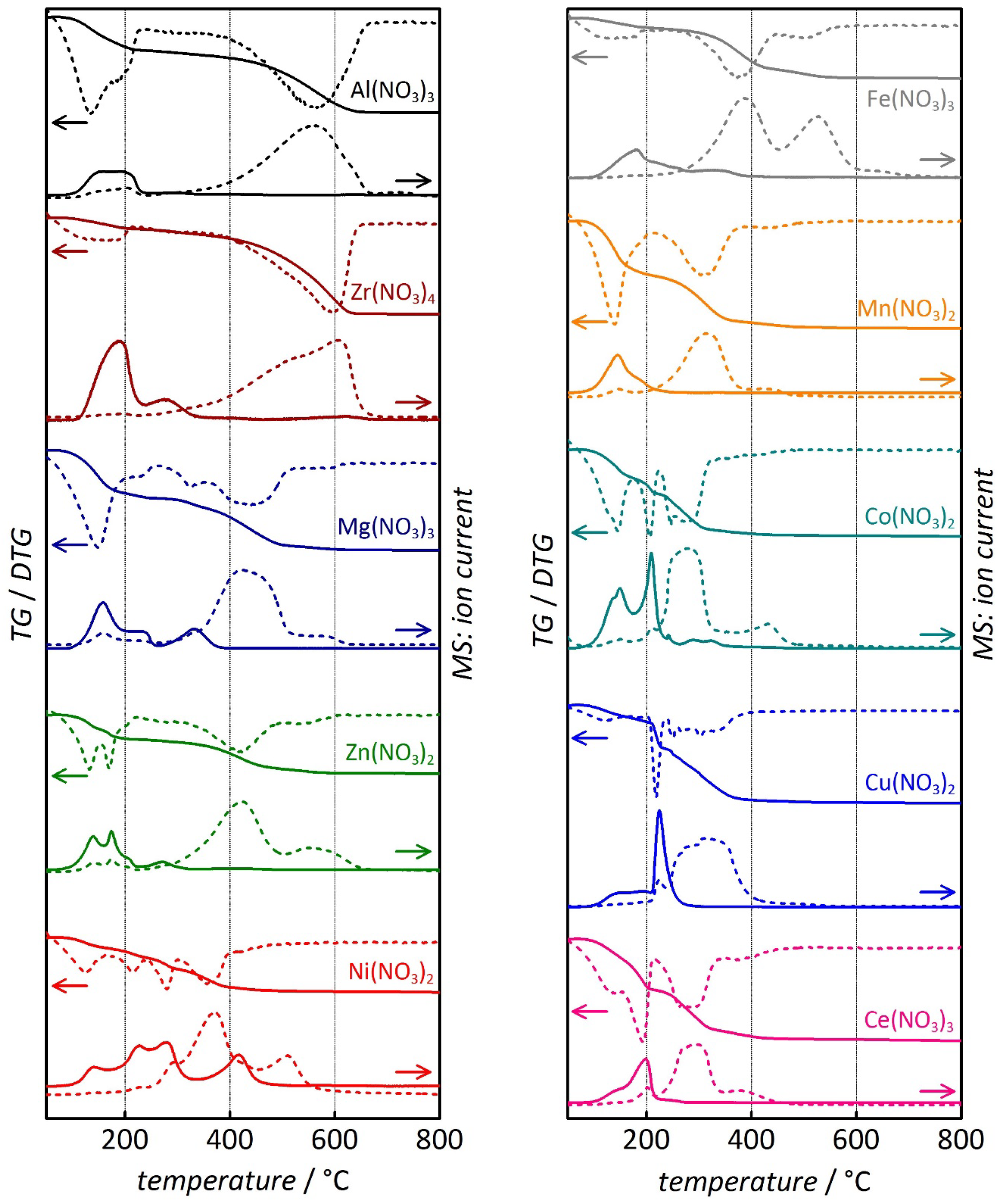
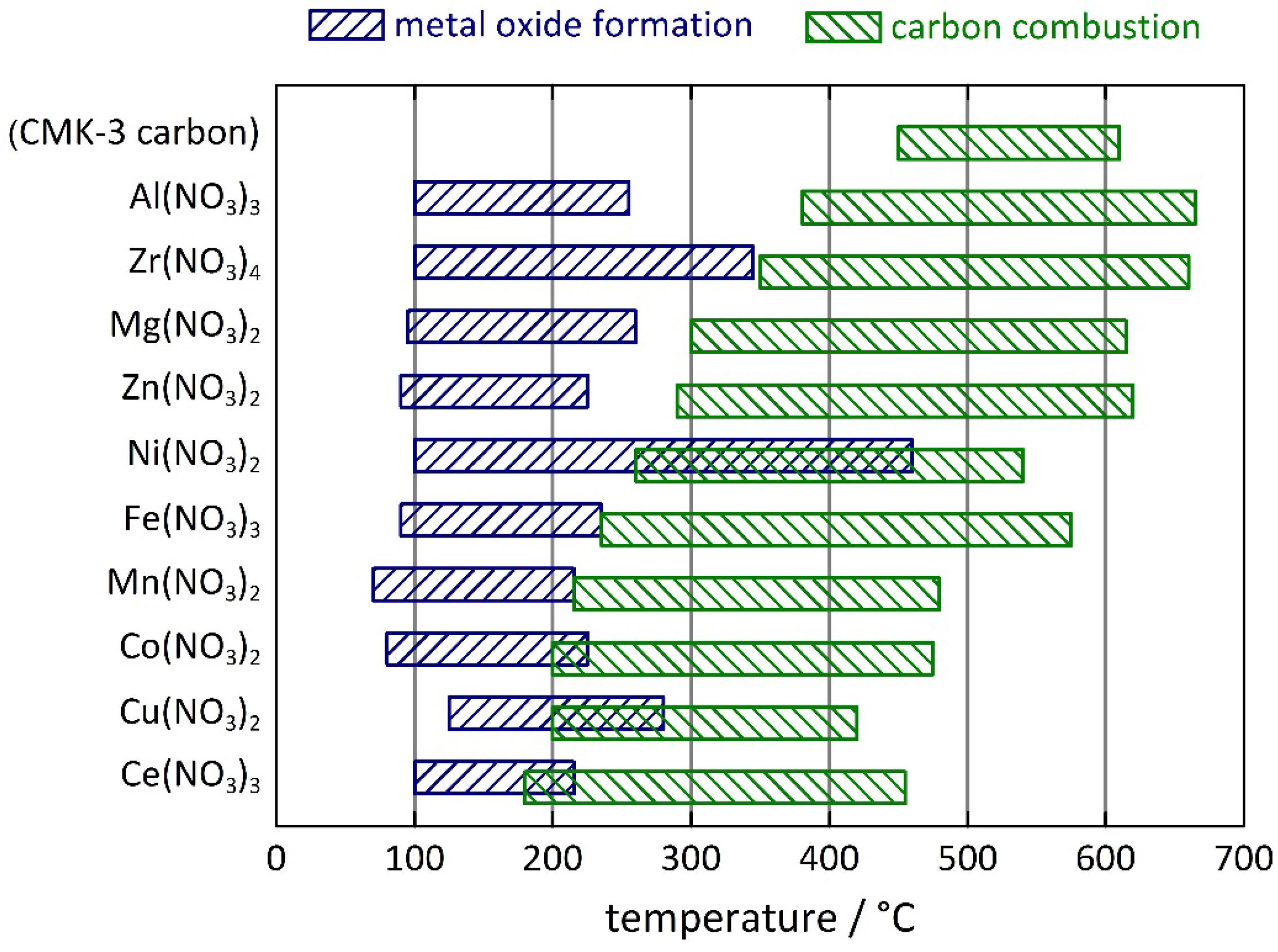
| Sample | Temperature (°C) of metal oxide formation | Temperature (°C) of carbon combustion | |||
|---|---|---|---|---|---|
| onset | offset | Literature * | onset | offset | |
| CMK-3 carbon | - | - | - | 450 | 610 |
| Al(NO3)3 | 100 | 255 | 167 | 380 | 665 |
| Zr(NO3)4 | 100 | 345 | - | 350 | 660 |
| Mg(NO3)2 | 95 | 260 | 450 | 300 | 615 |
| Zn(NO3)2 | 90 | 225 | 317 | 290 | 620 |
| Ni(NO3)2 | 100 | 460 | 307 | 260 | 540 |
| Fe(NO3)3 | 90 | 235 | 167 | 235 | 575 |
| Mn(NO3)2 | 70 | 215 | 207 | 215 | 480 |
| Co(NO3)2 | 80 | 225 | 242 | 200 | 475 |
| Cu(NO3)2 | 125 | 280 | 247 | 200 | 420 |
| Ce(NO3)3 | 100 | 215 | 297 | 180 | 455 |
3. Experimental Section
3.1. Synthesis of SBA-15 Silica
3.2. Synthesis of CMK-3 Carbon
3.3. Impregnation of CMK-3 Carbon with Metal Nitrates
3.4. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Schüth, F. Endo- and Exotemplating to Create High-Surface-Area Inorganic Materials. Angew. Chem. Int. Ed. 2003, 42, 3604–3622. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.H.; Schüth, F. Nanocasting: A Versatile Strategy for Creating Nanostructured Porous Materials. Adv. Mater. 2006, 18, 1793–1805. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, D. Synthesis of Replica Mesostructures by the Nanocasting Strategy. J. Mater. Chem. 2005, 15, 1217–1231. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, H.; Zhao, D. “Host-Guest” Chemistry in the Synthesis of Ordered Nonsiliceous Mesoporous Materials. Acc. Chem. Res. 2006, 39, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, M. Repeated Templating. Chem. Mater. 2007, 20, 961–971. [Google Scholar] [CrossRef]
- Wagner, T.; Haffer, S.; Weinberger, C.; Klaus, D.; Tiemann, M. Mesoporous Materials as Gas Sensors. Chem. Soc. Rev. 2013, 42, 4036–4053. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered Mesoporous Metal Oxides: Synthesis and Applications. Chem. Soc. Rev. 2012, 41, 4909–4927. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Schüth, F. Synthesis of Non-Siliceous Mesoporous Oxides. Chem. Soc. Rev. 2014, 43, 313–344. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Joo, S.H.; Ryoo, R.; Kruk, M.; Jaroniec, M.; Liu, Z.; Ohsuna, T.; Terasaki, O. Synthesis of New, Nanoporous Carbon with Hexagonally Ordered Mesostructure. J. Am. Chem. Soc. 2000, 122, 10712–10713. [Google Scholar] [CrossRef]
- Weinberger, C.; Haffer, S.; Wagner, T.; Tiemann, M. Fructose as a Precursor for Mesoporous Carbon: Straightforward Solvent-Free Synthesis by Nanocasting. In The Science and Function of Nanomaterials: From Synthesis to Application; Harper-Leatherman, A., Solbrig, C.M., Eds.; ACS: Washington, DC, USA, 2014; pp. 3–12. [Google Scholar]
- Weinberger, C.; Haffer, S.; Wagner, T.; Tiemann, M. Fructose and Urea as Precursors for N-/O-Modified Mesoporous Carbon with Enhanced Sorption Capacity for Heavy Metal Ions. Eur. J. Inorg. Chem. 2014, 2014, 2787–2792. [Google Scholar] [CrossRef]
- Roggenbuck, J.; Tiemann, M. Ordered Mesoporous Magnesium Oxide with High Thermal Stability Synthesized by Exotemplating Using CMK-3 Carbon. J. Am. Chem. Soc. 2005, 127, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, J.; Koch, G.; Tiemann, M. Synthesis of Mesoporous Magnesium Oxide by CMK-3 Carbon Structure Replication. Chem. Mater. 2006, 18, 4151–4156. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Roggenbuck, J.; Tiemann, M.; Ivanova, L.; Paneva, D.; Mitov, I.; Minchev, C. Iron oxide nanoparticles supported on mesoporous MgO and CeO2: A comparative physicochemical and catalytic study. Microporous Mesoporous Mater. 2007, 110, 339–346. [Google Scholar] [CrossRef]
- Waitz, T.; Tiemann, M.; Klar, P.J.; Sann, J.; Stehr, J.; Meyer, B.K. Crystalline ZnO with an Enhanced Surface Area Obtained by Nanocasting. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Wagner, T.; Waitz, T.; Roggenbuck, J.; Fröba, M.; Kohl, C.D.; Tiemann, M. Ordered Mesoporous ZnO for Gas Sensing. Thin Solid Films 2007, 515, 8360–8363. [Google Scholar] [CrossRef]
- Schwalm, M.; Horst, S.; Chernikov, A.; Rühle, W.W.; Lautenschläger, S.; Klar, P.J.; Meyer, B.; Waitz, T.; Tiemann, M.; Chatterjee, S. Time-Resolved Photoluminescence Study of in ZnO Nanostructures. Phys. Status Solidi C 2009, 6, 542–545. [Google Scholar] [CrossRef]
- Chernikov, A.; Horst, S.; Waitz, T.; Tiemann, M.; Chatterjee, S. Photoluminescence Properties of Ordered Mesoporous ZnO. J. Phys. Chem. C 2011, 115, 1375–1379. [Google Scholar] [CrossRef]
- Polarz, S.; Orlov, A.V.; Schüth, F.; Lu, A.-H. Preparation of High-Surface-Area Zinc Oxide with Ordered Porosity, Different Pore Sizes, and Nanocrystalline Walls. Chem. Eur. J. 2007, 13, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, J.; Schäfer, H.; Tsoncheva, T.; Minchev, C.; Hanss, J.; Tiemann, M. Mesoporous CeO2: Synthesis by Nanocasting, Characterisation and Catalytic Properties. Microporous Mesoporous Mater. 2007, 101, 335–341. [Google Scholar] [CrossRef]
- Dong, A.; Ren, N.; Tang, Y.; Wang, Y.; Zhang, Y.; Hua, W.; Gao, Z. General Synthesis of Mesoporous Spheres of Metal Oxides and Phosphates. J. Am. Chem. Soc. 2003, 125, 4976–4978. [Google Scholar] [CrossRef] [PubMed]
- Haffer, S.; Weinberger, C.; Tiemann, M. Mesoporous Al2O3 by Nanocasting: Relationship between Crystallinity and Mesoscopic Order. Eur. J. Inorg. Chem. 2012, 3283–3288. [Google Scholar] [CrossRef]
- Birnbaum, W.; Weinberger, C.; Schill, V.; Haffer, S.; Tiemann, M.; Kuckling, D. Synthesis of Mesoporous Alumina through Photo Cross-Linked Poly(dimethylacrylamide) Hydrogels. Colloid Polym. Sci. 2014, 292, 3055–3060. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, A.; Wang, X.; Zhang, T. Ordered Crystalline Alumina Molecular Sieves Synthesized via a Nanocasting Route. Chem. Mater. 2006, 18, 5153–5155. [Google Scholar] [CrossRef]
- Lai, X.; Li, X.; Geng, W.; Tu, J.; Li, J.; Qiu, S. Ordered Mesoporous Copper Oxide with Crystalline Walls. Angew. Chem. Int. Ed. 2007, 46, 738–741. [Google Scholar] [CrossRef] [PubMed]
- Roggenbuck, J.; Waitz, T.; Tiemann, M. Synthesis of Mesoporous Metal Oxides by Structure Replication: Strategies of Impregnating Porous Matrices with Metal Salts. Microporous Mesoporous Mater. 2008, 113, 575–582. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Fan-Yuan, L.; Tsong-Huei, C.; Chuin-Tih, Y. Thermal Decomposition of Metal Nitrates in Air and Hydrogen Environments. J. Phys. Chem. B 2003, 107, 1044–1047. [Google Scholar] [CrossRef]
- Sietsma, J.R.A.; Meeldijk, J.D.; den Breejen, J.P.; Versluijs-Helder, M.; van Dillen, A.J.; de Jongh, P.E.; de Jong, K.P. The Preparation of Supported NiO and Co3O4 Nanoparticles by the Nitric Oxide Controlled Thermal Decomposition of Nitrates. Angew. Chem. Int. Ed. 2007, 46, 4547–4549. [Google Scholar] [CrossRef] [PubMed]
- Schwickardi, M.; Johann, T.; Schmidt, W.; Schüth, F. High-Surface-Area Oxides Obtained by an Activated Carbon Route. Chem. Mater. 2002, 14, 3913–3919. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weinberger, C.; Roggenbuck, J.; Hanss, J.; Tiemann, M. Synthesis of Mesoporous Metal Oxides by Structure Replication: Thermal Analysis of Metal Nitrates in Porous Carbon Matrices. Nanomaterials 2015, 5, 1431-1441. https://doi.org/10.3390/nano5031431
Weinberger C, Roggenbuck J, Hanss J, Tiemann M. Synthesis of Mesoporous Metal Oxides by Structure Replication: Thermal Analysis of Metal Nitrates in Porous Carbon Matrices. Nanomaterials. 2015; 5(3):1431-1441. https://doi.org/10.3390/nano5031431
Chicago/Turabian StyleWeinberger, Christian, Jan Roggenbuck, Jan Hanss, and Michael Tiemann. 2015. "Synthesis of Mesoporous Metal Oxides by Structure Replication: Thermal Analysis of Metal Nitrates in Porous Carbon Matrices" Nanomaterials 5, no. 3: 1431-1441. https://doi.org/10.3390/nano5031431






