New Insights into Understanding Irreversible and Reversible Lithium Storage within SiOC and SiCN Ceramics
Abstract
:1. Introduction
2. Results and Discussion
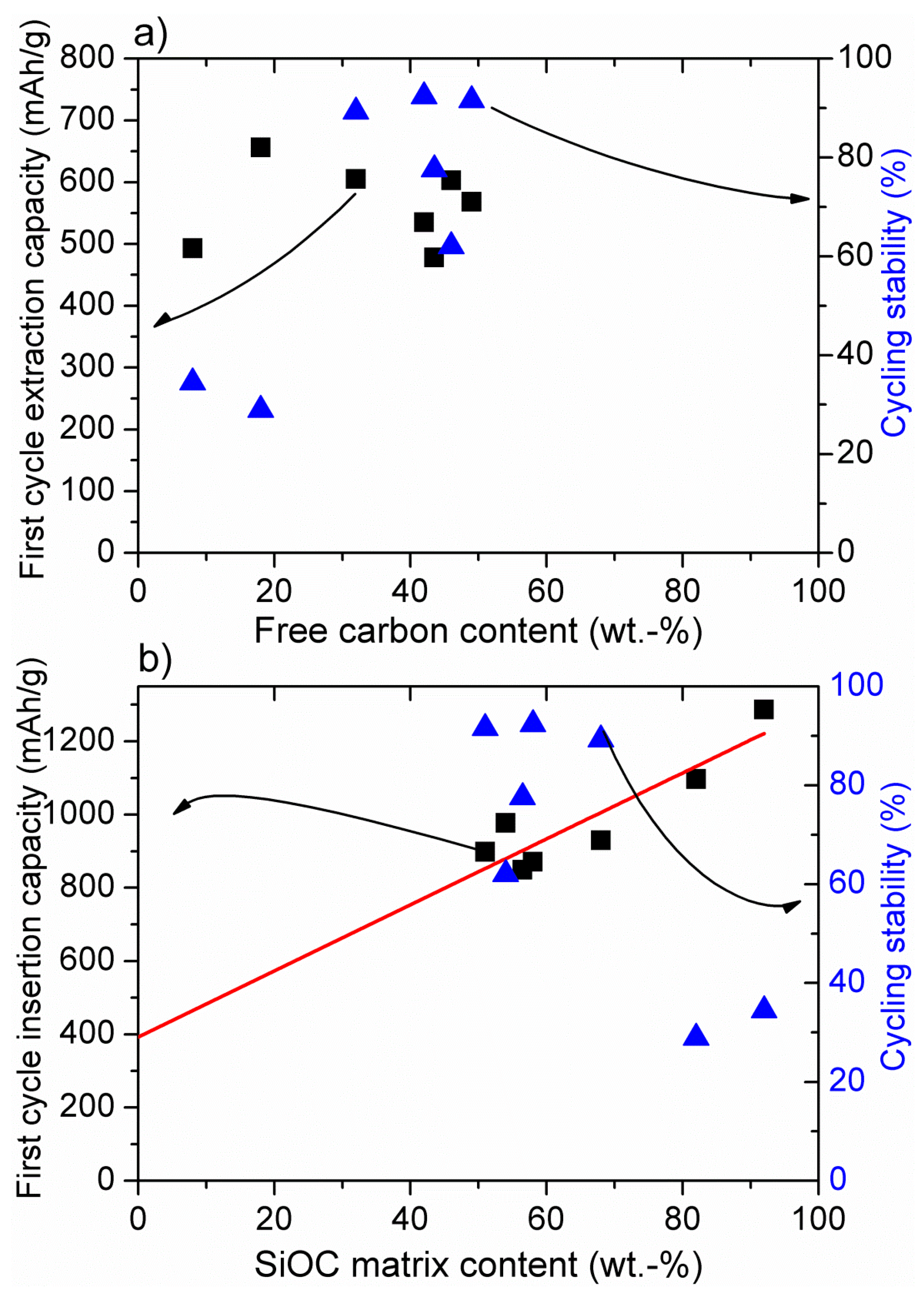
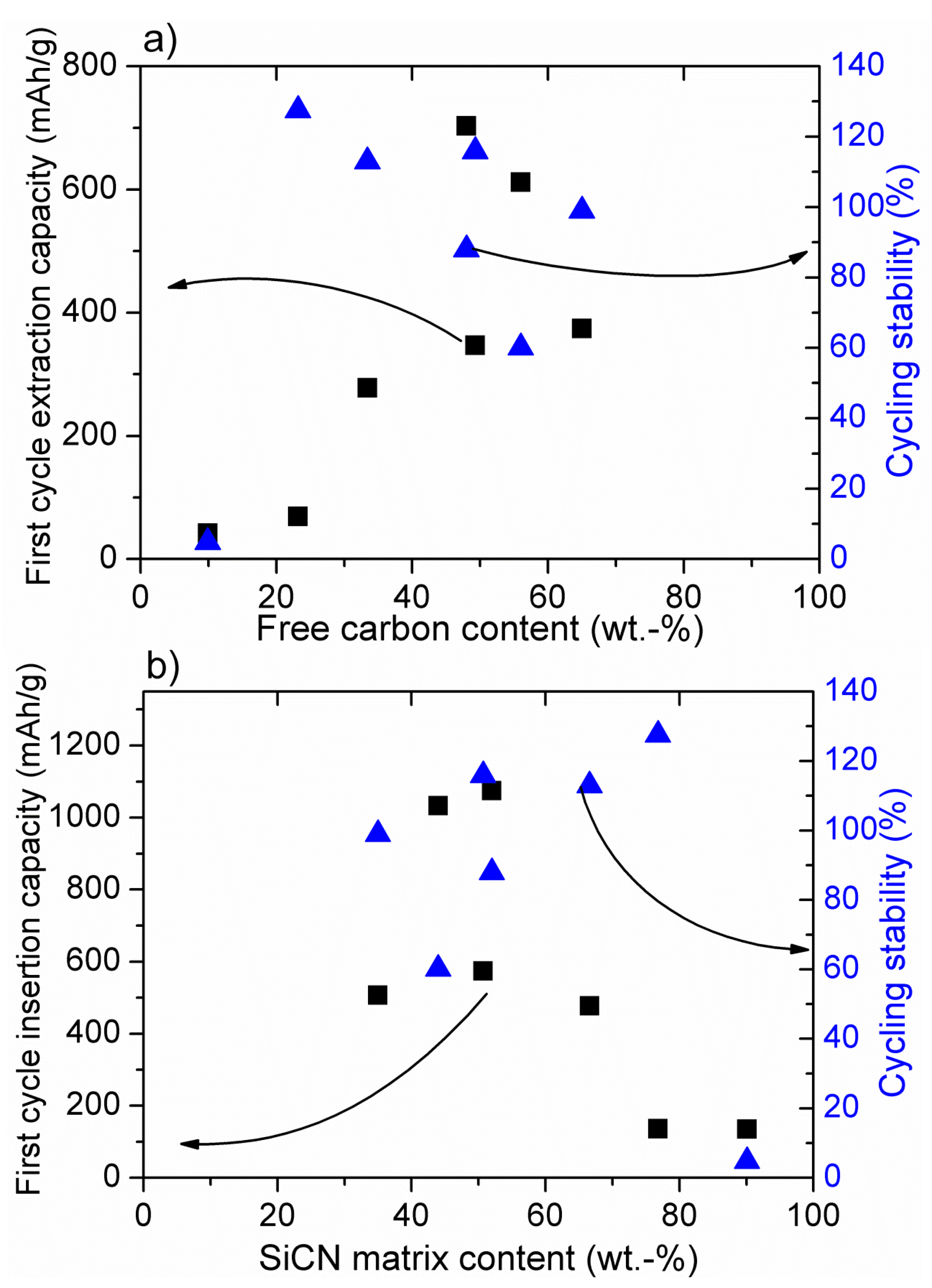
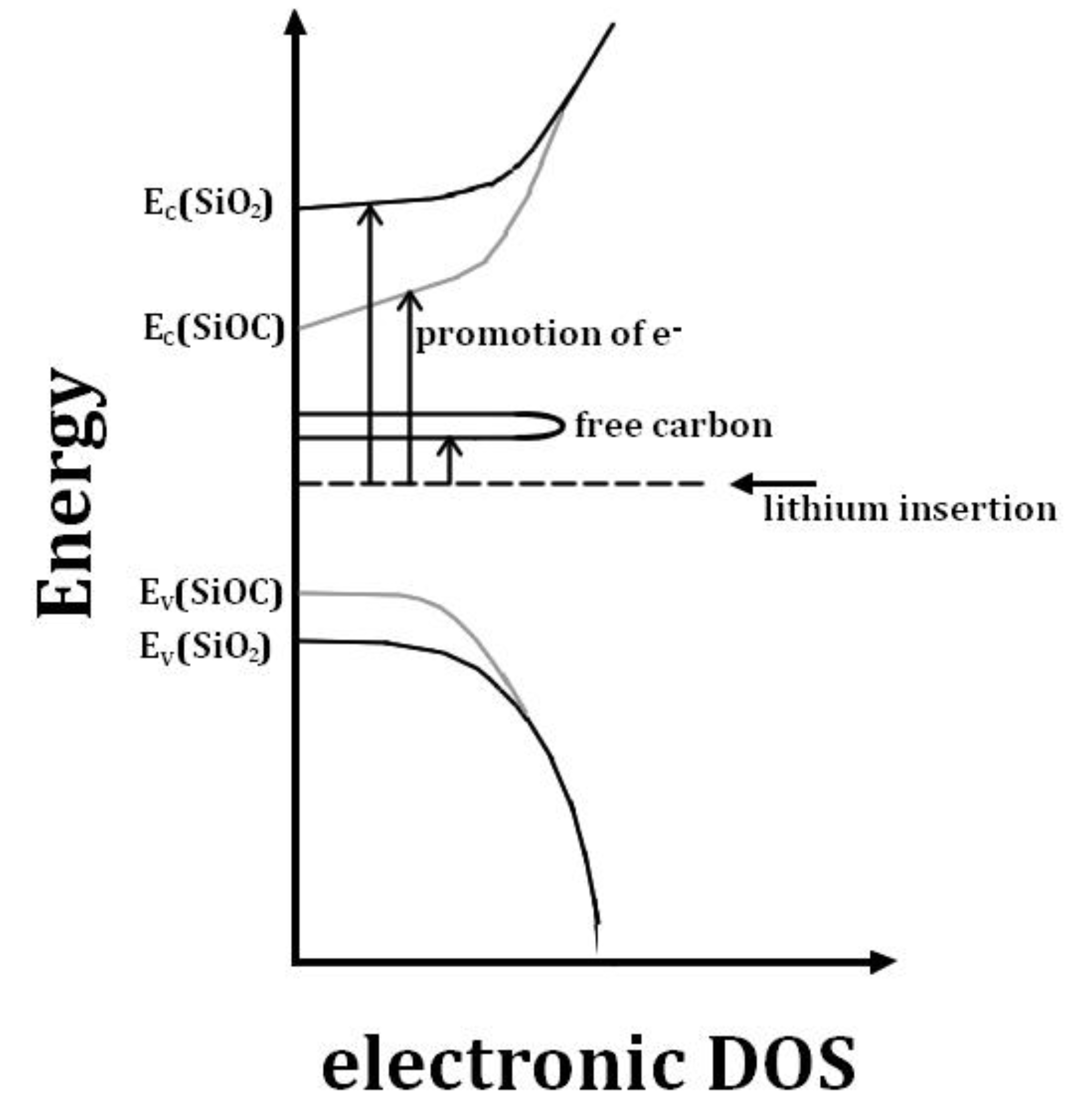
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bugga, R.V.; Smart, M.C. Lithium plating behavior in lithium-ion cells. ECS Trans. 2010, 25, 241–252. [Google Scholar]
- Smart, M.C.; Ratnakumar, B.V. Effects of electrolyte composition on lithium plating in lithium-ion cells. J. Electrochem. Soc. 2011, 158, A379–A389. [Google Scholar] [CrossRef]
- Honbo, H.; Takei, K.; Ishii, Y.; Nishida, T. Electrochemical properties and Li deposition morphologies of surface modified graphite after grinding. J. Power Sources 2009, 189, 337–343. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Key, B.; Chen, H.; Best, A.S.; Hollenkamp, A.F.; Grey, C.P. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 2010, 9, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Agubra, V.; Fergus, J. Lithium ion battery anode aging mechanisms. Materials 2013, 6, 1310–1325. [Google Scholar] [CrossRef]
- Harris, S.J.; Timmons, A.; Baker, D.R.; Monroe, C. Direct in situ measurements of Li transport in Li-ion battery negative electrodes. Chem. Phys. Lett. 2010, 485, 265–274. [Google Scholar] [CrossRef]
- Markovsky, B.; Levi, M.D.; Aurbach, D. The basic electroanalytical behavior of practical graphite-lithium intercalation electrodes. Electrochim. Acta 1998, 43, 2287–2304. [Google Scholar] [CrossRef]
- Aurbach, D.; Levi, M.D.; Levi, E.; Teller, H.; Markovsky, B.; Salitra, G.; Heider, U.; Heider, L. Common electroanalytical behavior of Li intercalation processes into graphite and transition metal oxides. J. Electrochem. Soc. 1998, 145, 3024–3034. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Weissman, I.; Levi, E.; Ein-Eli, Y. On the correlation between surface chemistry and performance of graphite negative electrodes for Li ion batteries. Electrochim. Acta 1999, 45, 67–86. [Google Scholar] [CrossRef]
- Aurbach, D.; Gnanaraj, J.S.; Levi, M.D.; Levi, E.A.; Fischer, J.E.; Claye, A. On the correlation among surface chemistry, 3D structure, morphology, electrochemical and impedance behavior of various lithiated carbon electrodes. J. Power Sources 2001, 97, 92–96. [Google Scholar] [CrossRef]
- Gnanaraj, J.S.; Levi, M.D.; Levi, E.; Salitra, G.; Aurbach, D.; Fischer, J.E.; Claye, A. Comparison between the electrochemical behavior of disordered carbons and graphite electrodes in connection with their structure. J. Electrochem. Soc. 2001, 148, A525–A536. [Google Scholar] [CrossRef]
- Aurbach, D.; Teller, H.; Koltypin, M.; Levi, E. On the behavior of different types of graphite anodes. J. Power Sources 2003, 119, 2–7. [Google Scholar] [CrossRef]
- Levi, M.D.; Wang, C.; Gnanaraj, J.S.; Aurbach, D. Electrochemical behavior of graphite anode at elevated temperatures in organic carbonate solutions. J. Power Sources 2003, 119, 538–542. [Google Scholar] [CrossRef]
- Markovsky, B.; Rodkin, A.; Cohen, Y.S.; Palchik, O.; Levi, E.; Aurbach, D.; Kim, H.J.; Schmidt, M. The study of capacity fading processes of Li-ion batteries: Major factors that play a role. J. Power Sources 2003, 119, 504–510. [Google Scholar] [CrossRef]
- Markervich, E.; Salitra, G.; Levi, M.D.; Aurbach, D. Capacity fading of lithiated graphite electrodes studied by a combination of electroanalytical methods, Raman spectroscopy and SEM. J. Power Sources 2005, 146, 146–150. [Google Scholar] [CrossRef]
- Ning, G.; Haran, B.; Popov, B.N. Capacity fade study of lithium-ion batteries cycled at high discharge rates. J. Power Sources 2003, 117, 160–169. [Google Scholar] [CrossRef]
- Novak, P.; Joho, F.; Lanz, M.; Rykart, B.; Panitz, J.-C.; Alliata, D.; Katz, R.; Haas, O. The complex electrochemistry of graphite electrodes in lithium-ion batteries. J. Power Sources 2001, 97, 39–46. [Google Scholar] [CrossRef]
- Shim, J.; Striebel, K.A. Effect of electrode density on cycle performance and irreversible capacity loss for natural graphite anode in lithium-ion batteries. J. Power Sources 2003, 119, 934–937. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Lithium insertion into carbon-rich SiOC ceramics: Influence of pyrolysis temperature on electrochemical properties. J. Power Sources 2013, 244, 450–455. [Google Scholar] [CrossRef]
- Haluschka, C.; Kleebe, H.J.; Franke, R.; Riedel, R. Silicon carbonitride ceramics derived from polysilazanes Part I. Investigation of compositional and structural properties. J. Eur. Ceram. Soc. 2000, 20, 1355–1364. [Google Scholar] [CrossRef]
- Mera, G.; Riedel, R.; Poli, F.; Müller, K. Carbon-rich sicn ceramics derived from phenyl-containing poly(silylcarbodiimides). J. Eur. Ceram. Soc. 2009, 29, 2873–2883. [Google Scholar] [CrossRef]
- Mera, G.; Navrotsky, A.; Sen, S.; Kleebe, H.-J.; Riedel, R. Polymer-derived SiCN and SiOC ceramics—Structure and energetics at the nanoscale. J. Mater. Chem. A 2013, 1, 3826–3836. [Google Scholar] [CrossRef]
- Wilson, A.M.; Reimers, J.N.; Fuller, E.W.; Dahn, J.R. Lithium insertion in pyrolyzed siloxane polymers. Solid State Ionics 1994, 74, 249–254. [Google Scholar] [CrossRef]
- Xing, W.; Wilson, A.M.; Zank, G.; Dahn, J.R. Pyrolysed pitch-polysilane blends for use as anode materials in lithium ion batteries. Solid State Ionics 1997, 93, 239–244. [Google Scholar] [CrossRef]
- Xing, W.; Wilson, A.M.; Eguchi, K.; Zank, G.; Dahn, J.R. Pyrolyzed polysiloxanes for use as anode materials in lithium-ion batteries. J. Electrochem. Soc. 1997, 144, 2410–2416. [Google Scholar] [CrossRef]
- Wilson, A.M.; Zank, G.; Eguchi, K.; Xing, W.; Yates, B.; Dahn, J.R. Polysiloxane pyrolysis. Chem. Mater. 1997, 9, 1601–1606. [Google Scholar] [CrossRef]
- Wilson, A.M.; Zank, G.; Eguchi, K.; Xing, W.; Dahn, J.R. Pyrolysed silicon-containing polymers as high capacity anodes for lithium-ion batteries. J. Power Sources 1997, 68, 195–200. [Google Scholar] [CrossRef]
- Wilson, A.M.; Xing, W.; Zank, G.; Yates, B.; Dahn, J.R. Pyrolysed pitch-polysilane blends for use as anode materials in lithium ion batteries II: The effect of oxygen. Solid State Ionics 1997, 100, 259–266. [Google Scholar] [CrossRef]
- Saha, A.; Raj, R.; Williamson, D.L. A model for the nanodomains in polymer-derived SiOC. J. Am. Chem. Soc. 2006, 89, 2188–2195. [Google Scholar]
- Sanchez-Jimenez, P.E.; Raj, R. Lithium insertion in polymer-derived silicon oxycarbide ceramics. J. Am. Ceram. Soc. 2010, 93, 1127–1135. [Google Scholar] [CrossRef]
- Ahn, D.; Raj, R. Thermodynamic measurements pertaining to the hysteretic intercalation of lithium in polymer-derived silicon oxycarbide. J. Power Sources 2010, 195, 3900–3906. [Google Scholar] [CrossRef]
- Shen, J.; Raj, R. Silicon-oxycarbide based thin film anodes for lithium ion batteries. J. Power Sources 2011, 196, 5945–5950. [Google Scholar] [CrossRef]
- Ahn, D.; Raj, R. Cyclic stability and c-rate performance of amorphous silicon and carbon based anodes for electrochemical storage of lithium. J. Power Sources 2011, 196, 2179–2186. [Google Scholar] [CrossRef]
- Fukui, H.; Hisashi, O.; Hino, T.; Kanamura, K. A Si–O–C composite anode: High capability and proposed mechanism of lithium storage associated with microstructural characteristics. Appl. Mater. Interfaces 2010, 4, 998–1008. [Google Scholar] [CrossRef]
- Fukui, H.; Nakata, N.; Dokko, K.; Takemura, B.; Ohsuka, H.; Hino, T.; Kanamura, K. Lithiation and delithiation of silicon oxycarbide single particles with a unique microstructure. ACS Appl. Mater. Interf. 2011, 3, 2318–2322. [Google Scholar] [CrossRef]
- Fukui, H.; Ohsuka, H.; Hino, T.; Kanamura, K. Influence of polystyrene/phenyl substituents in precursors on microstructures of Si–O–C composite anodes for lithium-ion batteries. J. Power Sources 2011, 196, 371–378. [Google Scholar] [CrossRef]
- Fukui, H.; Ohsuka, H.; Hino, T.; Kanamura, K. Polysilane/acenaphthylene blends toward Si–O–C composite anodes for rechargeable lithium-ion batteries. J. Electrochem. Soc. 2011, 158, A550–A555. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Toma, L.; Fasel, C.; Riedel, R. Carbon-rich SiOC anodes for lithium-ion batteries: Part I. Influence of material UV-pre-treatment on high power properties. Solid State Ionics 2012, 225, 522–526. [Google Scholar] [CrossRef]
- Dibandjo, P.; Graczyk-Zajac, M.; Riedel, R.; Pradeep, V.S.; Soraru, G.D.A. Lithium insertion into dense and porous carbon-rich polymer-derived SiOC ceramics. J. Eur. Ceram. Soc. 2012, 32, 2495–2503. [Google Scholar] [CrossRef]
- Fukui, H.; Eguchi, K.; Ohsuka, H.; Hino, T.; Kanamura, K. Structures and lithium storage performance of SiOC composite materials depending on pyrolysis temperatures. J. Power Sources 2013, 243, 152–158. [Google Scholar] [CrossRef]
- Fukui, H.; Ohsuka, H.; Hino, T.; Kanamura, K. Silicon oxycarbides in hard-carbon microstructures and their electrochemical lithium storage. J. Electrochem. Soc. 2013, 160, A1276–A1281. [Google Scholar] [CrossRef]
- Pradeep, V.S.; Graczyk-Zajac, M.; Wilamowska, M.; Riedel, R.; Soraru, G.D. Influence of pyrolysis atmosphere on the lithium storage properties of carbon-rich polymer derived SiOC ceramic anodes. Solid State Ionics 2013, 262, 22–24. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Determination of the chemical diffusion coefficient of Li-ions in carbon-rich silicon oxycarbide anodes by electro-analytical methods. Electrochim. Acta 2014, 115, 665–670. [Google Scholar] [CrossRef]
- Dahn, J.R.; Wilson, A.M.; Xing, W.; Zank, G.A. Electrodes for Lithium Ion Batteries Using Polysilazanes Ceramic with Lithium. U.S. Patent 5631106(A), 20 May 1997. [Google Scholar]
- Su, D.; Li, Y.-L.; Feng, Y.; Jin, J. Electrochemical properties of polymer-derived SiCN materials as the anode in lithium ion batteries. J. Am. Ceram. Soc. 2009, 92, 2962–2968. [Google Scholar] [CrossRef]
- Feng, Y. Electrochemical properties of heat-treated polymer-derived sicn anode for lithium ion batteries. Electrochim. Acta 2010, 55, 5860–5866. [Google Scholar] [CrossRef]
- Kaspar, J.; Mera, G.; Nowak, A.P.; Graczyk-Zajac, M.; Riedel, R. Electrochemical study of lithium insertion into carbon-rich polymer-derived silicon carbonitride ceramics. Electrochim. Acta 2010, 56, 174–182. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Mera, G.; Kaspar, J.; Riedel, R. Electrochemical studies of carbon-rich polymer-derived sicn ceramics as anode materials for lithium-ion batteries. J. Eur. Ceram. Soc. 2010, 30, 3235–3243. [Google Scholar] [CrossRef]
- Reinold, L.M.; Graczyk-Zajac, M.; Gao, Y.; Mera, G.; Riedel, R. Carbon-rich sicn ceramics as high capacity/high stability anode material for lithium-ion batteries. J. Power Sources 2013, 236, 224–229. [Google Scholar] [CrossRef]
- Baek, S.-H.; Reinold, L.M.; Graczyk-Zajac, M.; Riedel, R.; Hammerath, F.; Büchner, B.; Grafe, H.-J. Lithium dynamics in carbon-rich polymer-derived SiCN ceramics probed by nuclear magnetic resonance. J. Power Sources 2014, 253, 342–348. [Google Scholar] [CrossRef]
- Graczyk-Zajac, M.; Fasel, C.; Riedel, R. Polymer-derived-SiCN ceramic/graphite composite as anode material with enhanced rate capability for lithium ion batteries. J. Power Sources 2011, 196, 6412–6418. [Google Scholar] [CrossRef]
- Reinold, L.M.; Graczyk-Zajac, M.; Fasel, C.; Riedel, R. Prevention of solid electrolyte interphase damaging on silicon by using polymer derived SiCN ceramics. ECS Trans. 2011, 35, 37–44. [Google Scholar]
- Wilamowska, M.; Graczyk-Zajac, M.; Riedel, R. Composite materials based on polymer-derived SiCN ceramic and disordered hard carbons as anodes for lithium-ion batteries. J. Power Sources 2013, 24, 80–86. [Google Scholar] [CrossRef]
- Kaspar, J.; Graczyk-Zajac, M.; Riedel, R. Carbon-rich SiOC anodes for lithium-ion batteries: Part II. Role of thermal cross-linking. Solid State Ionics 2012, 225, 527–531. [Google Scholar] [CrossRef]
- Kolb, R.; Fasel, C.; Liebau-Kunzmann, V.; Riedel, R. SiCN/C-ceramic composite as anode material for lithium ion batteries. J. Eur. Ceram. Soc. 2006, 26, 3903–3908. [Google Scholar] [CrossRef]
- Liu, G.; Kaspar, J.; Reinold, L.M.; Graczyk-Zajac, M.; Riedel, R. Electrochemical performance of DVB-modified sioc and sicn polymer-derived negative electrodes for lithium-ion batteries. Electrochim. Acta 2013, 106, 101–108. [Google Scholar] [CrossRef]
- Pradeep, V.S.; Graczyk-Zajac, M.; Riedel, R.; Soraru, G.D. New insights in to the lithium storage mechanism in polymer derived SiOC anode materials. Electrochim. Acta 2014, 119, 78–85. [Google Scholar] [CrossRef]
- Mera, G.; Tamayo, A.; Nguyen, H.; Sen, S.; Riedel, R. Nanodomain structure of carbon-rich silicon carbonitride polymer-derived ceramics. J. Am. Ceram. Soc. 2010, 93, 1169–1175. [Google Scholar] [CrossRef]
- Morcos, R.; Mera, G.; Navrotsky, A.; Varga, T.; Riedel, R.; Poli, F.; Müller, K. Enthalpy of formation of carbon-rich polymer-derived amorphous SiCN ceramics. J. Am. Ceram. Soc. 2008, 91, 3349–3354. [Google Scholar] [CrossRef]
- Widgeon, S.; Mera, G.; Gao, Y.; Stoyanov, E.; Sen, S.; Navrotsky, A.; Riedel, R. Nanostructure and energetics of carbon-rich SiCN ceramics derived from polysilylcarbodiimides: Role of the nanodomain interfaces. Chem. Mater. 2012, 24, 1181–1191. [Google Scholar] [CrossRef]
- Widgeon, S.J.; Sen, S.; Mera, G.; Ionescu, E.; Riedel, R.; Navrotsky, A. 29Si and 13C solid-state NMR spectroscopic study of nanometer-scale structure and mass fractal characteristics of amorphous polymer derived silicon oxycarbide ceramics. Chem. Mater. 2010, 22, 6221–6228. [Google Scholar] [CrossRef]
- Blum, Y.D.; MacQueen, D.B.; Kleebe, H.-J. Synthesis and characterization of carbon-enriched silicon oxycarbides. J. Eur. Ceram. Soc. 2005, 25, 143–149. [Google Scholar] [CrossRef]
- Gregori, G.; Kleebe, H.-J.; Blum, Y.D.; Babonneau, F. Evolution of C-rich sioc ceramics: Part II. Characterization by high lateral resolution techniques electron energy-loss spectroscopy high-resolution TEM and energy-filtered TEM. Int. J. Mat. Res. 2006, 97, 710–720. [Google Scholar]
- Kleebe, H.-J.; Gregori, G.; Blum, Y.D.; Babonneau, F. Evolution of C-rich sioc ceramics. Part I. Characterization by integral spectroscopic techniques solid-state NMR and Raman spectroscopy. Int. J. Mat. Res. 2006, 97, 699–709. [Google Scholar]
- Soraru, G.D.; Modena, S.; Guadagnino, E.; Colombo, P.; Egan, J.; Pantano, C. Chemical durability of silicon oxycarbide glasses. J. Am. Ceram. Soc. 2002, 85, 1529–1536. [Google Scholar] [CrossRef]
- Kroll, P. Tracing reversible and irreversible Li insertion in SiCO ceramics with modeling and ab-initio simulations. MRS Proc. 2011, 1313, 1–6. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk-Zajac, M.; Reinold, L.M.; Kaspar, J.; Sasikumar, P.V.W.; Soraru, G.-D.; Riedel, R. New Insights into Understanding Irreversible and Reversible Lithium Storage within SiOC and SiCN Ceramics. Nanomaterials 2015, 5, 233-245. https://doi.org/10.3390/nano5010233
Graczyk-Zajac M, Reinold LM, Kaspar J, Sasikumar PVW, Soraru G-D, Riedel R. New Insights into Understanding Irreversible and Reversible Lithium Storage within SiOC and SiCN Ceramics. Nanomaterials. 2015; 5(1):233-245. https://doi.org/10.3390/nano5010233
Chicago/Turabian StyleGraczyk-Zajac, Magdalena, Lukas Mirko Reinold, Jan Kaspar, Pradeep Vallachira Warriam Sasikumar, Gian-Domenico Soraru, and Ralf Riedel. 2015. "New Insights into Understanding Irreversible and Reversible Lithium Storage within SiOC and SiCN Ceramics" Nanomaterials 5, no. 1: 233-245. https://doi.org/10.3390/nano5010233




