Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods
Abstract
:1. General Issues of Nanostructured and Nanocomposite Materials
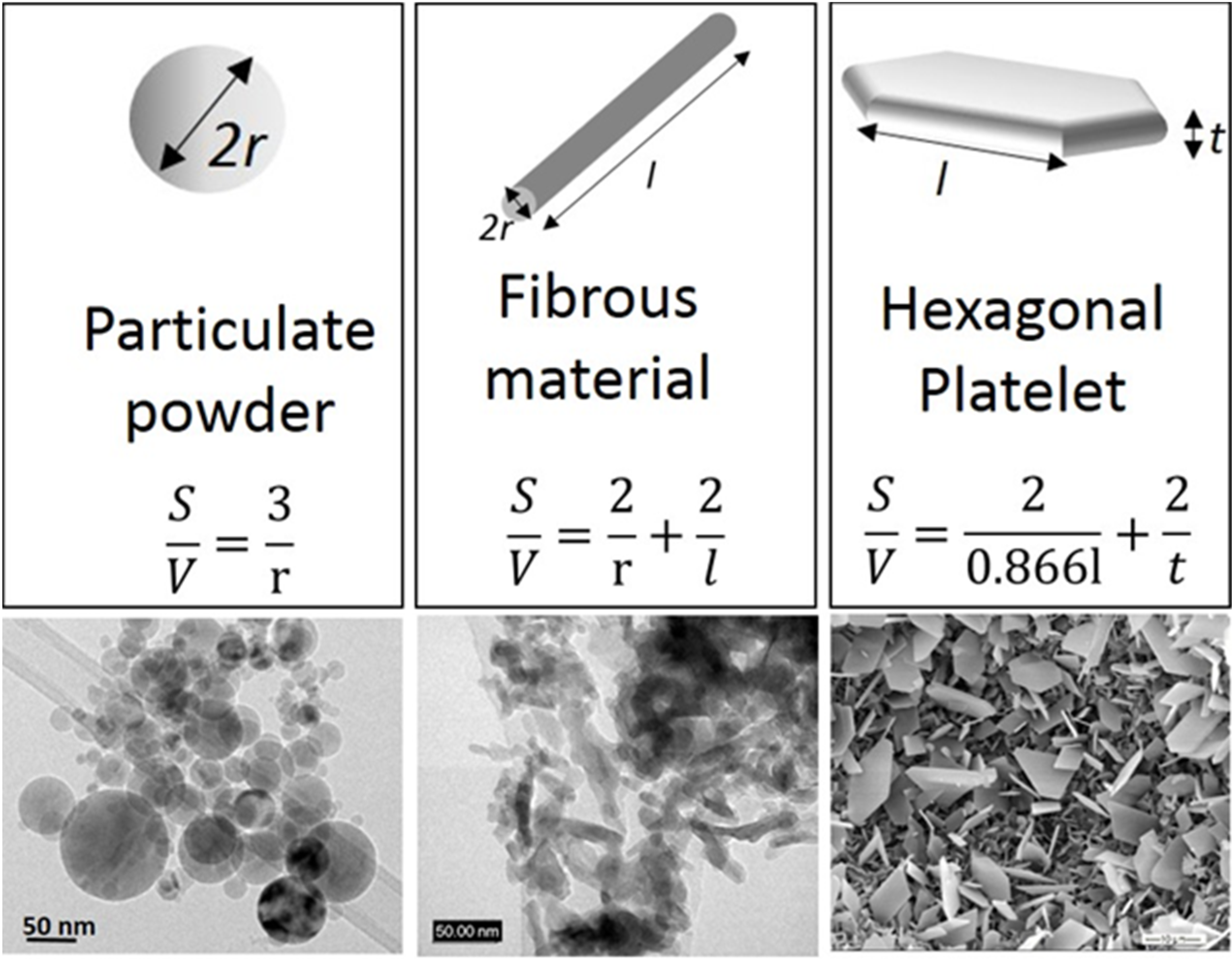
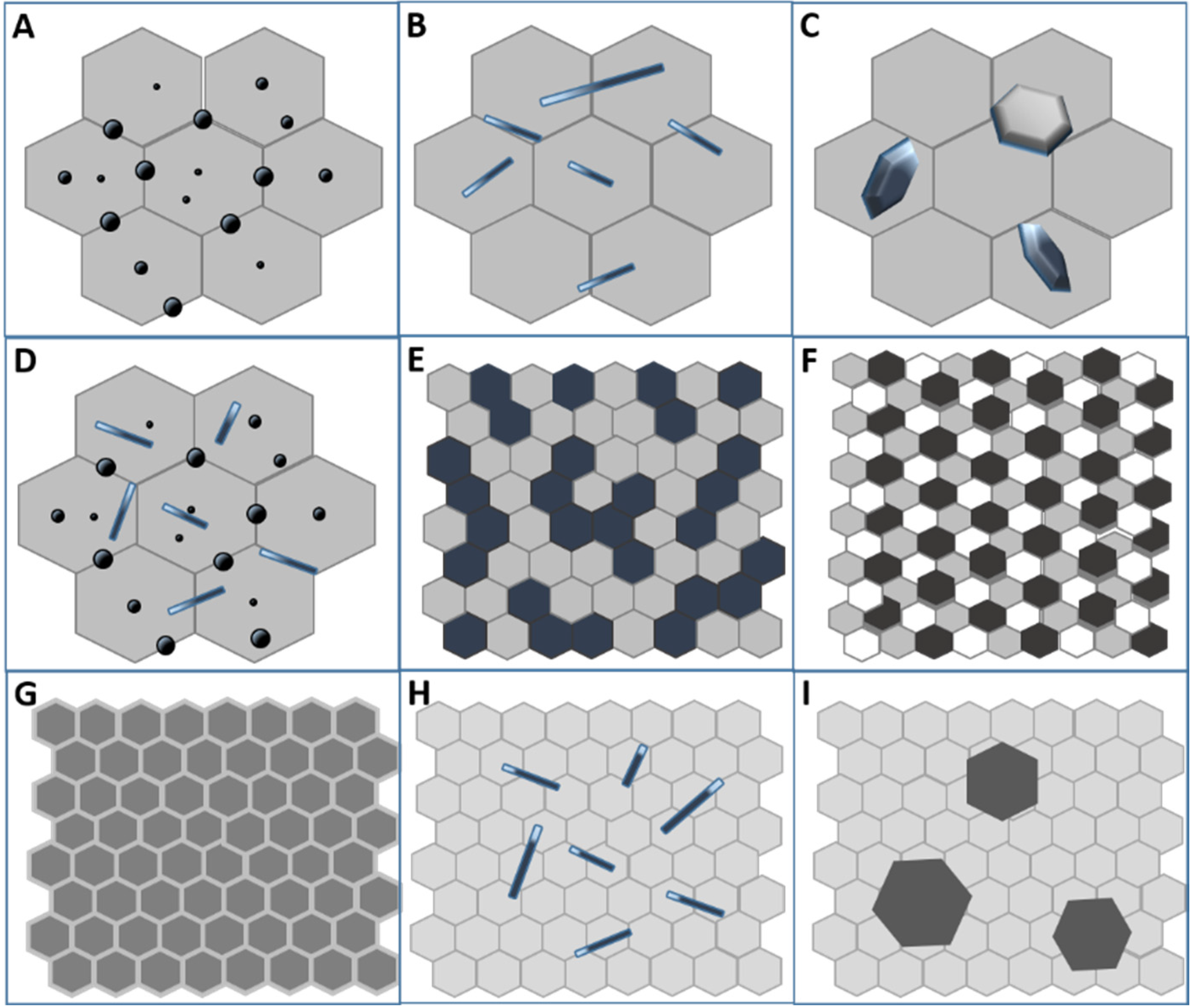
2. Why Nanostructured Ceramics?
2.1. Sintering and Grain Growth of Nanocrystalline Powders
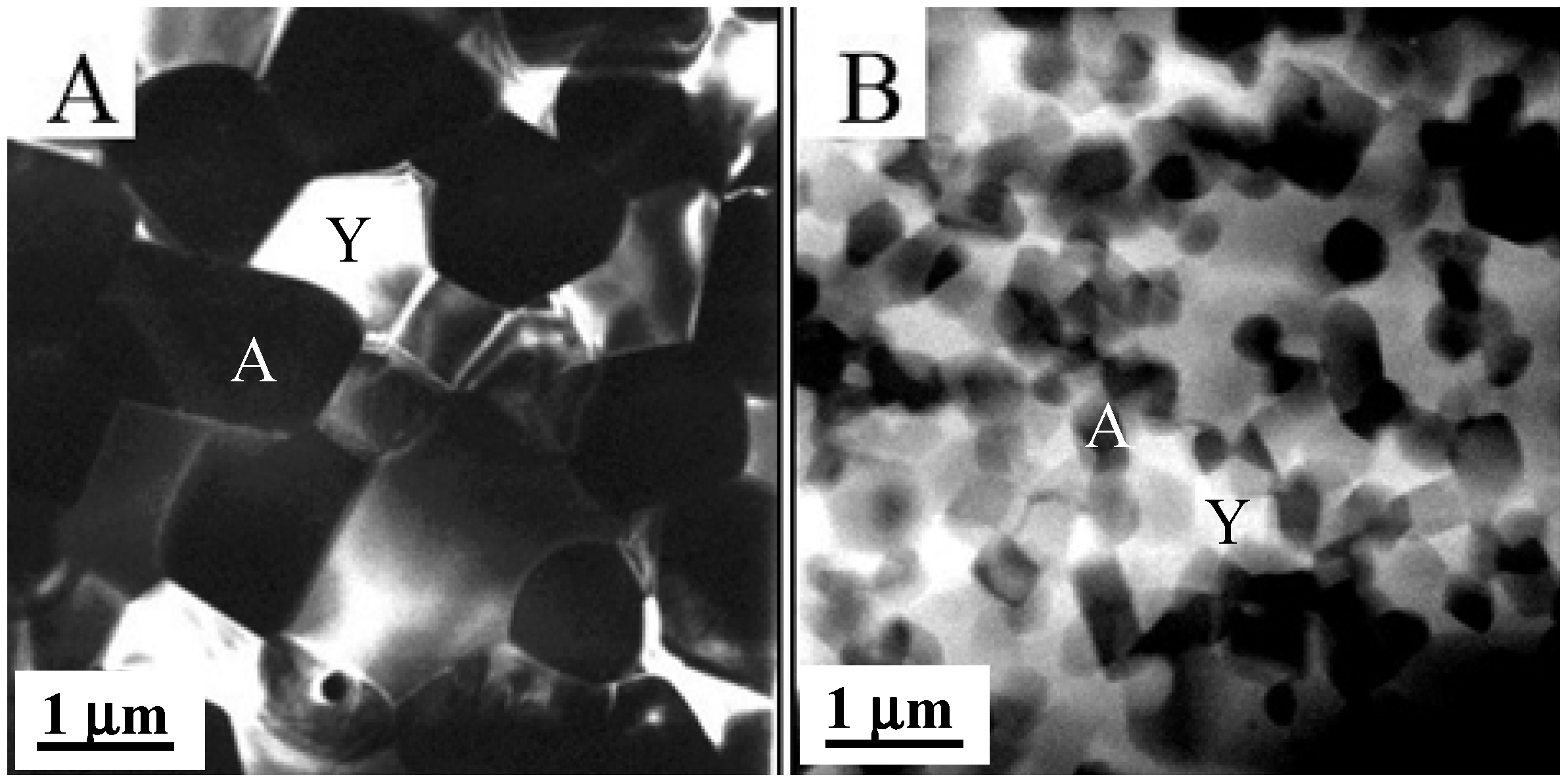
2.2. Mechanical Behavior of Nanocrystalline Ceramics
2.2.1. Hardness and Strength
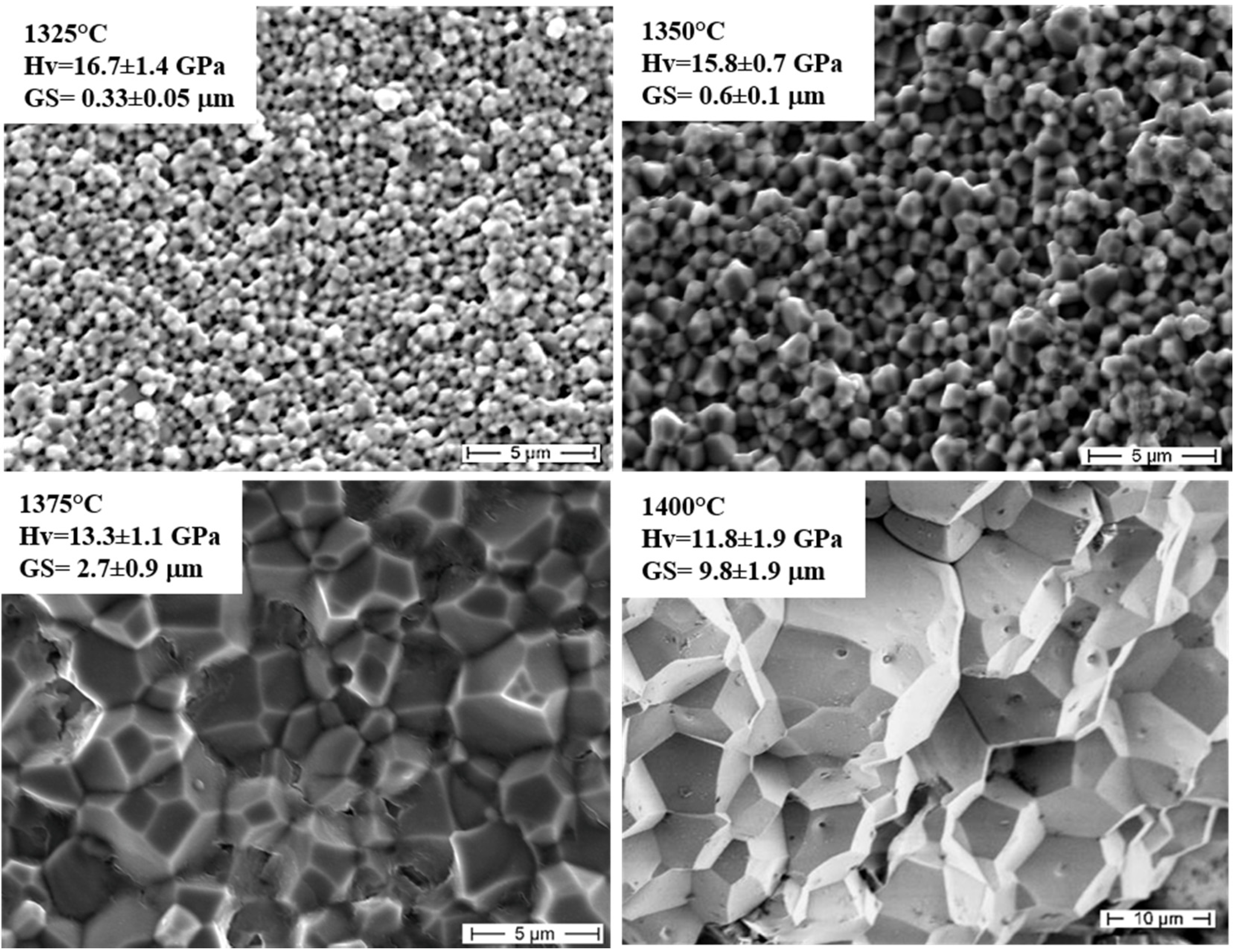
2.2.2. High-Temperature Mechanical Properties
3. Why Nanocomposites Ceramics?
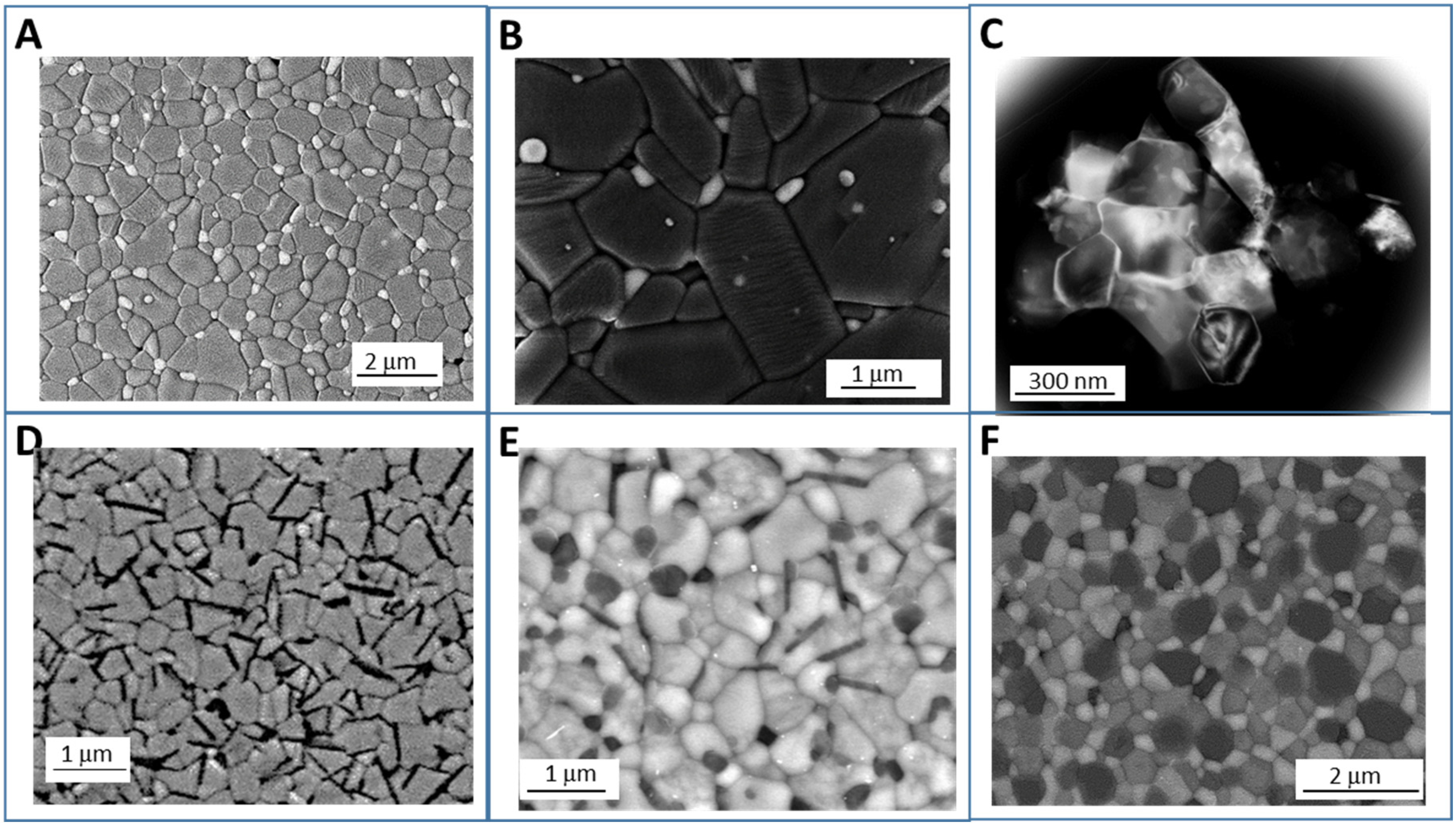
3.1. Sintering and Grain Growth of Nanocomposite Ceramics
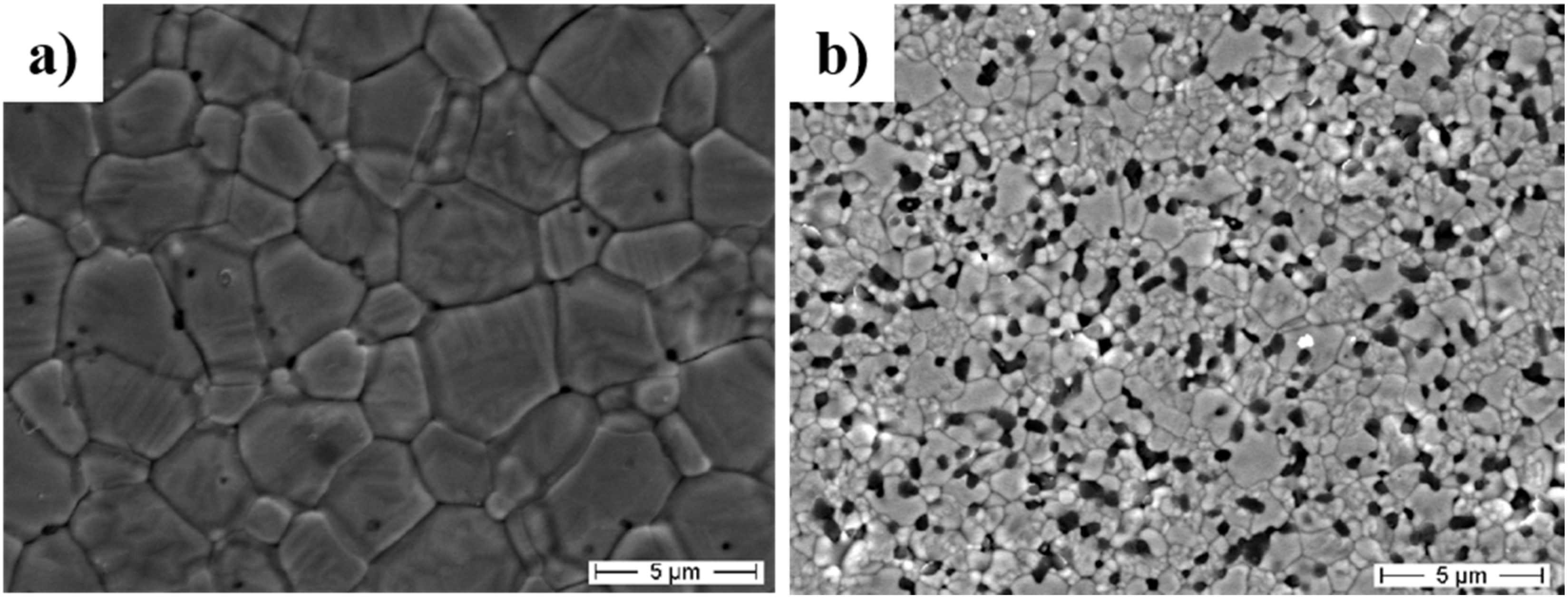
3.2. Mechanical Properties of Nanocomposite Ceramics
3.2.1. Hardness and Strength


3.2.2. Fracture Toughness
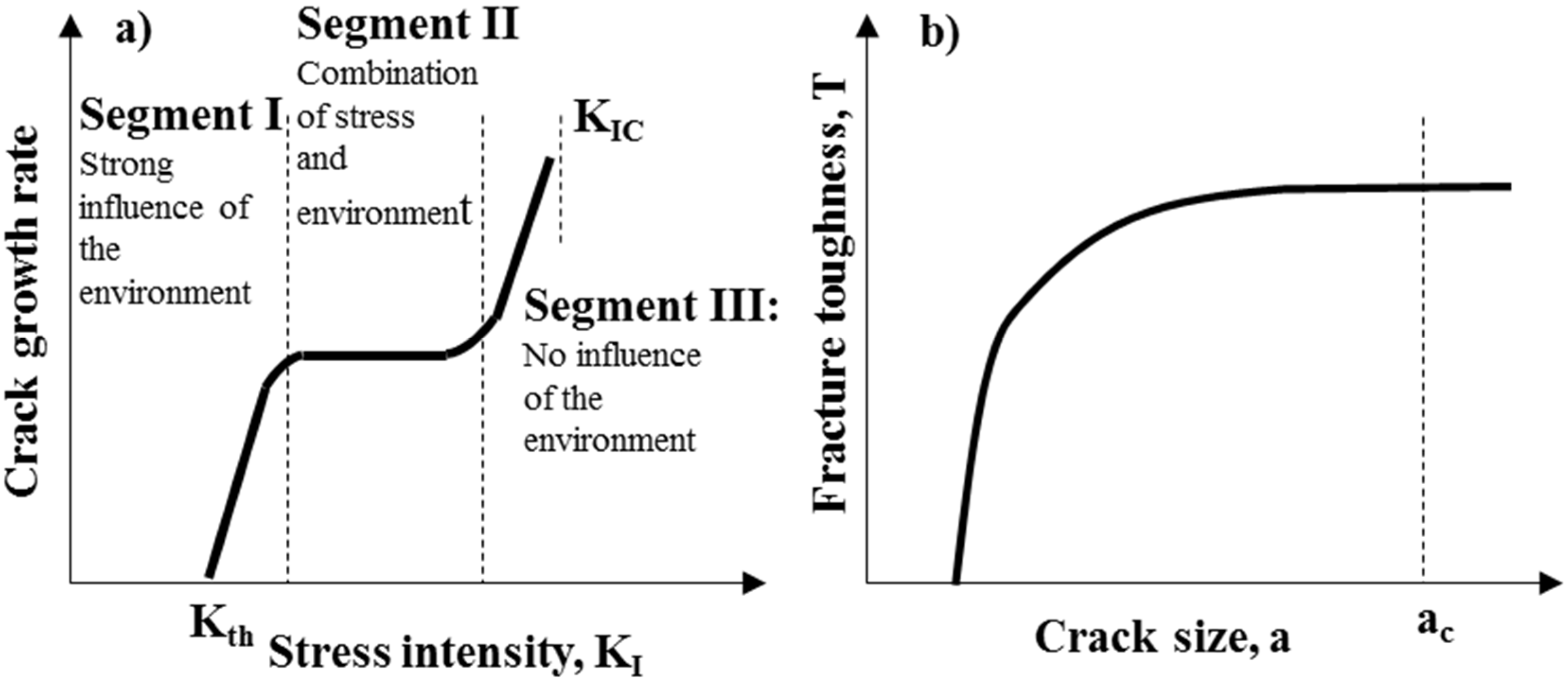
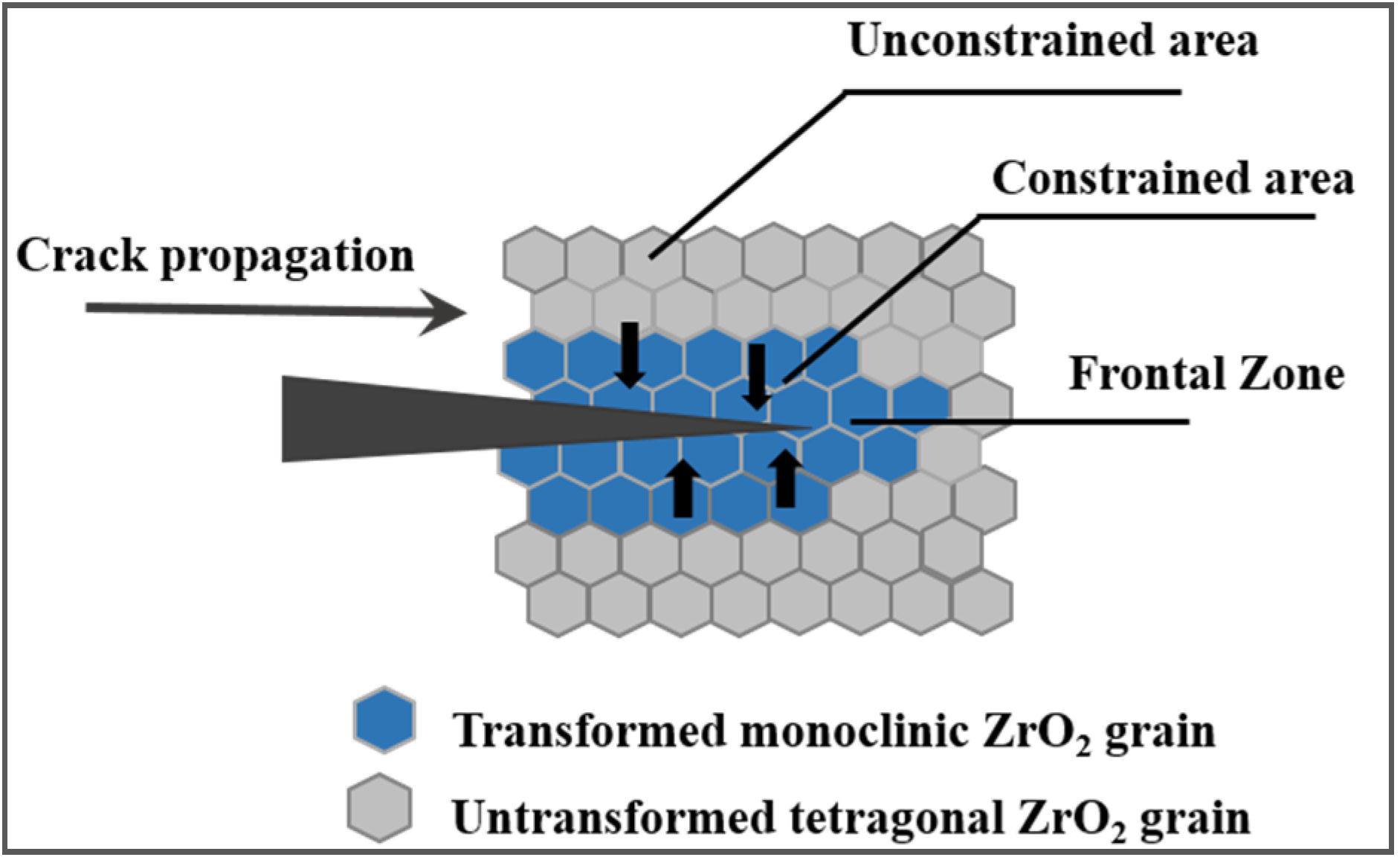
- (i)
- The ZrO2 particle size;
- (ii)
- The stabilizing phase concentration;
- (iii)
- The particle size distribution;
- (iv)
- The particle-matrix thermal expansion mismatch.
3.2.3. High-Temperature Mechanical Properties
4. Synthesis of Nanocomposite Ceramic Powders
| Synthesis Route | Type of Composite | Composition | References |
|---|---|---|---|
| Mechanochemical | Oxide/oxide | HA/MgTiO3/MgO; β-CP/MgTiO3/MgO | [101] |
| Oxide/non-oxide | Al2O3/ZrB2/ZrO2; Al2O3/TiB2 | [102,103] | |
| Non-oxide/non-oxide | B4C/SiC, NbC/NbB2 | [104,105] | |
| Polymer precursor | Oxide/non-oxide | Al2O3/SiC; Mullite/SiC | [71,106,107,108] |
| Non-oxide/non-oxide | ZrC/SiC; Si3N4/SiC | [109,110,111,112,113,114,115,116] | |
| Vapor Phase | Oxide/oxide | ZrO2/SiO2; TiO2/V2O5 | [71,117] |
| Non-oxide/non-oxide | Si3N4/SiC | [71] | |
| SHS | Oxide/non-oxide Non-oxide/non-oxide | Al2O3/SiC; Mullite/TiB2 | [118,119] [120,121,122,123,124] |
| Si3N4/TiN;Si3N4/MoSi2; Si3N4/SiC; | |||
| TiN–SiC–Si3N4; ZrB2–SiC–ZrC–ZrSi | |||
| Sol-gel | Oxide/oxide | Al2O3/ZrO2;Al2O3/Y3Al5O12; | [125,126,127,128,129,130] |
| Mullite/ZrO2; Mullite/TiO2 | |||
| Oxide/non-oxide | Al2O3/SiC; Mullite/SiCAlN/BN | [131,132,133,134] | |
| Non-oxide/non-oxide | Mullite/SiCAlN/BN | [135] | |
| Co-precipitation | Oxide/oxide | Al2O3/ZrO2;Al2O3/Y3Al5O12; ZrO2/Gd2O3; Al2O3/LaAl11O18; Ca10(PO4)6(OH)2]/Fe2O3/Mullite/Al2O3. | [34,136,137,138,139,140,141,142,143] |
| Solution combustion/ | Oxide/oxide | Al2O3/ZrO2; CeO2–MxOy; MOx–ZnO; | [73,144,145,146,147,148,149,150,151] |
| Spray decomposition | γ-Fe2O3–TiO2; Al2O3/ZrO2/MgAl2O4 | ||
| Surface modification route | Oxide/oxide | Al2O3/ZrO2; Al2O3/Y3Al5O12; Al2O3/Mullite; Al2O3/SiO2; ZrO2/MgAl2O4; Al2O3/ZrO2/Y3Al5O12; ZrO2/Al2O3/SrAl12O19 | [14,16,21,62,70,88,152,153,154,155,156,157,158,159] |
| Oxide/non-oxide | SiC/Al2O3; SiC/Y2O3 | [160] |
4.1. Conventional Powder Route
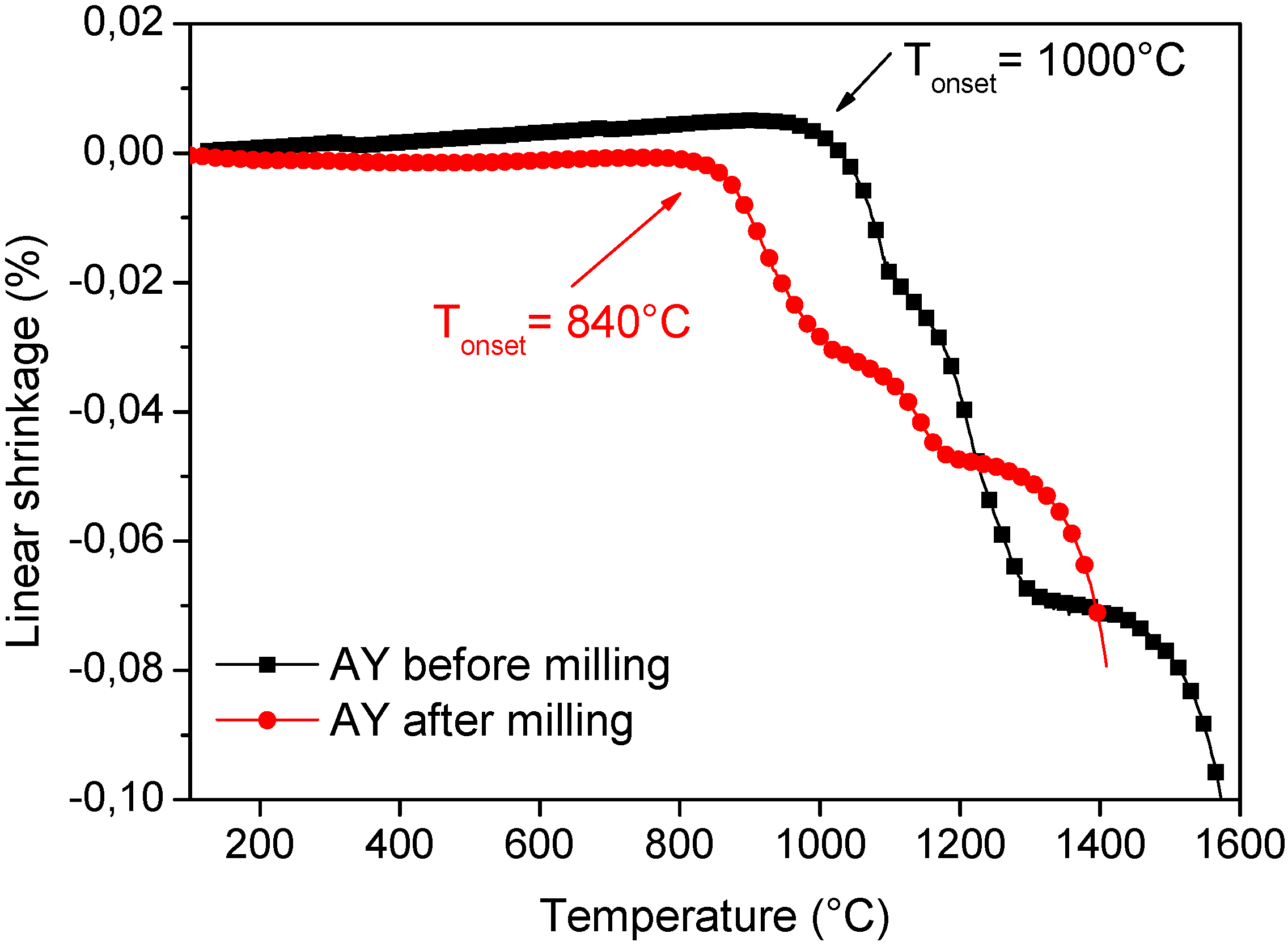
4.2. Mechanochemical Synthesis
4.3. Polymer Precursor Route
4.4. Vapor Phase Reaction Technique
4.5. Self-Propagating High-Temperature Synthesis (SHS) and Combustion Synthesis (CS)
4.6. Solution Techniques
4.6.1. Sol-Gel
| Synthesis Route | Bulk Density (g/cm3) | Vickers Hardness (Hv) | Bending Strength (MPa) |
|---|---|---|---|
| Sol-gel | 3.90 | 1385.5 | 219.8 |
| Mechanical mixing | 3.62 | 1076.5 | 184.6 |
4.6.2. Co-Precipitation
| Synthesis Route | Sintering Temperature (1500 °C) | Sintering Temperature (1650 °C) | ||
|---|---|---|---|---|
| Bending Strength (MPa) | Fracture Toughness (MPa√m) | Bending Strength (MPa) | Fracture Toughness (MPa√m) | |
| Co-precipitation | 604 ± 25 | 5.0 ± 0.5 | 402 ± 21 | 4.1 ± 0.1 |
| Precipitation | 485 ± 28 | 4.2 ± 0.5 | 284 ± 4 | 4.0 ± 0.1 |
| Milling | 432 ± 140 | 4.2 ± 0.6 | 111 ± 14 | 3.5 ± 0.4 |

4.6.3. Spray Decomposition
4.6.4. Solution Combustion
4.7. Surface Modification Methods
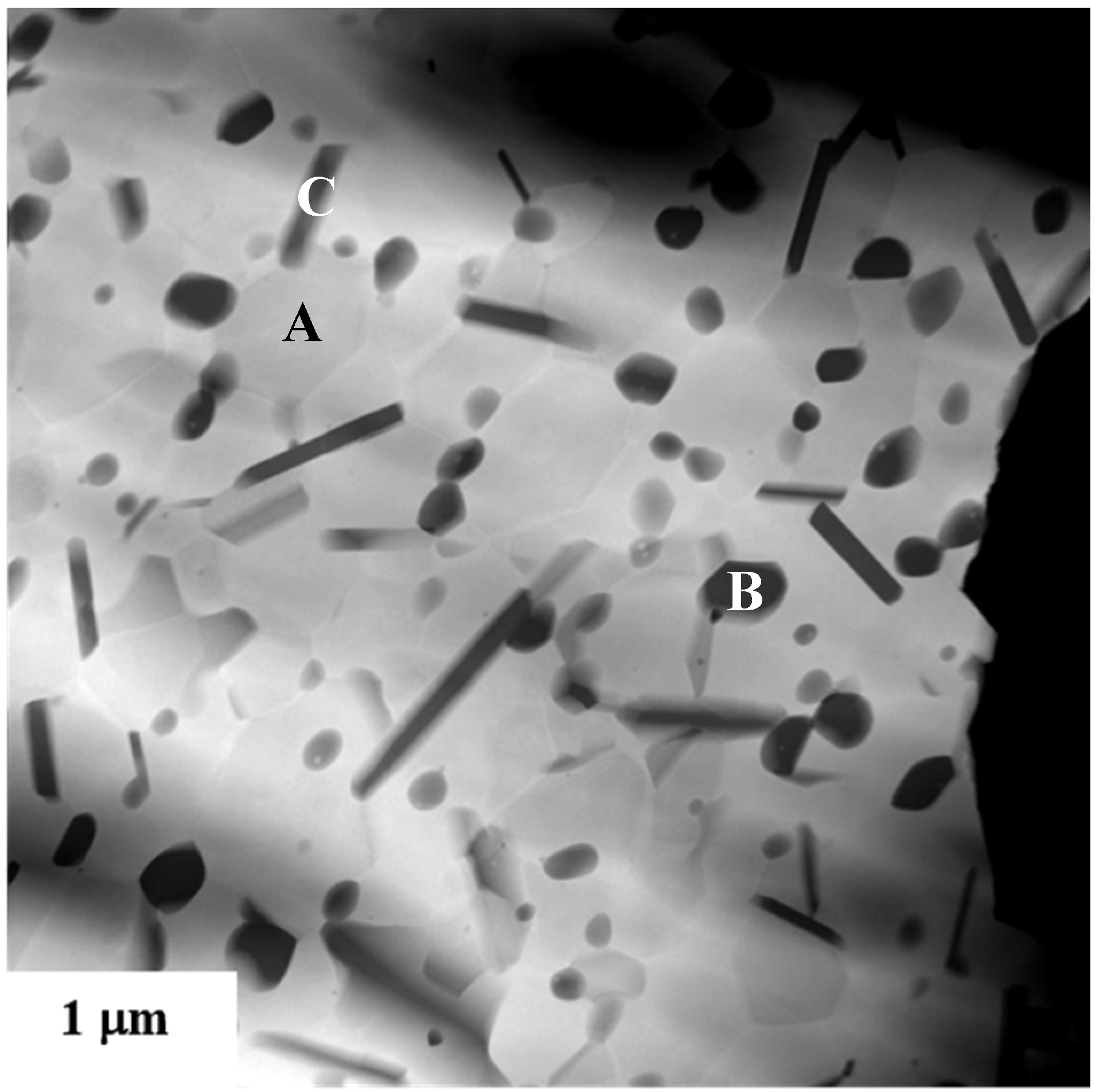
4.8. Industrial Production of Ceramic Composite Powders
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mayo, M.J. Processing of nanocrystalline ceramics from ultrafine particles. Int. Mater. Rev. 1996, 41, 85–115. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T.W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Ovid’ko, I.A.; Sheinerman, A.G. Micromechanisms for improved fracture toughness in nanoceramics. Rev. Adv. Mater. Sci. 2011, 29, 105–115. [Google Scholar]
- Niihara, K. New design concept of structural ceramics/ceramic nanocomposites. J. Ceram. Soc. Jpn. 1991, 99, 974–982. [Google Scholar] [CrossRef]
- Kuntz, J.D.; Zhan, G.D.; Mukherjee, A.K. Nanocrystalline-matrix ceramic composites for improved fracture toughness. MRS Bull. 2004, 29, 22–27. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Niihara, K. Microstructure and properties of alumina-silicon carbide nanocomposites fabricated by pressureless sintering and post hot-isostatic pressing. Trans. Nonferrous Metals Soc. China 2011, 21, s1–s6. [Google Scholar] [CrossRef]
- Yang, J.F.; Ohji, T.; Sekino, T.; Li, C.L.; Niihara, K. Phase transformation, microstructure and mechanical properties of Si3N4/SiC composite. J. Eur. Ceram. Soc. 2001, 21, 2179–2183. [Google Scholar] [CrossRef]
- Yoshimura, M.; Ohji, T.; Sando, M.; Choa, Y.H.; Sekino, T.; Niihara, K. Oxidation-induced strengthening and toughening behavior in micro- and nano-composites of Y2O3/SiC system. Mater. Lett. 1998, 35, 139–143. [Google Scholar] [CrossRef]
- Rong, S.F.; Ji, Z.S.; Zhu, Y.C.; Zhang, J.Q. Effect of rod-like grain on properties and toughening mechanism of 3Y-TZP/Al2O3 ceramics. Nonferrous Metals Soc. China 2008, 18, 388–392. [Google Scholar] [CrossRef]
- Nakamura, M.; Hirao, K.; Yamauchi, Y.; Kanzaki, S. Wear behavior of β-Si3N4 ceramics reinforced by rod-like β-Si3N4 grains. Wear 2003, 254, 94–102. [Google Scholar] [CrossRef]
- Becher, P.F.; Hsueh, C.H.; Angelini, P.; Tiegs, T.N. Toughening behavior in whiskers-reinforced ceramic matrix composites. J. Am. Ceram. Soc. 1988, 71, 1050–1061. [Google Scholar] [CrossRef]
- Becher, P.F. Microstructural design of toughening ceramics. J. Am. Ceram. Soc. 1991, 74, 255–269. [Google Scholar] [CrossRef]
- BIOLOX®delta. Available online: https://www.exac.com/products/hip/revision-femoral/biolox (accessed on 22 February 2015).
- Palmero, P.; Fornabaio, M.; Montanaro, L.; Reveron, H.; Esnouf, C.; Chevalier, J. Towards long lasting zirconia-based composites for dental implants. Part I: Innovative synthesis, microstructural characterization and in vitro stability. Biomaterials 2015, 50, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Kocagoz, S.; Arnholt, C.; Huet, R.; Ueno, M.; Walter, W.L. Advances in zirconia toughened alumina biomaterials for total joint replacement. J. Mech. Behav. Biomed. Mater. 2014, 31, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Palmero, P.; Sola, A.; Naglieri, V.; Bellucci, D.; Lombardi, M.; Cannillo, V. Elaboration and mechanical characterization of multi-phase alumina-based ultra-fine composites. J. Mater. Sci. 2012, 47, 1077–1084. [Google Scholar] [CrossRef]
- Liu, J.; Yan, H.; Jiang, K. Mechanical properties of graphene platelet-reinforced alumina ceramic composites. Ceram. Int. 2013, 39, 6215–6221. [Google Scholar] [CrossRef]
- Palmero, P.; Kern, F.; Lombardi, M.; Gadow, R. Role of immiscible and miscible second phases on the sintering kinetics and microstructural development of nano-crystalline α-Al2O3-based materials. Ceram. Int. 2011, 37, 3547–3556. [Google Scholar] [CrossRef]
- Tatarko, P.; Grasso, S.; Chlup, Z.; Porwal, H.; Kašiarová, M.; Dlouhy, I.; Reece, M.J. Toughening effect of multi-walled boron nitride nanotubes and their influence on the sintering behavior of 3Y-TZP zirconia ceramics. J. Eur. Ceram. Soc. 2014, 34, 1829–1843. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.M.; Oh, Y.S.; Yang, Y.H.; Lim, Y.S.; Yoon, D.H.; Lee, C.; Kim, Y.J.; Ruoff, R.S. Unoxidized graphene/alumina nanocomposite: Fracture and wear-resistance effects of graphene on alumina matrix. Sci. Rep. 2014, 4, 1–10. [Google Scholar]
- Palmero, P.; Naglieri, V.; Spina, V.; Lombardi, M. Microstructural design and elaboration of multiphase ultra-fine ceramics. Ceram. Int. 2012, 37, 139–144. [Google Scholar] [CrossRef]
- Zhang, J.; Tu, R.; Goto, T. Densification of SiO2–cBN composites by using Ni nanoparticle and SiO2 nanolayer coated cBN powder. Ceram. Int. 2012, 38, 4961–4966. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Wang, M.; Wu, G.; Sun, X.; Liu, M. Effect of SiC whiskers on microstructure and mechanical properties of the MoSi2–SiCw composites. Int. J. Refract. Metals Hard Mater. 2013, 41, 489–494. [Google Scholar] [CrossRef]
- Gleiter, H. Nanocrystalline materials. Progr. Mater. Sci. 1989, 33, 223–315. [Google Scholar] [CrossRef]
- Groza, J.R. Nanosintering. Nanostructured Mater. 1999, 12, 987–992. [Google Scholar] [CrossRef]
- Suryanarayana, C. Nanocrystalline materials. Int. Mater. Rev. 1995, 40, 41–64. [Google Scholar] [CrossRef]
- Gutmanas, E.Y. Materials with fine microstructures by advanced powder metallurgy. Progr. Mater. Sci. 1990, 34, 261–366. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E. Phase Transformations in Metals and Alloys, 2nd ed.; Chapman and Hall: London, UK, 1992; p. 113. [Google Scholar]
- Hansen, J.D.; Rusin, R.P.; Teng, M.H.; Johnson, D.L. Combined-stage sintering model. J. Am. Ceram. Soc. 1992, 75, 1129–1135. [Google Scholar] [CrossRef]
- Rabe, T.; Wasche, R. Sintering behavior of nanocrystalline titanium nitride powders. Nanostructured Mater. 1995, 6, 357–360. [Google Scholar] [CrossRef]
- Yan, M.F.; Rhodes, W.W. Low temperature sintering of TiO2. Mater. Sci. Eng. 1983, 61, 59–66. [Google Scholar] [CrossRef]
- Hahn, H. Microstructure and properties of nanostructured oxides. Nanostructured Mater. 1993, 2, 251–265. [Google Scholar] [CrossRef]
- Skandan, G. Processing of nanostructured zirconia ceramics. Nanostructured Mater. 1995, 5, 111–126. [Google Scholar] [CrossRef]
- Palmero, P.; Simone, A.; Esnouf, C.; Fantozzi, G.; Montanaro, L. Comparison among different sintering routes for preparing alumina-YAG nanocomposites. J. Eur. Ceram. Soc. 2006, 26, 941–947. [Google Scholar] [CrossRef]
- Herring, C. Effect of change of scale on sintering phenomena. J. Appl. Phys. 1950, 21, 301–303. [Google Scholar] [CrossRef]
- Zeng, W.; Gao, L.; Gui, L.; Guo, J. Sintering kinetics of α-Al2O3 powder. Ceram. Int. 1999, 25, 723–726. [Google Scholar] [CrossRef]
- Lourenc, M.A.; Cunto, G.G.; Figueiredo, F.M.; Frade, J.R. Model of two-step sintering conditions for yttria-substituted zirconia powders. Mater. Chem. Phys. 2011, 126, 262–271. [Google Scholar] [CrossRef]
- Chen, I.W.; Wang, H.X. Sintering dense nanocrystalline ceramics without final-stage grain growth. Nature 2002, 404, 168–171. [Google Scholar] [CrossRef]
- Pande, C.S.; Cooper, K.P. Nanomechanics of Hall-Petch relationship in nanocrystalline materials. Progr. Mater. Sci. 2009, 54, 689–706. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Progr. Mater. Sci. 2006, 51, 427–556. [Google Scholar]
- Wollmershauser, J.A.; Feigelson, B.N.; Gorzkowski, E.P.; Ellis, C.T.; Goswami, R.; Qadri, S.B.; Tischler, J.G.; Kub, F.J.; Everett, R.K. An extended hardness limit in bulk nanoceramics. Acta Mater. 2014, 69, 9–16. [Google Scholar] [CrossRef]
- Armstrong, R.W. Grain size dependent alumina fracture mechanics stress intensity. Int. J. Refract. Metals Hard Mater. 2001, 19, 251–255. [Google Scholar] [CrossRef]
- Spina, G.; Bonnefont, G.; Palmero, P.; Fantozzi, G.; Chevalier, J.; Montanaro, L. Transparent YAG obtained by spark plasma sintering of co-precipitated powder. Influence of dispersion route and sintering parameters on optical and microstructural characteristics. J. Eur. Ceram. Soc. 2012, 32, 2957–2964. [Google Scholar] [CrossRef]
- Palmero, P.; Bonelli, B.; Fantozzi, G.; Bonnefont, G.; Montanaro, L.; Chevalier, J. Surface and mechanical properties of transparent polycrystalline YAG fabricated by SPS. Mater. Res. Bull. 2013, 7, 2589–2597. [Google Scholar] [CrossRef]
- Wollmershauser, J.A.; Feigelson, B.N.; Qadri, S.B.; Villalobos, G.R.; Hunt, M.; Imam, M.A.; Sanghera, J.S. Transparent nanocrystalline spinel by room temperature high-pressure compaction. Scripta Mater. 2013, 69, 334–337. [Google Scholar] [CrossRef]
- Lawn, B. Fracture of Brittle Solids, 2nd ed.; Cambridge Solid State Science Series: Cambridge, UK, 1993. [Google Scholar]
- Mohamed, F.A. Deformation mechanism maps for micro-grained, ultrafine-grained and nano-grained materials. Mater. Sci. Eng. 2011, A528, 1431–1435. [Google Scholar] [CrossRef]
- Nabarro, F.R.N. Report on a Conference on Strength of Solids; Physical Society: London, UK, 1948; pp. 75–90. [Google Scholar]
- Herring, C. Diffusional viscosity of a polycrystalline solid. J. Appl. Phys. 1950, 21, 437–444. [Google Scholar] [CrossRef]
- Coble, R.L. A model for boundary diffusion controlled creep in polycrystalline materials. J. Appl. Phys. 1963, 34, 1679–1681. [Google Scholar] [CrossRef]
- Mohamed, A.F.; Li, Y. Creep and superplasticity in nanocrystalline materials: Current understanding and future prospects. Mater. Sci. Eng. 2001, A298, 1–15. [Google Scholar] [CrossRef]
- Nieman, G.W.; Weertman, R.J.; Siegel, R.W. Mechanical behavior of nanocrystalline Cu and Pd. J. Mater. Res. 1991, 6, 1012–1027. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Sha, Z.D.; Branicio, P.S.; Zhang, Y.W.; Sorkin, V.; Peia, Q.X.; Srolovitz, D.J. Superplastic nanocrystalline ceramics at room temperature and high strain rates. Scripta Mater. 2013, 69, 525–528. [Google Scholar] [CrossRef]
- Karch, J.; Birringer, R.; Gleiter, H. Ceramics ductile at low temperature. Nature 1987, 330, 556–558. [Google Scholar] [CrossRef]
- Höfler, H.J.; Averback, R.S. Grain growth in nanocrystalline TiO2 and its relation to Vickers hardness and fracture toughness. Scr. Metall. Mater. 1990, 24, 2401–2406. [Google Scholar] [CrossRef]
- Mayo, M.J.; Siegel, R.W.; Liao, Y.X.; Nix, W.D. Nanoindentation of nanocrystalline ZnO. J. Mater. Res. 1992, 7, 973–979. [Google Scholar] [CrossRef]
- Mayo, M.J.; Siegel, R.W.; Narayanasamy, A.; Nix, W.D. Mechanical properties of nanophase TiO2 as determined by nanoindentation. J. Mater. Res. 1990, 5, 1073–1082. [Google Scholar] [CrossRef]
- Anderson, M.P.; Grest, G.S.; Doherty, R.D.; Li, K.; Srolovitz, D.J. Inhibition of grain growth by second phase particles: Three dimensional Monte Carlo computer simulation. Scr. Metall. 1989, 23, 753–758. [Google Scholar] [CrossRef]
- Ryum, N.; Hunderi, O.; Nes, E. On grain boundary drag from second phase particles. Scr. Metall. 1983, 17, 1281–1283. [Google Scholar] [CrossRef]
- Palmero, P.; Traverso, R. Co-precipitation of YAG powders for transparent materials: Effect of the synthesis parameters on processing and microstructure. Materials 2014, 7, 7145–7156. [Google Scholar] [CrossRef]
- Lach, R.; Haberko, K.; Bućko, M.M.; Szumera, M.; Grabowski, G. Ceramic matrix composites in the alumina/5–30 vol.% YAG system. J. Eur. Ceram. Soc. 2011, 10, 1889–1895. [Google Scholar] [CrossRef]
- Kern, F.; Palmero, P.; Marro, F.G.; Mestra, A. Processing of alumina-zirconia composites by surface modification route with enhanced hardness and wear resistance. Ceram. Int. 2015, in press. [Google Scholar] [CrossRef]
- Kern, F.; Palmero, P. Microstructure and mechanical properties of alumina 5 vol% zirconia nanocomposites prepared by powder coating and powder mixing routes. Ceram. Int. 2013, 39, 673–682. [Google Scholar] [CrossRef]
- Novak, S.; Kosmač, T.; Ribitsch, V. Investigation of the powder characteristics and microstructures of alumina-zirconia composites. Mater. Sci. Eng. 1995, A194, 235–241. [Google Scholar] [CrossRef]
- Casellas, D.; Nagl, M.M.; Llanes, L.; Anglada, M. Fracture toughness of alumina and ZTA ceramics: Microstructural coarsening effects. J. Mater. Process. Technol. 2003, 143–144, 148–152. [Google Scholar] [CrossRef]
- Zhao, J.; Stearns, L.C.; Harmer, M.P.; Chan, H.M.; Miller, G.A. Mechanical-behavior of alumina silicon-carbide nanocomposites. J. Eur. Ceram. Soc. 1993, 76, 503–510. [Google Scholar] [CrossRef]
- Nakahira, A.; Niihara, K.; Ohkijima, J.; Hirai, T. Al2O3/Si3N4 nano-composites. J. Jpn. Soc. Powder Powder Metall. 1998, 36, 239–242. [Google Scholar] [CrossRef]
- Acchar, W.; Segadães, A.M. Properties of sintered alumina reinforced with niobium carbide. Int. J. Refract. Metals Hard Mater. 2009, 27, 427–430. [Google Scholar] [CrossRef]
- Naglieri, V.; Palmero, P.; Montanaro, L. Preparation and characterization of alumina-doped powders for the design of multi-phasic nano-microcomposites. J. Therm. Anal. Calorim. 2009, 97, 231–237. [Google Scholar] [CrossRef]
- Fornabaio, M.; Palmero, P.; Traverso, R.; Esnouf, C.; Reveron, H.; Chevalier, J.; Montanaro, L. Zirconia-based composites for biomedical applications: Role of second phases on composition, microstructure and zirconia transformability. J. Eur. Ceram. Soc. 2015. accepted for publication. [Google Scholar]
- Sternitzke, M. Review: Structural ceramic nanocomposites. J. Eur. Ceram. Soc. 1997, 17, 1061–1082. [Google Scholar] [CrossRef]
- Cannon, W.R.; Gugel, E.; Leimer, G.; Woetting, G.; Heimann, R.G. Ceramics, Advanced Structural Products. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA Ed.: Weinheim, Germany, 2011; pp. 1–34. [Google Scholar]
- Bengisu, M. Properties of Ceramic Materials and Their Evaluation. In Engineering Ceramics; Bengisu, M., Ed.; Stringer-Verlag: Berlin, Germany, 2001; pp. 226–252. [Google Scholar]
- Zambetakis, T.; Guille, J.L.; Willer, B.; Daire, M. Mechanical properties of pressure-sintered Al2O3-ZrC composites. J. Mater. Sci. 1987, 22, 1135–1140. [Google Scholar] [CrossRef]
- Ohji, T.; Jeong, Y.K.; Choa, H.H.; Niihara, K. Strengthening and toughening mechanisms of ceramic nanocomposites. J. Am. Ceram. Soc. 1998, 81, 1453–1460. [Google Scholar] [CrossRef]
- Ferroni, L.P.; Pezzotti, G. Evidence for bulk residual stress strengthening in Al2O3/SiC nanocomposites. J. Am. Ceram. Soc. 2002, 85, 2033–2038. [Google Scholar] [CrossRef]
- Selsing, J. Internal stresses in ceramics. J. Am. Ceram. Soc. 1961, 44, 419–426. [Google Scholar] [CrossRef]
- Buren, S.J. Ceramic cutting tools. Ceram. Eng. Sci. Proc. 1982, 3, 35–359. [Google Scholar]
- Freiman, S. Brittle fracture behavior of ceramics. Am. Ceram. Soc. Bull. 1988, 67, 392–402. [Google Scholar]
- Lange, F.F. The interaction of a crack front with a second phase dispersion. Philos. Mag. 1970, 22, 983–992. [Google Scholar] [CrossRef]
- Green, D.J. Fracture toughness prediction for crack bowing in brittle particulate composites. J. Am. Ceram. Soc. 1893, 66, C-4–C-5. [Google Scholar]
- Faber, K.T.; Evans, A.G. Intergranular crack defection toughening in silicon carbide. J. Am. Ceram. Soc. 1983, 66, C-94–C-96. [Google Scholar] [CrossRef]
- Mah, T.I.; Mendiratta, M.G. Fracture toughness and strength of Si3N4-TiC composites. Am. Ceram. Soc. Bull. 1981, 60, 1229–1240. [Google Scholar]
- Faber, K.T.; Evans, A.G. Crack defection process-II. Experiment. Acta Metall. 1983, 31, 577–584. [Google Scholar] [CrossRef]
- Tan, H.; Yang, W. Toughening mechanisms of nano-composite ceramics. Mech. Mater. 1998, 30, 111–123. [Google Scholar] [CrossRef]
- Awaji, H.; Choi, S.M.; Yagi, E. Mechanisms of toughening and strengthening in ceramic-based nanocomposites. Mech. Mater. 2002, 34, 411–422. [Google Scholar] [CrossRef]
- Ruhle, M.; Evans, A.G.; McMeeking, M.R.; Charalmbides, P.G.; Hutchinson, J.W. Microcrack toughening in alumina/zirconia. Acta Metall. 1987, 35, 2701–2710. [Google Scholar] [CrossRef]
- Chevalier, J.; Taddei, P.; Gremillard, L.; Deville, S.; Fantozzi, G.; Bartolomé, J.F.; Pecharroman, C.; Moya, J.S.; Diaz, L.A.; Torrecillas, R.; et al. Reliability assessment in advanced nanocomposite materials for orthopaedic applications. J. Mech. Behav. Biomed. Mater. 2011, 4, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Ruhle, M.; Claussen, N.; Heuer, A.H. Transformation and microcrack toughening as complementary processes in ZrO2-toughened Al2O3. J. Am. Ceram. Soc. 1986, 69, 195–197. [Google Scholar] [CrossRef]
- Wilkinson, D.S. Creep mechanism in multiphase ceramic materials. J. Am. Ceram. Soc. 1998, 81, 275–299. [Google Scholar] [CrossRef]
- Wilkinson, D.S. Oxide Ceramics, Creep and Creep Rupture of. In Encyclopedia of Materials: Science and Technology; Elsevier Science Ltd.: Cambridge, UK, 2001; pp. 6592–6595. [Google Scholar]
- Tai, Q.; Mocellin, A. Review: High temperature deformation of Al2O3-based ceramic particle or whisker composites. Ceram. Int. 1999, 25, 395–408. [Google Scholar] [CrossRef]
- Reveron, H.; Zaafrani, O.; Fantozzi, G. Microstructure development, hardness, toughness and creep behavior of pressureless sintered alumina/SiC micro-nanocomposites obtained by slip-casting. J. Eur. Ceram. Soc. 2010, 30, 1351–1357. [Google Scholar] [CrossRef]
- Palmero, P.; Fantozzi, G.; Lomello, F.; Bonnefont, G.; Montanaro, L. Creep behavior of alumina/YAG composites prepared by different sintering routes. Ceram. Int. 2012, 38, 433–441. [Google Scholar] [CrossRef]
- Descamps, P.; O’Sullivan, D.; Poorteman, M.; Descamps, J.C.; Leriche, A.; Cambier, F. Creep behavior of Al2O3-SiC nanocomposites. J. Eur. Ceram. Soc. 1999, 19, 2475–2485. [Google Scholar] [CrossRef]
- Galusek, D.; Galusková, G. Alumina matrix composites with non-oxide nanoparticle addition and enhanced functionalities. Nanomaterials 2015, 5, 115–143. [Google Scholar] [CrossRef]
- Ohji, T.; Hirano, T.; Nakahira, A.; Niihara, K. Particle/matrix interface and its role in creep inhibition in alumina/silicon carbide nanocomposites. J. Am. Ceram. Soc. 1996, 79, 33–45. [Google Scholar] [CrossRef]
- Parthasarathy, T.A.; Mah, T.I.; Keller, K. Creep mechanism of polycrystalline yttrium-aluminum-garnet. J. Am. Ceram. Soc. 1992, 75, 1756–1759. [Google Scholar] [CrossRef]
- Duong, H.; Wolfestine, J. Creep behavior of fine-grained two-phase Al2O3–Y3Al5O12 materials. Mater. Sci. Eng. A 1993, 172, 173–179. [Google Scholar] [CrossRef]
- Maglia, F.; Tredici, I.G.; Anselmi-Tamburini, U. Densification and properties of bulk nanocrystalline functional ceramics with grain size below 50 nm. J. Eur. Ceram. Soc. 2013, 33, 1045–1066. [Google Scholar] [CrossRef]
- Fahamin, A.; Nasiri-Tabrizi, B.; Ebrahimi-Kahrizsangi, R. Synthesis of calcium phosphate-based composite nanopowders by mechanochemical process and subsequent thermal treatment. Ceram. Int. 2012, 38, 6729–6738. [Google Scholar] [CrossRef]
- Abbasi, B.J.; Zakeri, M.; Tayebifard, S.A. Mechanochemical synthesis of Al2O3–ZrB2–ZrO2 nanocomposite powder. Mater. Res. Bull. 2014, 49, 672–676. [Google Scholar] [CrossRef]
- Sharifi, E.M.; Karimzadeh, F.; Enayati, M.H. Preparation of Al2O3–TiB2 nanocomposite powder by mechanochemical reaction between Al, B2O3 and Ti. Adv. Powder Technol. 2011, 22, 526–531. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, X.; Wang, J.; Wang, W.; Wang, Y.; Fu, Z. Synthesis and structural evolution of B4C–SiC nanocomposite powders by mechanochemical processing and subsequent heat treatment. Powder Technol. 2014, 254, 131–136. [Google Scholar] [CrossRef]
- Torabin, O.; Naghibi, S.; Golabgir, M.H.; Tajizadegan, H.; Jamshidi, A. Mechanochemical synthesis of NbC–NbB2 nanocomposite from the Mg/B2O3/Nb/C powder mixtures. Ceram. Int. 2015, 41, 5362–5369. [Google Scholar] [CrossRef]
- Su, B.; Sternitzke, M. A Novel Processing Route for Alumina/SiC Nanocomposites by Si-Polymer Pyrolysis. In Fourth Euro. Ceramics; Bellosi, A., Ed.; Gruppo Editoriale Faenza Editrice S.p.A.: Faenza, Italy, 1995; Volume 4, pp. 109–l16. [Google Scholar]
- Galusek, D.; Sedláček, J.; Švančárek, P.; Riedel, R.; Satet, R.; Hoffmann, M. The influence of post-sintering HIP on the microstructure, hardness, and indentation fracture toughness of polymer-derived Al2O3–SiC nanocomposites. J. Eur. Ceram. Soc. 2007, 27, 1237–1245. [Google Scholar] [CrossRef]
- Riedel, R.; Toma, L.; Fasel, C.; Miehe, G. Polymer-derived mullite–SiC-based nanocomposites. J. Eur. Ceram. Soc. 2007, 29, 3079–3090. [Google Scholar] [CrossRef]
- Pizon, D.; Charpentier, L.; Lucas, R.; Foucaud, S.; Maître, A.; Balat-Pichelin, M. Oxidation behavior of spark plasma sintered ZrC–SiC composites obtained from the polymer-derived ceramics route. Ceram. Int. 2014, 40, 5025–5031. [Google Scholar] [CrossRef]
- Riedel, R.; Strecker, K.; Petzow, G. In situ polysilane-derived silicon carbide particulates dispersed in silicon nitride composite. J. Am. Ceram. Soc. 1989, 72, 2071–2077. [Google Scholar] [CrossRef]
- Riedel, R.; Seher, M.; Becker, G. Sintering of amorphous polymer-derived Si, N and C containing composite powders. J. Eur. Ceram. Soc. 1989, 5, 113–122. [Google Scholar] [CrossRef]
- Wan, J.; Duan, R.G.; Gasch, M.J.; Mukherjee, A.K. Methods of processing Si3N4/SiC nano-nanocomposites from polymer precursor. Mater. Sci. Eng. A 2006, 424, 105–116. [Google Scholar] [CrossRef]
- Hirano, T.; Niihara, K. Microstructure and mechanical properties of Si3N4/SiC composites. Mater. Lett. 1995, 22, 249–254. [Google Scholar] [CrossRef]
- Niihara, K.; Hirano, T.; Nakahira, A.; Izaki, K. The Correlation Between Interface Structure and Mechanical Properties for Silicon Nitride based Nanocomposites. In Grain Boundary Controlled Properties of Fine Ceramics; Ishizaki, K., Niihara, K., Isotani, M., Ford, R.G., Eds.; Elsevier Applied Science: London, UK, 1992; pp. 103–111. [Google Scholar]
- Riedel, R.; Seher, M.; Mayer, J.; Szabo, D.V. Polymer-derived Si-based bulk ceramics, part I: Preparation, processing and properties. J. Eur. Ceram. Soc. 1995, 15, 703–715. [Google Scholar] [CrossRef]
- Mayer, J.; Szabo, D.V.; Rtihle, M.; Seher, M.; Riedel, R. Polymer-derived Si-based bulk ceramics, part II: Microstructural characterisation by electron spectroscopic imaging. J. Eur. Ceram. Soc. 1995, 15, 717–727. [Google Scholar] [CrossRef]
- Choi, S.; Lee, M.S.; Park, D.W. Photocatalytic performance of TiO2/V2O5 nanocomposite powder prepared by DC arc plasma. Ceram. Int. 2007, 33, 379–383. [Google Scholar] [CrossRef]
- Zedda, D. Synthesis of alumina-silicon carbide composites by chemically activated self-propagation reaction. Ceram. Int. 2001, 27, 163–169. [Google Scholar] [CrossRef]
- Zaki, Z.I. Combustion synthesis of mullite-titanium boride composite. Ceram. Int. 2009, 35, 673–678. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Kharatyan, S.L.; Blugan, G.; Kuebler, J. Combustion synthesis and compaction of Si3N4/TiN composite powder. Ceram. Int. 2007, 33, 379–383. [Google Scholar] [CrossRef]
- Kharatyan, S.L.; Manukyan, K.V.; Nersisyan, H.H.; Khachatryan, H.L. Macrokinetic laws of activated combustion during synthesis of composite ceramic powders based on silicon nitride. Int. J. SHS 2003, 12, 19–34. [Google Scholar]
- Han, J.C.; Chen, Q.C.; Du, S.Y.; Wood, Y.V. Synthesis of Si3N4–TiN–SiC composites by combustion reaction under high nitrogen pressures. J. Eur. Ceram. Soc. 2000, 20, 927–932. [Google Scholar] [CrossRef]
- Wanbao, H.; Baolin, Z.; Hanrui, Z.; Wenlan, L. Combustion synthesis of Si3N4–TiN composite powders. Ceram. Int. 2004, 8, 2211–2214. [Google Scholar] [CrossRef]
- Ryu, H.Y.; Nersisyan, H.H.; Lee, J.H. Preparation of zirconium-based ceramic and composite fine-grained powders. Int. J. Refract. Metals Hard Mater. 2012, 30, 133–138. [Google Scholar] [CrossRef]
- Jayaseelan, D.D.; Rani, D.A.; Nishikawa, T.; Awaji, H.; Gnanam, F.D. Powder characteristics, sintering behavior and microstructure of sol-gel derived ZTA composites. J. Eur. Ceram. Soc. 2000, 20, 267–275. [Google Scholar] [CrossRef]
- Naga, S.M.; Abdelbary, E.M.; Awaad, M.; El-Shaer, Y.I.; Abd-Elwaha, H.S. Effect of the preparation route on the mechanical properties of Yttria–Ceria doped Tetragonal Zirconia/Alumina composites. Ceram. Int. 2013, 39, 1835–1840. [Google Scholar] [CrossRef]
- Towata, A.; Hwang, H.J.; Yasuoka, M.; Sando, M.; Niihara, K. Preparation of polycrystalline alumina/YAG composite fibers by sol-gel method. Compos. A 2001, 32, 1127–1131. [Google Scholar] [CrossRef]
- Wang, W.; Weng, D.; Wu, X.D. Preparation and thermal stability of zirconia-doped mullite fibers via sol-gel method. Progr. Nat. Sci. Mater. Int. 2001, 21, 117–121. [Google Scholar] [CrossRef]
- Naga, S.M.; El-Maghraby, A. Preparation and characterization of porous fibrous mullite bodies doped with TiO2. Mater. Charact. 2011, 6, 174–180. [Google Scholar] [CrossRef]
- Urretavizcaya, G.; Porto Lopez, J.M.; Cavalieri, A.L. Pressureless sintering of sol–gel alumina matrix composites. Mater. Lett. 2000, 43, 281–285. [Google Scholar] [CrossRef]
- Xu, Y.; Nakahira, A.; Niihara, K. Characteristics of Al2O3-SiC nanocomposite prepared by sol-gel processing. J. Ceram. Soc. Jpn. 1994, 102, 312–315. [Google Scholar] [CrossRef]
- Gao, L.; Wang, H.Z.; Hong, J.S.; Miyamoto, H.; Miyamoto, K.; Nishikawa, Y.; Torre, S.D.D.L. Mechanical properties and microstructure of nano-SiC-Al2O3 composites densified by spark plasma sintering. J. Eur. Ceram. Soc. 1999, 19, 609–613. [Google Scholar] [CrossRef]
- Wang, H.Z.; Gao, L.; Guo, J.K. The effect of nanoscale SiC particles on the microstructure of Al2O3 ceramics. Ceram. Int. 2000, 26, 391–396. [Google Scholar] [CrossRef]
- Warrier, K.G.K.; Anilkumar, G.M. Densification of mullite–SiC nanocomposite sol-gel precursors by pressureless sintering. Mater. Chem. Phys. 2001, 67, 263–266. [Google Scholar] [CrossRef]
- Strutt, P.R.; Xiao, T.D.; Gonsalves, K.E.; Boland, R. Chemical synthesis of an aluminum nitride/boron nitride nanostructured composite material. Nanostructured Mater. 1993, 2, 347–353. [Google Scholar] [CrossRef]
- Rana, P.R.; Pratihar, S.K.; Bhattacharyya, S. Powder processing and densification behavior of alumina–high zirconia nanocomposites using chloride precursors. J. Mater. Process. Technol. 2007, 190, 350–357. [Google Scholar] [CrossRef]
- Balmer, M.L.; Lange, F.F.; Jayaram, V.; Levi, C.G. Development of nano-nomposite microstructures in ZrO2-Al2O3 via the solution precursor method. J. Am. Ceram. Soc. 1995, 78, 1489–1495. [Google Scholar] [CrossRef]
- Han, X.; Liang, Z.; Feng, L.; Wang, W.; Chen, J.; Xue, C.; Zhao, H. Co-precipitated synthesis of Al2O3–ZrO2 composite ceramic nanopowders by precipitant and drying method regulation: A systematic study. Ceram. Int. 2015, 41, 505–513. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L.; Shen, Z.; Nygren, M. Mechanical properties and microstructures of Al2O3—vol% YAG composites. J. Eur. Ceram. Soc. 2001, 21, 779–783. [Google Scholar] [CrossRef]
- Wang, H.; Huang, H.; Liang, J.; Liu, J. Preparation of ZrO2/Gd2O3 composite ceramic materials by co-precipitation method. Ceram. Int. 2014, 40, 3995–3999. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zhang, Y.F.; Wang, S.W.; Guo, J.K. In situ synthesis of rodlike LaAl11O18 in Al2O3 powder by a coprecipitation method. J. Eur. Ceram. Soc. 2001, 21, 919–923. [Google Scholar] [CrossRef]
- Trandafir, D.L.; Mirestean, C.; Turcu, R.V.F.; Frentiu, B.; Eniu, D.; Simon, S. Structural characterization of nanostructured hydroxyapatite–iron oxide composites. Ceram. Int. 2014, 40, 11071–11078. [Google Scholar] [CrossRef]
- Zhou, M.; Ferreira, J.M.F.; Fonseca, A.T.; Baptista, J.L. In situ formed-alumina platelets in a mullite-alumina composite. J. Eur. Ceram. Soc. 1998, 18, 495–500. [Google Scholar] [CrossRef]
- Chandradass, J.; Kim, M.H.; Bae, D.S. Influence of citric acid to aluminum nitrate molar ratio on the combustion synthesis of alumina–zirconia nanopowders. J. Alloys Compd. 2009, 470, L9–L12. [Google Scholar] [CrossRef]
- Bhaduri, S.; Bhaduri, S.B.; Zhou, E. Auto ignition synthesis and consolidation of Al2O3-ZrO2 nano/nano composite powders. J. Mater. Res. 1998, 13, 156–165. [Google Scholar] [CrossRef]
- Tahmasebi, K.; Paydar, M.H. Microwave assisted solution combustion synthesis of alumina–zirconia, ZTA, nanocomposite powder. J. Alloys Compd. 2011, 509, 1192–1196. [Google Scholar] [CrossRef]
- Reddy, L.H.; Reddy, G.K.; Devaiah, D.; Reddy, B.M. A rapid microwave-assisted solution combustion synthesis of CuO promoted CeO2–MxOy (M = Zr, La, Pr and Sm) catalysts for CO oxidation. Appl. Catal. A 2012, 445–446, 297–305. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Ohashi, N.; Hishita, S.; Yoshikawa, Y. Synthesis of nanosized nitrogen-containing MOx–ZnO (M = W, V, Fe) composite powders by spray pyrolysis and their visible-light-driven photocatalysis in gas-phase acetaldehyde decomposition. Catal. Today 2004, 93–95, 895–901. [Google Scholar] [CrossRef]
- Harra, J.; Nikkanen, J.P.; Aromaa, M.; Suhonen, H.; Honkanen, M.; Salminen, T.; Heinonen, S.; Levänen, E.; Mäkelä, J.M. Gas phase synthesis of encapsulated iron oxide-titanium dioxide composite nanoparticles by spray pyrolysis. Powder Technol. 2031, 243, 46–52. [Google Scholar] [CrossRef]
- Khoshkalam, M.; Faghihi-Sani, M.A. An investigation on mechanical properties of Alumina-Zirconia-Magnesia spinel composite ceramics fabricated by gel-casting using solution combustion synthesized powder. Mater. Sci. Eng. 2013, A587, 336–343. [Google Scholar] [CrossRef]
- Kingsley, J.J.; Patil, K.C. A novel combustion process for the synthesis of fine particles α-alumina and related oxide materials. Mater. Lett. 1988, 6, 427–432. [Google Scholar] [CrossRef]
- Cortesi, P.; Bowen, H.K. Continuous coating of alumina particles with alkoxide-derived zirconia particles. Ceram. Int. 1989, 15, 173–177. [Google Scholar] [CrossRef]
- Schehl, M.; Diaz, J.A.; Torrecillas, R. Alumina nanocomposites form powder-alkoxide mixtures. Acta Materall. 2002, 50, 1125–1139. [Google Scholar] [CrossRef]
- De Aza, A.H.; Chevalier, J.; Fantozzi, G.; Schehl, M.; Torrecillas, R. Crack growth resistance of alumina, zirconia and zirconia toughened alumina ceramics for joint prostheses. Biomaterials 2002, 23, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Vleugels, J.; van Der Biest, O. Synthesis and characterization of CeO2-coated ZrO2 powder-based TZP. Mater. Lett. 2000, 46, 249–254. [Google Scholar] [CrossRef]
- Palmero, P.; Montanaro, L.; Reveron, H.; Chevalier, J. Surface coating of oxide powders: A new synthesis method to process biomedical grade nano-composites. Materials 2014, 7, 5012–5037. [Google Scholar] [CrossRef]
- Palmero, P.; Esnouf, C. Phase and microstructural evolution of yttrium-doped nanocrystalline alumina: A contribution of advanced microscopy techniques. J. Eur. Ceram. Soc. 1998, 18, 495–500. [Google Scholar] [CrossRef]
- Addad, A.; Crampon, J.; Duclos, R. High temperature deformation of a 5 wt.% zirconia-spinel composite: Influence of a threshold stress. J. Eur. Ceram. Soc. 2002, 22, 329–335. [Google Scholar] [CrossRef]
- Tang, J; Ling, Z.; Lu, Y.; Li, A.; Ling, H.; Wang, Y.; Shao, Q. Preparation of α-Al2O3-SiO2 by heterogeneous nucleation-and-growth processing. Mater. Lett. 2002, 56, 46–449. [Google Scholar]
- Zhang, J.X.; Gao, L.Q. Nanocomposite powders from coating with heterogeneous nucleation processing. Ceram. Int. 2001, 27, 143–147. [Google Scholar] [CrossRef]
- Palmero, P.; Lombardi, M.; Montanaro, L.; Azar, M.; Chevalier, J.; Garnier, V.; Fantozzi, G. Effect of heating rate on phase and microstructural evolution during pressureless sintering of a nanostructured transition alumina. Int. J. Appl. Ceram. Technol. 2009, 6, 420–430. [Google Scholar]
- Aghili, S.E.; Enayati, M.H.; Karimzadeh, F. Synthesis of (Fe,Cr)3Al–Al2O3 nanocomposite through mechanochemical combustion reaction induced by ball milling of Cr, Al and Fe2O3 powders. Adv. Powder Technol. 2014, 25, 408–414. [Google Scholar] [CrossRef]
- The Homepage of Tosoh Corporation. Available online: http://www.tosoh.com (accessed on 5 March 2015).
- The Website of Index of Materials-Ceramic-Powders. Available online: http://www.goodfellow.com/E/Ceramic-Powders.html (accessed on 5 March 2015).
- The Homepage of Ogekkt Nateruaks Inc. Available online: http://www.phelly.com (accessed on 5 March 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmero, P. Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods. Nanomaterials 2015, 5, 656-696. https://doi.org/10.3390/nano5020656
Palmero P. Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods. Nanomaterials. 2015; 5(2):656-696. https://doi.org/10.3390/nano5020656
Chicago/Turabian StylePalmero, Paola. 2015. "Structural Ceramic Nanocomposites: A Review of Properties and Powders’ Synthesis Methods" Nanomaterials 5, no. 2: 656-696. https://doi.org/10.3390/nano5020656






