One-Pot Mechanochemical Synthesis of Carbons with High Microporosity and Ordered Mesopores for CO2 Uptake at Ambient Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Microporous–Mesoporous Carbons
2.3. Measurements and Characterization of Microporous–Mesoporous Carbons
2.4. Calculations
2.5. CO2 Activation
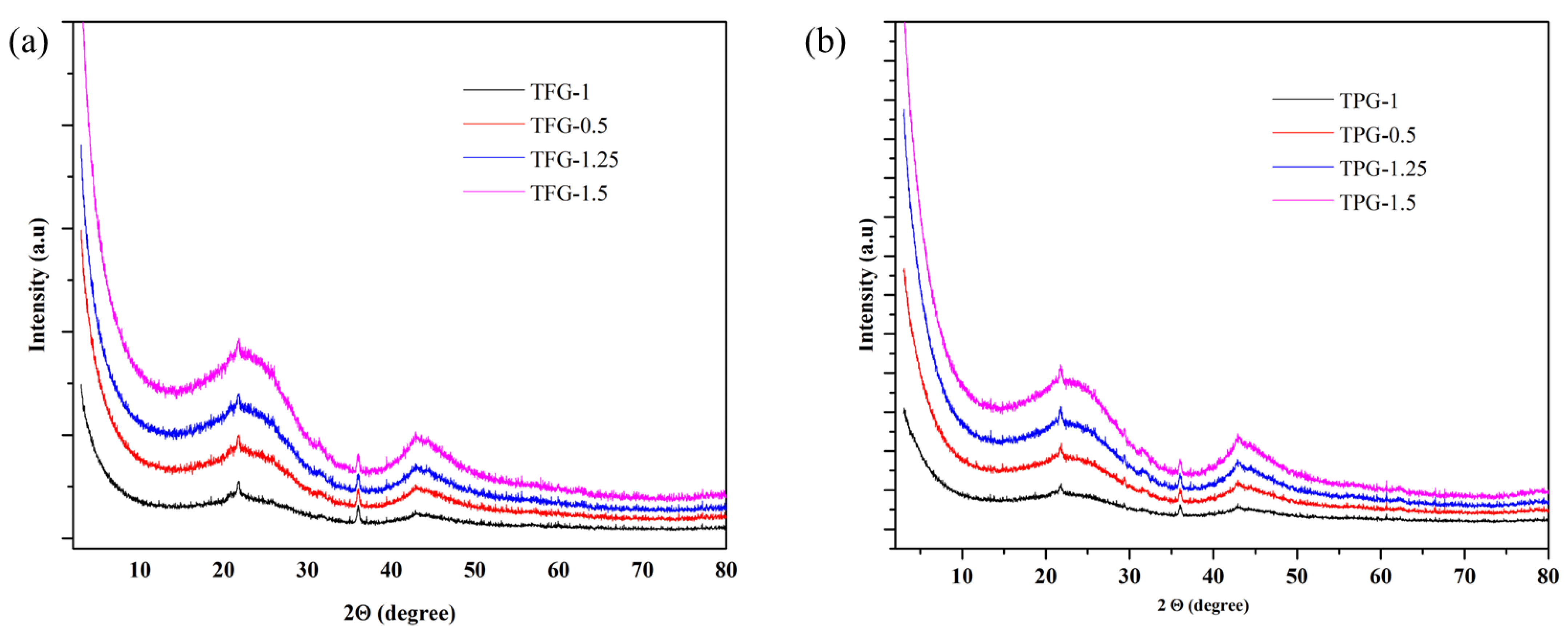
3. Results and Discussion
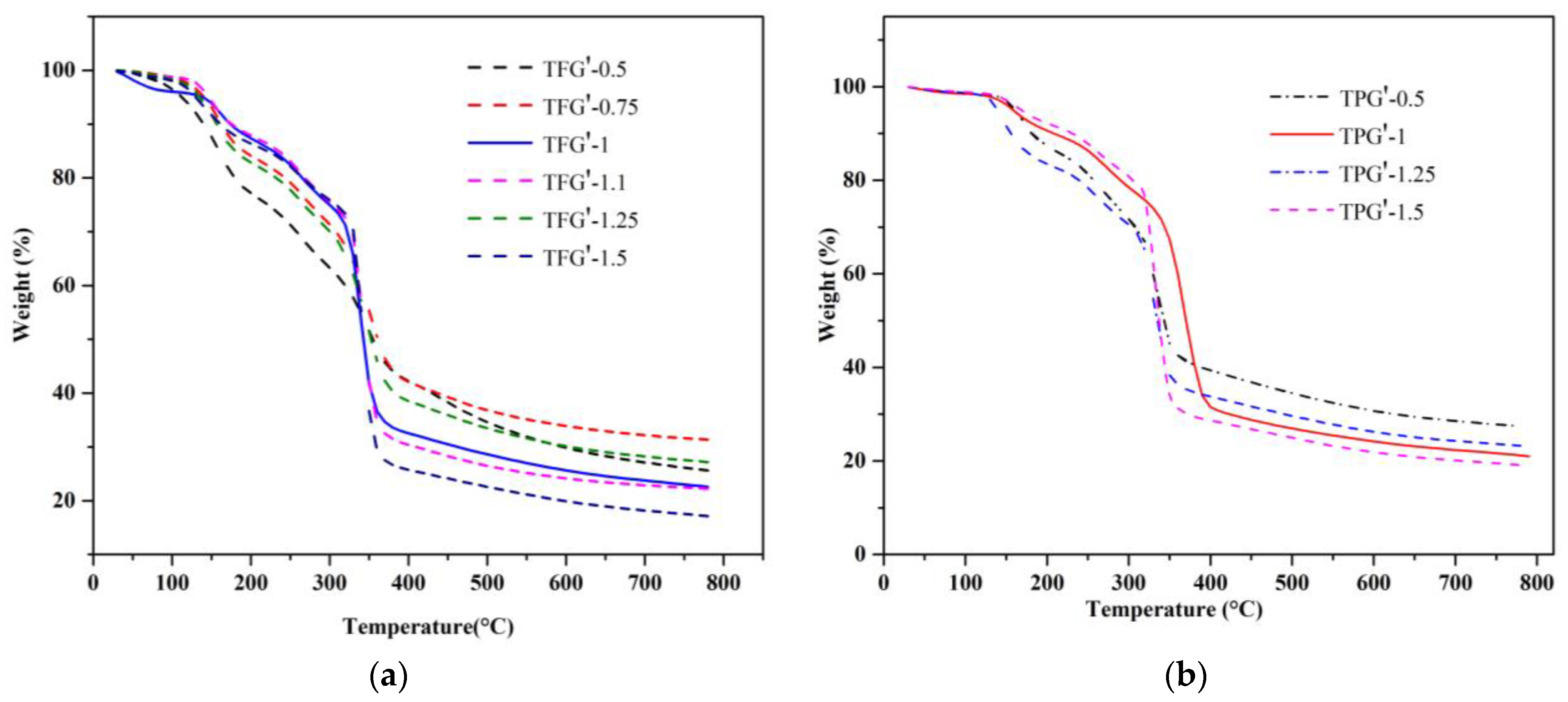
3.1. Thermogravimetric Studies
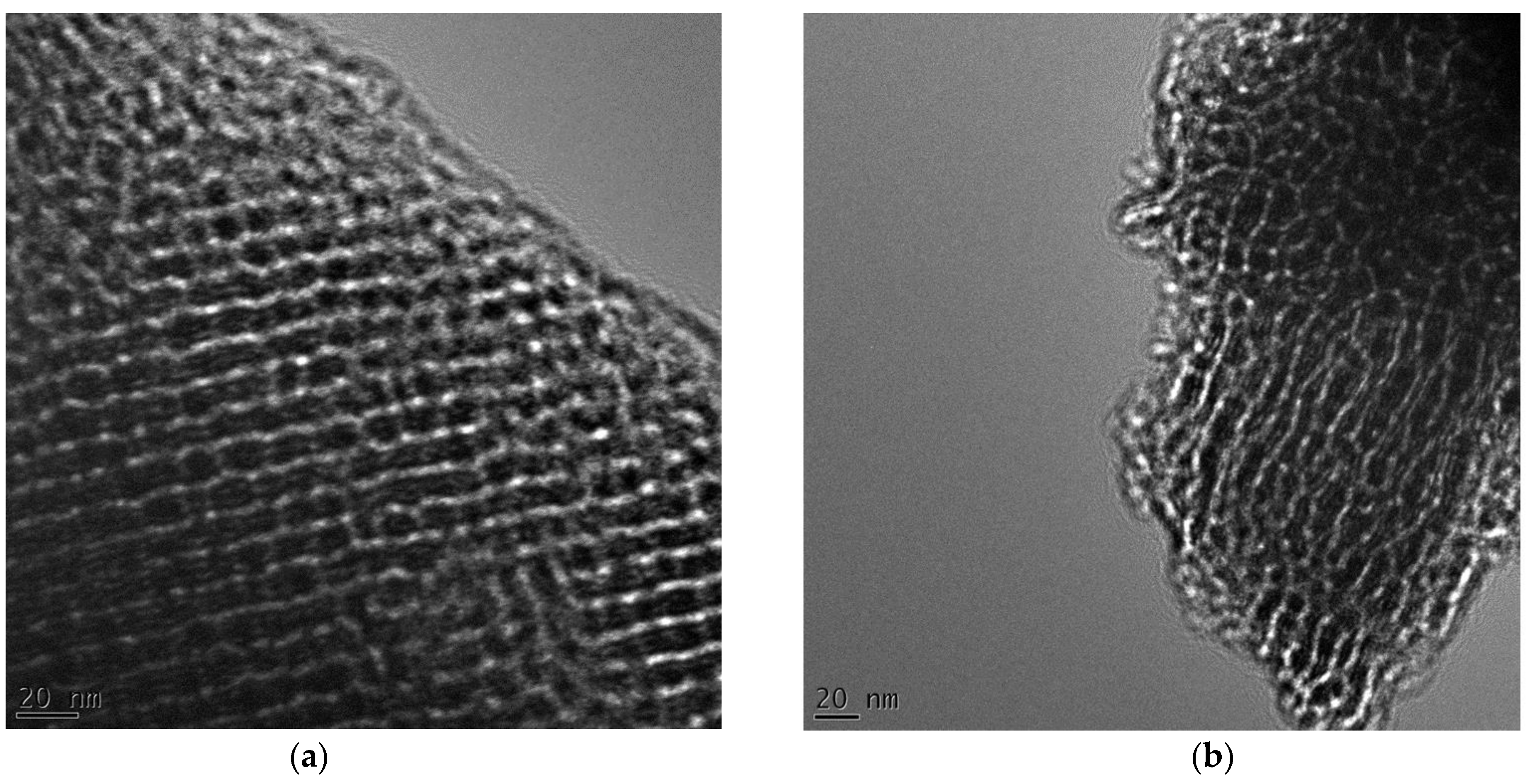
3.2. TEM Imaging of Mesostructures
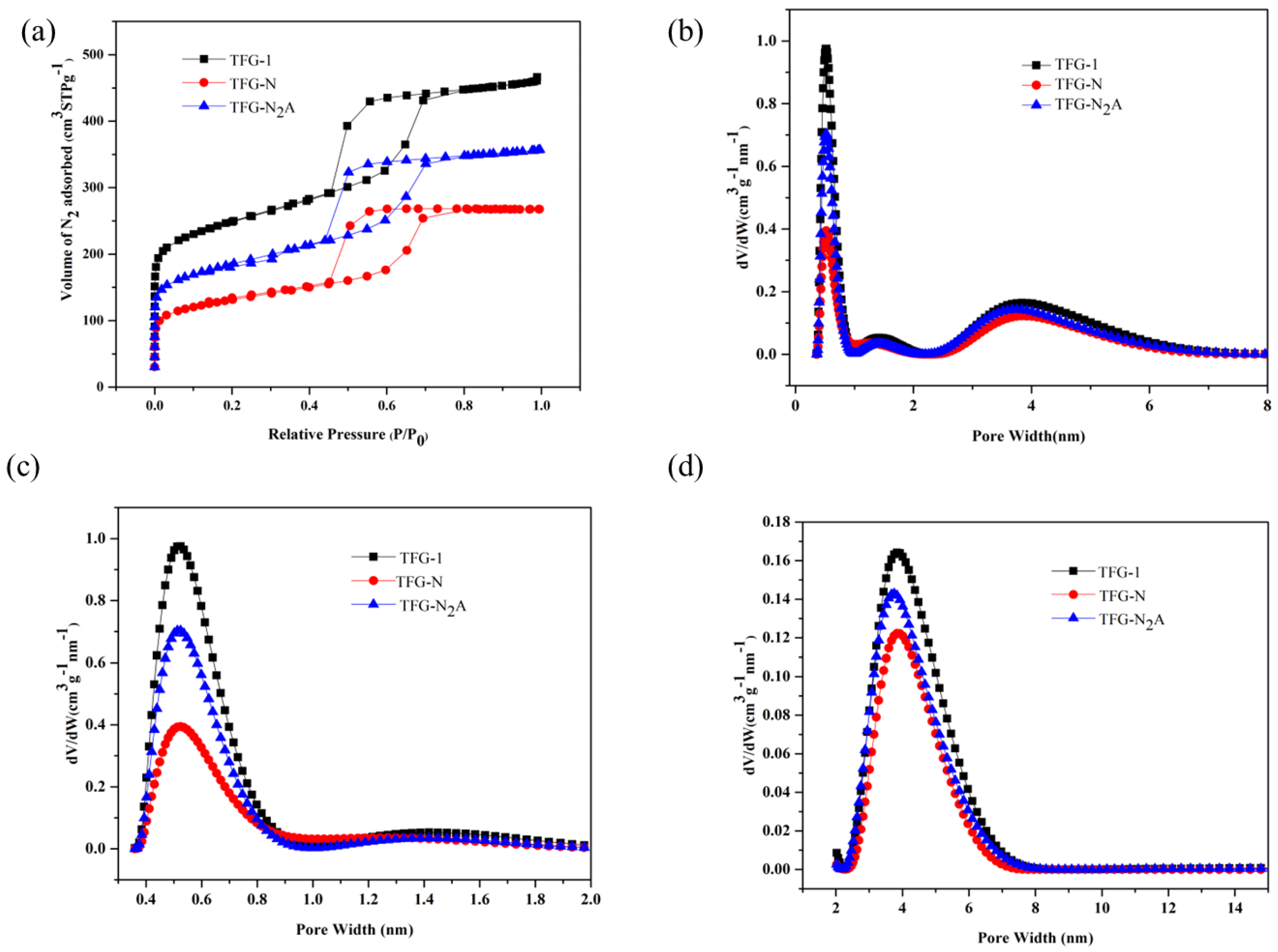
3.3. Nitrogen Adsorption Studies
3.4. Carbon Dioxide Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehdipour-Ataei, S.; Aram, E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts 2022, 13, 2–30. [Google Scholar]
- Xu, H.; Li, H.; Xie, L.; Zhao, D.; Kong, B. Interfacial Assembly of Functional Mesoporous Carbon-Based Materials into Films for Batteries and Electrocatalysis. Adv. Mater. Interfaces 2022, 9, 2101998–21022031. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Patwardhan, S.B.; Pandit, S.; Kumar Gupta, P.; Kumar Jha, N.; Rawat, J.; Joshi, H.C.; Priya, K.; Gupta, M.; Lahiri, D.; Nag, M.; et al. Recent Advances in the Application of Biochar in Microbial Electrochemical Cells. Fuel 2022, 311, 122501–122517. [Google Scholar]
- Wang, J.; Chaemchuen, S.; Klomkliang, N.; Verpoort, F. In Situ Thermal Solvent-Free Synthesis of Zeolitic Imidazolate Frameworks with High Crystallinity and Porosity for Effective Adsorption and Catalytic Applications. Cryst. Growth Des. 2021, 21, 5349–5359. [Google Scholar]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.Y.; Albahily, K.; Vinu, A. Biomass Derived Porous Carbon for CO2 Capture. Carbon 2019, 148, 164–186. [Google Scholar]
- Ghimire, P.P.; Zhang, L.; Kinga, U.A.; Guo, Q.; Jiang, B.; Jaroniec, M. Development of Nickel-Incorporated MCM-41-Carbon Composites and Their Application in Nitrophenol Reduction. J. Mater. Chem. A Mater. 2019, 7, 9618–9628. [Google Scholar]
- Stein, A.; Rudisill, S.G.; Petkovich, N.D. Perspective on the Influence of Interactions between Hard and Soft Templates and Precursors on Morphology of Hierarchically Structured Porous Materials. Chem. Mater. 2014, 26, 259–276. [Google Scholar]
- Parmentier, J.; Saadhallah, S.; Reda, M.; Gibot, P.; Roux, M.; Vidal, L.; Vix-Guterl, C.; Patarin, J. New Carbons with Controlled Nanoporosity Obtained by Nanocasting Using a SBA-15 Mesoporous Silica Host Matrix and Different Preparation Routes. J. Phys. Chem. Solids 2004, 65, 139–146. [Google Scholar]
- Braghiroli, F.L.; Fierro, V.; Parmentier, J.; Pasc, A.; Celzard, A. Easy and Eco-Friendly Synthesis of Ordered Mesoporous Carbons by Self-Assembly of Tannin with a Block Copolymer. Green Chem. 2016, 18, 3265–3271. [Google Scholar]
- Lesbayev, B.; Auyelkhankyzy, M.; Ustayeva, G.; Yeleuov, M.; Rakhymzhan, N.; Maltay, A.; Maral, Y. Recent advances: Biomass-derived porous carbon materials. S. Afr. J. Chem. Eng. 2023, 43, 327–336. [Google Scholar]
- Perondi, D.; Bassanesi, G.R.; Manera, C.; Lazzari, L.K.; Godinho, M.; Zattera, A.J.; Dotto, G.L. From Cellulose to Graphene-Like Porous Carbon Nanosheets. Microporous Mesoporous Mater. 2021, 323, 111217–111230. [Google Scholar]
- Kasturi, P.R.; Ramasamy, H.; Meyrick, D.; Sung Lee, Y.; Kalai Selvan, R. Preparation of Starch-Based Porous Carbon Electrode and Biopolymer Electrolyte for All Solid-State Electric Double Layer Capacitor. J. Colloid Interface Sci. 2019, 554, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ji, R.; Sun, Q.; Li, W.; Cheng, H.; Han, J.; Jiang, X.; Song, Y.; Xue, J. Self-Activation of Potassium/Iron Citrate-Assisted Production of Porous Carbon/Porous Biochar Composites from Macroalgae for High-Performance Sorption of Sulfamethoxazole. Bioresour. Technol. 2023, 369, 128361–128369. [Google Scholar] [CrossRef]
- Weidner, E.; Dubadi, R.; Samojeden, B.; Piasecki, A.; Jesionowski, T.; Jaroniec, M.; Ciesielczyk, F. Mechanochemical Synthesis of Alumina-Based Catalysts Enriched with Vanadia and Lanthana for Selective Catalytic Reduction of Nitrogen Oxides. Sci. Rep. 2022, 12, 21294–21304. [Google Scholar]
- Szczęśniak, B.; Borysiuk, S.; Choma, J.; Jaroniec, M. Mechanochemical Synthesis of Highly Porous Materials. Mater. Horiz. 2020, 7, 1457–1473. [Google Scholar]
- Casco, M.E.; Badaczewski, F.; Grätz, S.; Tolosa, A.; Presser, V.; Smarsly, B.M.; Borchardt, L. Mechanochemical Synthesis of Porous Carbon at Room Temperature with a Highly Ordered Sp2 Microstructure. Carbon 2018, 139, 325–333. [Google Scholar] [CrossRef]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C.W. Recent Advances in Mechanochemical Production of Chemicals and Carbon Materials from Sustainable Biomass Resources. Renew. Sustain. Energy Rev. 2020, 130, 109944–109964. [Google Scholar]
- Schneidermann, C.; Jäckel, N.; Oswald, S.; Giebeler, L.; Presser, V.; Borchardt, L. Solvent-Free Mechanochemical Synthesis of Nitrogen-Doped Nanoporous Carbon for Electrochemical Energy Storage. ChemSusChem 2017, 10, 2416–2424. [Google Scholar] [CrossRef]
- Chen, A.; Yu, Y.; Zhang, Y.; Zang, W.; Yu, Y.; Zhang, Y.; Shen, S.; Zhang, J. Aqueous-Phase Synthesis of Nitrogen-Doped Ordered Mesoporous Carbon Nanospheres as an Efficient Adsorbent for Acidic Gases. Carbon 2014, 80, 19–27. [Google Scholar]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beckt, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Sayari, A.; Yang, Y. SBA-15 Templated Mesoporous Carbon: New Insights into the SBA-15 Pore Structure. Chem. Mater. 2005, 17, 6108–6113. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Lota, G.; Centeno, T.A.; Frackowiak, E. Templated Mesoporous Carbons for Supercapacitor Application. Electrochim. Acta 2005, 50, 2799–2805. [Google Scholar] [CrossRef] [Green Version]
- Cha, W.S.; Talapaneni, S.N.; Kempaiah, D.M.; Joseph, S.; Lakhi, K.S.; Al-Enizi, A.M.; Park, D.H.; Vinu, A. Excellent supercapacitance performance of 3-D mesoporous carbon with large pores from FDU-12 prepared using a microwave method. RSC Adv. 2018, 8, 17017–17024. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S. Synthesis of Ordered Mesoporous Carbon by Soft Template Method. Mater. Today Proc. 2021, 81, 842–847. [Google Scholar] [CrossRef]
- Phuriragpitikhon, J.; Ghimire, P.; Jaroniec, M. Tannin-Derived Micro-Mesoporous Carbons Prepared by One-Step Activation with Potassium Oxalate and CO2. J. Colloid Interface Sci. 2019, 558, 55–67. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zeng, L.; Li, X.; Chen, N.; Bai, S.; He, H.; Wang, Q.; Zhang, C. A Review on Mechanochemistry: Approaching Advanced Energy Materials with Greener Force. Adv. Mater. 2022, 34, 2108327–2108357. [Google Scholar] [CrossRef]
- Dubadi, R.; Huang, S.D.; Jaroniec, M. Mechanochemical Synthesis of Nanoparticles for Potential Antimicrobial Applications. Materials 2023, 16, 1460. [Google Scholar] [CrossRef]
- Chalk, S.; McEwen, L. The IUPAC Gold Book. Chem. Int. 2017, 39, 25–30. [Google Scholar] [CrossRef]
- Xu, C.; De, S.; Balu, A.M.; Ojeda, M.; Luque, R. Mechanochemical Synthesis of Advanced Nanomaterials for Catalytic Applications. Chem. Comm 2015, 51, 6698–6713. [Google Scholar] [CrossRef]
- Amrute, A.P.; De Bellis, J.; Felderhoff, M.; Schüth, F. Mechanochemical Synthesis of Catalytic Materials. Chem.-A Eur. J 2021, 27, 6819–6847. [Google Scholar] [CrossRef] [PubMed]
- Dubadi, R.; Weidner, E.; Samojeden, B.; Jesionowski, T.; Ciesielczyk, F.; Huang, S.; Jaroniec, M. Exploring the Multifunctionality of Mechanochemically Synthesized γ-Alumina with Incorporated Selected Metal Oxide Species. Molecules 2023, 28, 2002. [Google Scholar] [CrossRef] [PubMed]
- Szczȩśniak, B.; Choma, J.; Jaroniec, M. Recent Advances in Mechanochemical Synthesis of Mesoporous Metal Oxides. Mater. Adv. 2021, 2, 2510–2523. [Google Scholar] [CrossRef]
- Szczęśniak, B.; Phuriragpitikhon, J.; Choma, J.; Jaroniec, M. Mechanochemical Synthesis of Three-Component Graphene Oxide/Ordered Mesoporous Carbon/Metal-Organic Framework Composites. J. Colloid Interface Sci. 2020, 577, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, L.; Yang, S.; Schott, J.A.; Liu, X.; Mahurin, S.M.; Huang, C.; Zhang, Y.; Fulvio, P.F.; Chisholm, M.F.; et al. Solid-State Synthesis of Ordered Mesoporous Carbon Catalysts via a Mechanochemical Assembly through Coordination Cross-Linking. Nat. Commun. 2017, 8, 15020–15030. [Google Scholar] [CrossRef] [Green Version]
- Castro-Gutiérrez, J.; Sanchez-Sanchez, A.; Ghanbaja, J.; Díez, N.; Sevilla, M.; Celzard, A.; Fierro, V. Synthesis of Perfectly Ordered Mesoporous Carbons by water assisted mechanochemical self-assembly of tannin. Green Chem. 2018, 20, 5123–5132. [Google Scholar] [CrossRef] [Green Version]
- Jaroniec, M.; Kruk, M.; Olivier, J.P. Standard Nitrogen Adsorption Data for Characterization of Nanoporous Silicas. Langmuir 1999, 15, 5410–5413. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M. Gas Adsorption Characterization of Ordered Organic-Inorganic Nanocomposite Materials. Chem. Mater. 2001, 12, 3169–3183. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Feng, D.; Zhao, Y.; Zhang, Y.; Sun, S.; Meng, S.; Guo, Y.; Huang, Y. Effects of K and Ca on Reforming of Model Tar Compounds with Pyrolysis Biochars under H2O or CO2. Chem. Eng. J. 2016, 306, 422–432. [Google Scholar] [CrossRef]
- Konai, N.; Raidandi, D.; Pizzi, A.; Girods, P.; Lagel, M.C.; Kple, M. Thermogravimetric Analysis of Anningre Tannin Resin. Maderas Cienc. Y Tecnol. 2016, 18, 245–252. [Google Scholar] [CrossRef]
- Tanaka, S.; Doi, A.; Nakatani, N.; Katayama, Y.; Miyake, Y. Synthesis of Ordered Mesoporous Carbon Films, Powders, and Fibers by Direct Triblock-Copolymer-Templating Method Using an Ethanol/Water System. Carbon 2009, 47, 2688–2698. [Google Scholar] [CrossRef]
- Shang, H.; Lu, Y.; Zhao, F.; Chao, C.; Zhang, B.; Zhang, H. Preparing high surface area porous carbon from biomass by carbonization in a molten salt medium. RSC Adv. 2015, 5, 75728–75734. [Google Scholar] [CrossRef]
- Zeng, H.; Qu, X.; Xu, D.; Luo, Y. Porous adsorption materials for carbon dioxide capture in industrial flue gas. Front. Chem. 2022, 10, 939701–939718. [Google Scholar] [CrossRef]
- Marszewska, J.; Jaroniec, M. Tailoring Porosity in Carbon Spheres for Fast Carbon Dioxide Adsorption. J. Colloid Interface Sci. 2017, 487, 162–174. [Google Scholar] [CrossRef]
- Wickramaratne, N.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Nelson, K.M.; Mahurin, S.M.; Mayes, R.T.; Williamson, B.; Teague, C.M.; Binder, A.J.; Baggetto, L.; Veith, G.M.; Dai, S. Preparation and CO2 adsorption properties of soft-templated mesoporous carbons derived from chestnut tannin precursors. Microporous Mesoporous Mater. 2016, 222, 94–103. [Google Scholar] [CrossRef]
- Saning, A.; Dubadi, R.; Chuenchom, L.; Dechtrirat, D.; Jaroniec, M. Microporous Carbons Obtained via Solvent-Free Mechanochemical Processing, Carbonization and Activation with Potassium Citrate and Zinc Chloride for CO2 Adsorption. Separations 2023, 10, 304. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, W.; Zhang, P.; Dai, S. Solvent-free and mechanochemical synthesis of N-doped mesoporous carbon from tannin and related gas sorption property. Chem. Eng. J. 2020, 381, 122579–122613. [Google Scholar] [CrossRef]




| Sample Notation | Tannin/Polymer wt. Ratio | Carbon Yield (g) | % Yield |
|---|---|---|---|
| TFG-0.5 | 1:0.5 | 1.22 | 61.2 |
| TFG-0.75 | 1:0.75 | 1.04 | 51.9 |
| TFG-1 | 1:1 | 1.16 | 58.1 |
| TFG-1.1 | 1:1.1 | 1.07 | 53.3 |
| TFG-1.25 | 1:1.25 | 1.22 | 61.1 |
| TFG-1.5 | 1:1.5 | 0.98 | 49.0 |
| TPG-0.5 | 1:0.5 | 0.91 | 45.6 |
| TPG-1 | 1:1 | 0.91 | 45.5 |
| TPG-1.25 | 1:1.25 | 0.86 | 43.0 |
| TPG-1.5 | 1:1.5 | 0.94 | 46.7 |
| TF-1 | 1:1 | 1.01 | 50.3 |
| TP-1 | 1:1 | 0.80 | 40.2 |
| T | - | 0.49 | 24.5 |
| Symbol | Carbon (%) | Hydrogen (%) |
|---|---|---|
| TFG-0.5 | 84.97 | 0.99 |
| TFG-0.75 | 87.50 | 1.16 |
| TFG-1N | 84.63 | 0.66 |
| TFG-1.1 | 85.50 | 0.89 |
| TFG-1.25 | 85.39 | 0.78 |
| TFG-1.5 | 83.80 | 0.79 |
| TPG-0.5 | 84.98 | 0.75 |
| TPG-1N | 85.71 | 1.17 |
| TPG-1.25 | 81.13 | 0.61 |
| TPG-1.5 | 85.74 | 0.68 |
| TF-1 | 84.59 | 0.94 |
| TP-1 | 87.18 | 0.83 |
| T | 77.30 | 0.49 |
| Sample | VT (cm3g−1) | V < 0.7 nm (cm3g−1) | V < 1 nm (cm3g−1) | V < 2 nm (cm3g−1) | Vme (cm3g−1) | Micropore Width at PSD Max, w (nm) | SBET (m2g−1) | nCO2 (25 °C) (mmolg−1) |
|---|---|---|---|---|---|---|---|---|
| TFG-0.5 | 0.59 | 0.16 | 0.19 | 0.22 | 0.37 | 0.55 | 663 | 2.87 |
| TFG-0.75 | 0.54 | 0.16 | 0.18 | 0.21 | 0.33 | 0.78 | 766 | 2.84 |
| TFG-1 | 0.72 | 0.22 | 0.25 | 0.29 | 0.43 | 0.52 | 868 | 2.93 |
| TFG-1.1 | 0.58 | 0.22 | 0.25 | 0.28 | 0.30 | 0.78 | 634 | 2.49 |
| TFG-1.25 | 0.73 | 0.22 | 0.26 | 0.30 | 0.43 | 0.76 | 883 | 2.01 |
| TFG-1.5 | 0.63 | 0.19 | 0.21 | 0.25 | 0.28 | 0.56 | 610 | 2.64 |
| TPG-1 | 0.63 | 0.25 | 0.30 | 0.33 | 0.30 | 0.52 | 888 | 3.20 |
| TPG-0.5 | 0.59 | 0.16 | 0.19 | 0.22 | 0.37 | 0.55 | 663 | 3.06 |
| TPG-1.25 | 0.73 | 0.22 | 0.26 | 0.30 | 0.43 | 0.76 | 883 | 3.18 |
| TPG-1.5 | 0.63 | 0.19 | 0.21 | 0.25 | 0.28 | 0.56 | 610 | 2.78 |
| Sample | Activator | SBET (m2g−1) | VT (cm3g−1) | CO2 Uptake (mmolg−1) at 25 °C and 1 bar | Refs. |
|---|---|---|---|---|---|
| 1 N1N40G30 | Anhydrous NH3 | 520 | 0.47 | 1.81 | [48] |
| 2 CTPC-A | PC, CO2, Mechanochemical | 1256 | 0.42 | 3.6 | [49] |
| 3 MC-Serine-0.3 | Zinc acetate, Mechanochemical | 471 | 0.51 | 2.5 | [50] |
| 4 N-OMCs-0.1 | KOH | 566 | 0.31 | 2.43 | [20] |
| 5 TG-C700-4K | PO, CO2 | 1192 | 0.90 | 3.6 | [26] |
| TFG-1 | One step CO2 | 868 | 0.72 | 2.93 | This work |
| TPG-1 | One step CO2 | 888 | 0.63 | 3.2 | This work |
| TPG-1.25 | One step CO2 | 883 | 0.73 | 3.18 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubadi, R.; Jaroniec, M. One-Pot Mechanochemical Synthesis of Carbons with High Microporosity and Ordered Mesopores for CO2 Uptake at Ambient Conditions. Nanomaterials 2023, 13, 2262. https://doi.org/10.3390/nano13152262
Dubadi R, Jaroniec M. One-Pot Mechanochemical Synthesis of Carbons with High Microporosity and Ordered Mesopores for CO2 Uptake at Ambient Conditions. Nanomaterials. 2023; 13(15):2262. https://doi.org/10.3390/nano13152262
Chicago/Turabian StyleDubadi, Rabindra, and Mietek Jaroniec. 2023. "One-Pot Mechanochemical Synthesis of Carbons with High Microporosity and Ordered Mesopores for CO2 Uptake at Ambient Conditions" Nanomaterials 13, no. 15: 2262. https://doi.org/10.3390/nano13152262





