Ni-Modified Ag/SiO2 Catalysts for Selective Hydrogenation of Dimethyl Oxalate to Methyl Glycolate
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation
2.3. Characterization
2.4. Reaction Testing
3. Results and Discussion
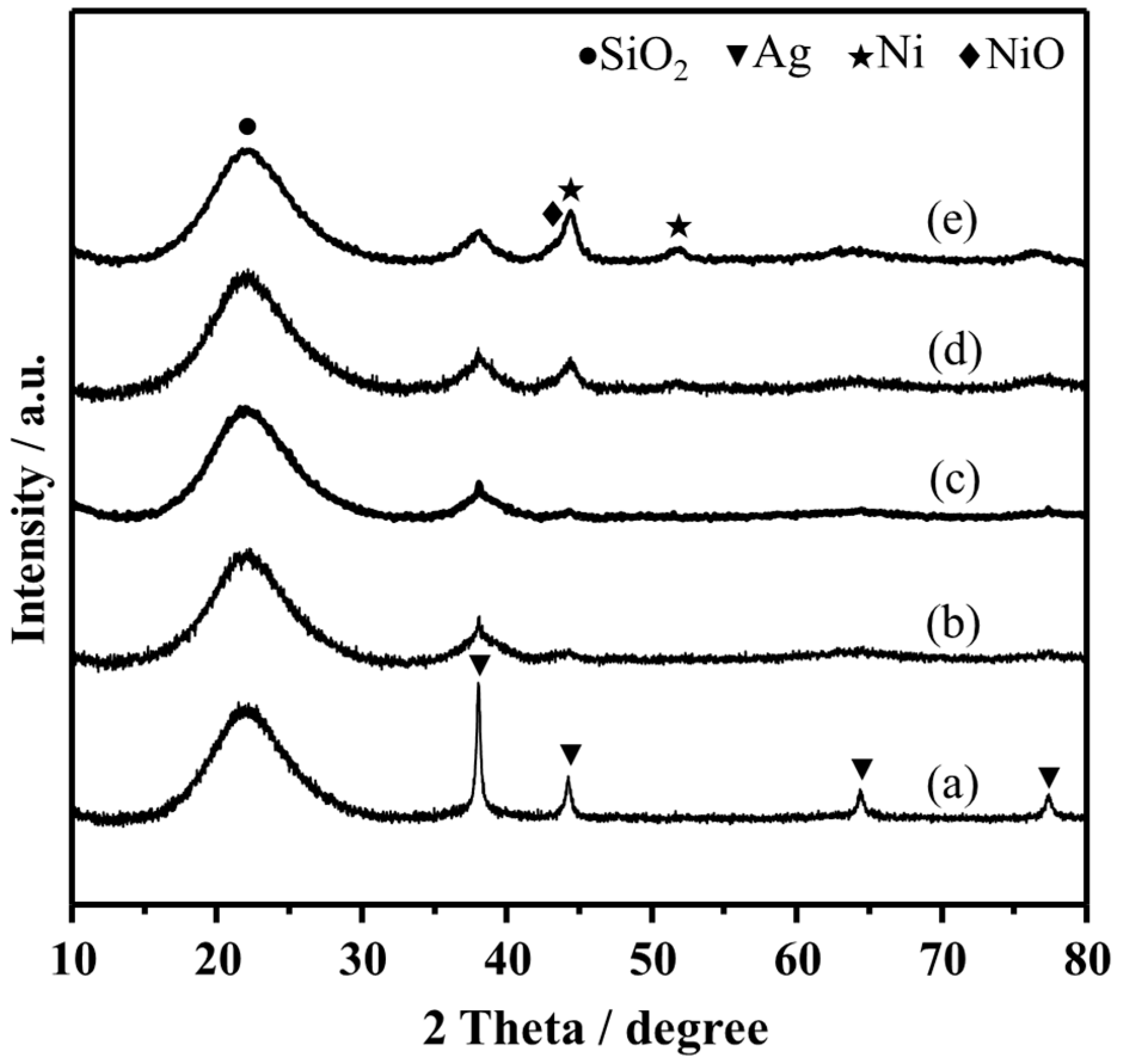
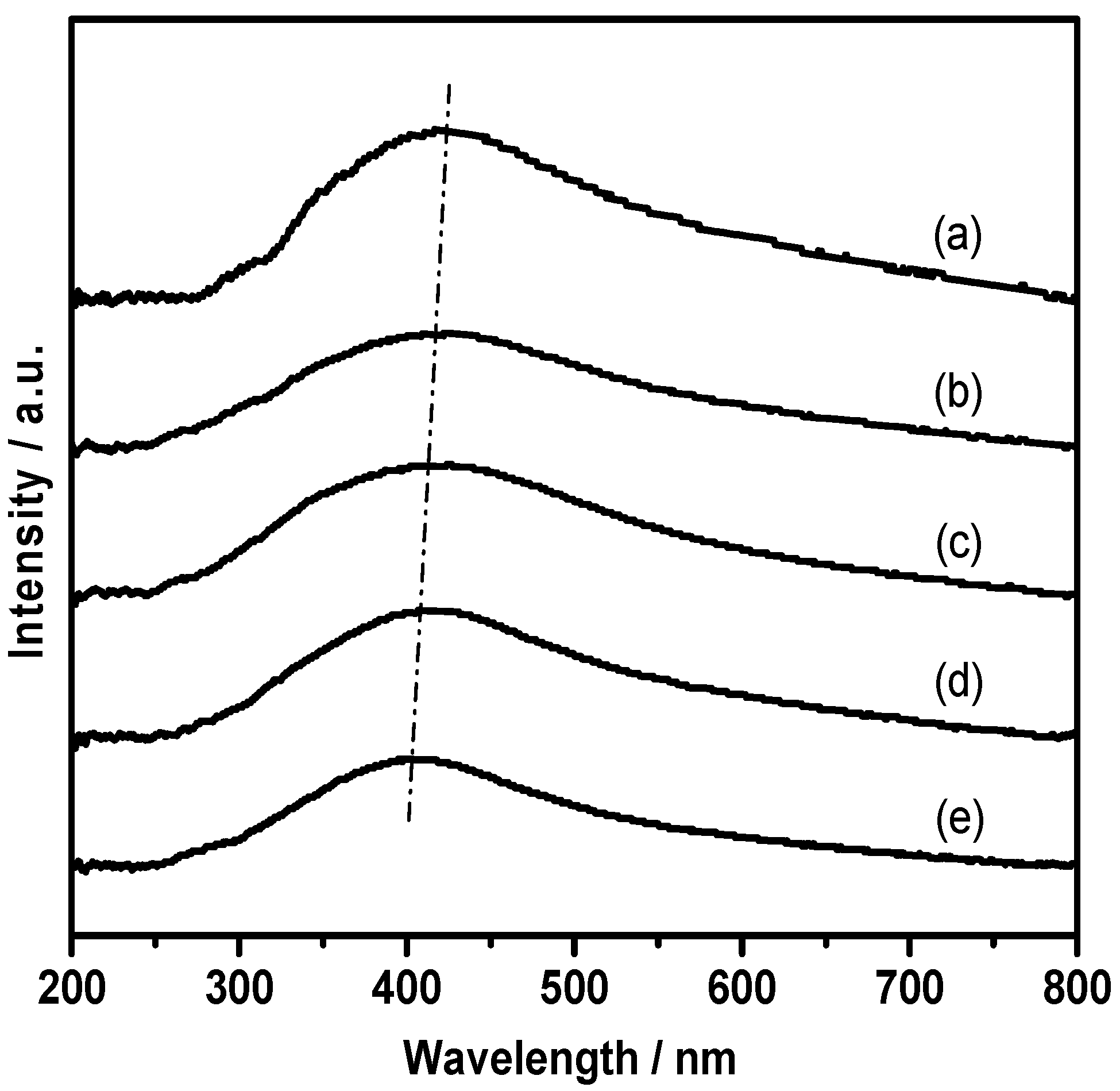
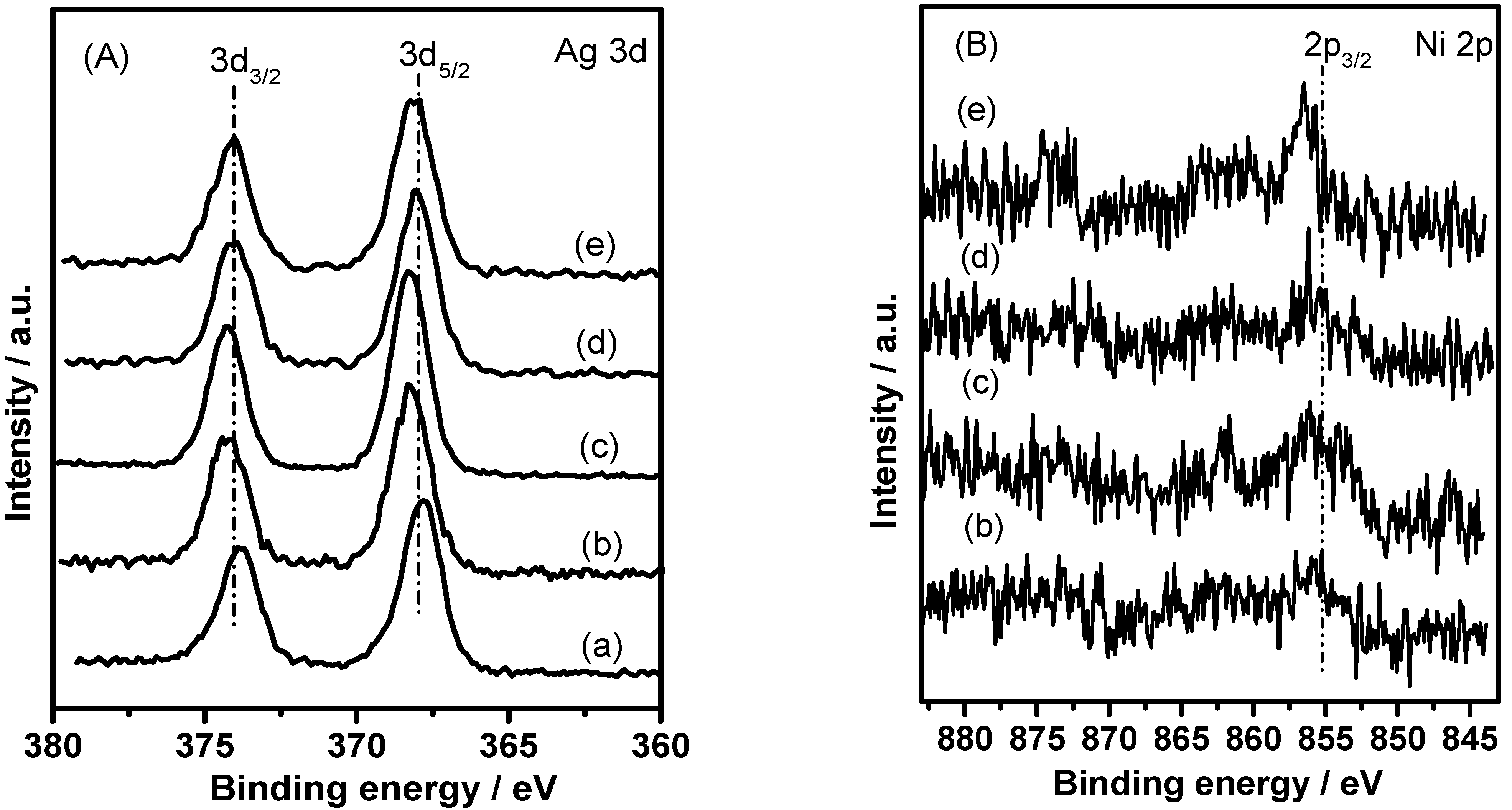
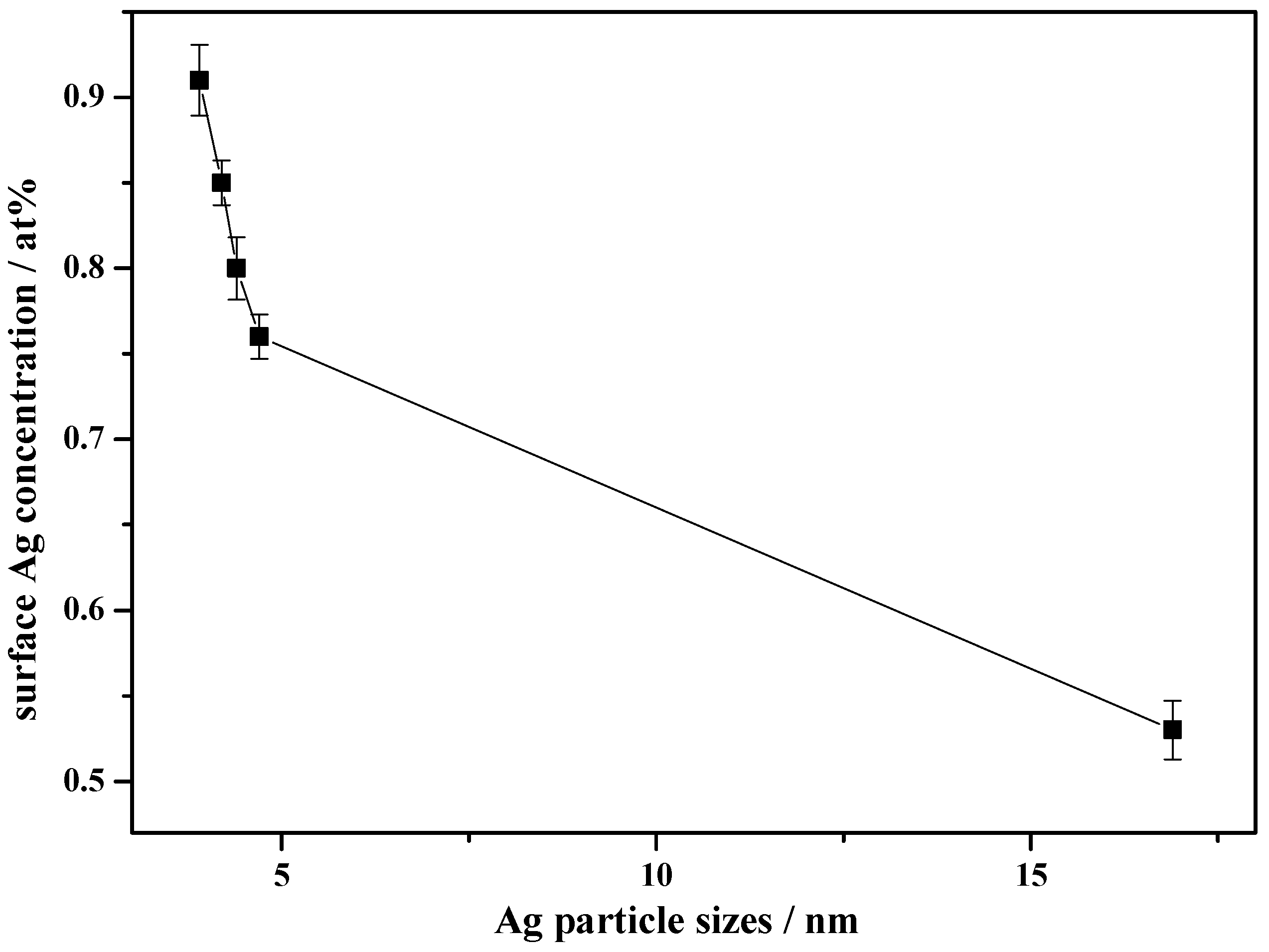
3.1. Characterization
3.2. Catalytic Performance
3.3. Catalyst Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, J.W.; Wang, X.H.; Zhao, J.X.; Jiang, S.H.; Quan, Y.H.; Pei, Y.L.; Wu, M.M.; Ren, J. Density-functional theory study on hydrogenation of dimethyl oxalate to methyl glycolate over copper catalyst: Effect of copper valence state. Mol. Catal. 2020, 482, 110667. [Google Scholar] [CrossRef]
- Zhu, J.; Cao, L.Q.; Li, C.Y.; Zhao, G.F.; Zhu, T.; Hu, W.; Sun, W.D.; Lu, Y. Nanoporous Ni3P evolutionarily structured onto a Ni foam for highly selective hydrogenation of dimethyl oxalate to methyl glycolate. ACS Appl. Mater. Interfaces 2019, 11, 37635–37643. [Google Scholar] [CrossRef]
- Huang, H.J.; Wang, B.; Wang, Y.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. Partial hydrogenation of dimethyl oxalate on Cu/SiO2 catalyst modified by sodium silicate. Catal. Today 2020, 358, 68–73. [Google Scholar] [CrossRef]
- Wang, B.W.; Xu, Q.; Song, H.; Xu, G.H. Synthesis of methyl glycolate by hydrogenation of dimethyl oxalate over Cu-Ag/SiO2 catalyst. J. Nat. Gas Chem. 2007, 16, 78–80. [Google Scholar] [CrossRef]
- Zhou, J.F.; Duan, X.P.; Ye, L.M.; Zheng, J.W.; Li, M.M.J.; Tsang, S.C.E.; Yuan, Y.Z. Enhanced chemoselective hydrogenation of dimethyl oxalate to methyl glycolate over bimetallic Ag–Ni/SBA-15 catalysts. Appl. Catal. A 2015, 505, 344–353. [Google Scholar] [CrossRef]
- Abbas, M.; Chen, Z.; Zhang, J.; Chen, J. Highly dispersed, ultra-small and noble metal-free Cu nanodots supported on porous SiO2 and their excellent catalytic hydrogenation of dimethyl oxalate to methyl glycolate. New J. Chem. 2018, 42, 10290–10299. [Google Scholar] [CrossRef]
- Dong, G.L.; Luo, Z.W.; Cao, Y.Q.; Zheng, S.N.; Zhou, J.H.; Li, W.; Zhou, X.G. Understanding size-dependent hydrogenation of dimethyl oxalate to methyl glycolate over Ag catalysts. J. Catal. 2021, 401, 252–261. [Google Scholar] [CrossRef]
- Chen, H.M.; Tan, J.J.; Zhu, Y.L.; Li, Y.W. An effective and stable Ni2P/TiO2 catalyst for the hydrogenation of dimethyl oxalate to methyl glycolate. Catal. Commun. 2016, 73, 46–49. [Google Scholar] [CrossRef]
- Chen, Y.F.; Han, L.P.; Zhu, J.; Chen, P.J.; Fan, S.Y.; Zhao, G.F.; Liu, Y.; Lu, Y. High-performance Ag-CuOx nanocomposite catalyst galvanically deposited onto a Ni-foam for gas-phase dimethyl oxalate hydrogenation to methyl glycolate. Catal. Commun. 2017, 96, 58–62. [Google Scholar] [CrossRef]
- Abbas, M.; Zhang, J.; Chen, J.G. Sonochemical engineering of highly efficient and robust Au nanoparticle-wrapped on Fe/ZrO2 nanorods and their controllable product selectivity in dimethyl oxalate hydrogenation. Catal. Sci. Technol. 2020, 10, 1125–1134. [Google Scholar] [CrossRef]
- Ye, R.P.; Lin, L.; Wang, L.C.; Ding, D.; Zhou, Z.F.; Pan, P.B.; Xu, Z.H.; Liu, J.; Adidharma, H.; Radosz, M.; et al. Perspectives on the active sites and catalyst design for the hydrogenation of dimethyl oxalate. ACS Catal. 2020, 10, 4465–4490. [Google Scholar] [CrossRef]
- Yin, A.Y.; Wen, C.; Dai, W.L.; Fan, K.N. Ag/MCM-41 as a highly efficient mesostructured catalyst for the chemoselective synthesis of methyl glycolate and ethylene glycol. Appl. Catal. B 2011, 108, 90–99. [Google Scholar] [CrossRef]
- Zheng, J.W.; Lin, H.Q.; Zheng, X.L.; Duan, X.P.; Yuan, Y.Z. Highly efficient mesostructured Ag/SBA-15 catalysts for the chemoselective synthesis of methyl glycolate by dimethyl oxalate hydrogenation. Catal. Commun. 2013, 40, 129–133. [Google Scholar] [CrossRef]
- Zheng, J.W.; Duan, X.P.; Lin, H.Q.; Gu, Z.Q.; Fang, H.H.; Li, J.H.; Yuan, Y.Z. Silver nanoparticles confined in carbon nanotubes: On the understanding of the confinement effect and promotional catalysis for the selective hydrogenation of dimethyl oxalate. Nanoscale 2016, 8, 5959–5967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, M.Y.; Wang, Y.; Zhang, J.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. Three dimensional Ag/KCC-1 catalyst with a hierarchical fibrous framework for the hydrogenation of dimethyl oxalate. RSC Adv. 2016, 6, 12788–12791. [Google Scholar] [CrossRef]
- Ouyang, M.Y.; Wang, J.; Peng, B.; Zhao, Y.J.; Wang, S.P.; Ma, X.B. Effect of Ti on Ag catalyst supported on spherical fibrous silica for partial hydrogenation of dimethyl oxalate. Appl. Surf. Sci. 2019, 466, 592–600. [Google Scholar] [CrossRef]
- Liu, Y.T.; Ding, J.; Yang, J.Y.; Bi, J.C.; Liu, K.F.; Chen, J.G. Stabilization of copper catalysts for hydrogenation of dimethyl oxalate by deposition of Ag clusters on Cu nanoparticles. Catal. Commun. 2017, 98, 43–46. [Google Scholar] [CrossRef]
- Hu, M.L.; Yan, Y.; Duan, X.P.; Ye, L.M.; Zhou, J.F.; Lin, H.Q.; Yuan, Y.Z. Effective anchoring of silver nanoparticles onto N-doped carbon with enhanced catalytic performance for the hydrogenation of dimethyl oxalate to methyl glycolate. Catal. Commun. 2017, 100, 148–152. [Google Scholar] [CrossRef]
- Li, M.M.J.; Ye, L.M.; Zheng, J.W.; Fang, H.H.; Kroner, A.; Yuan, Y.Z.; Tsang, S.C.E. Surfactant-free nickel–silver core@shell nanoparticles in mesoporous SBA-15 for chemoselective hydrogenation of dimethyl oxalate. Chem. Commun. 2016, 52, 2569–2572. [Google Scholar] [CrossRef]
- Duan, X.P.; Chen, T.Y.; Chen, T.X.; Huang, L.L.; Ye, L.; Lo, B.T.W.; Yuan, Y.Z.; Tsang, S.C.E. Intercalating lithium into the lattice of silver nanoparticles boosts catalytic hydrogenation of carbon–oxygen bonds. Chem. Sci. 2021, 12, 8791–8802. [Google Scholar] [CrossRef]
- Takanabe, K.; Nagaoka, K.; Nariai, K.; Aika, K.I. Titania-supported cobalt and nickel bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2005, 232, 268–275. [Google Scholar] [CrossRef]
- Yamamoto, R.; Sawayama, Y.; Shibahara, H.; Ichihashi, Y.; Nishiyama, S.; Tsuruya, S. Promoted partial oxidation activity of supported Ag catalysts in the gas-phase catalytic oxidation of benzyl alcohol. J. Catal. 2005, 234, 308–317. [Google Scholar] [CrossRef]
- Prakash, M.G.; Mahalakshmy, R.; Krishnamurthy, K.R.; Viswanathan, B. Studies on Ni–M (M=Cu, Ag, Au) bimetallic catalysts for selective hydrogenation of cinnamaldehyde. Catal. Today 2016, 263, 105–111. [Google Scholar] [CrossRef]
- Pei, G.X.; Liu, X.Y.; Wang, A.; Su, Y.; Li, L.; Zhang, T. Selective hydrogenation of acetylene in an ethylene-rich stream over silica supported Ag-Ni bimetallic catalysts. Appl. Catal. A 2017, 545, 90–96. [Google Scholar] [CrossRef]
- Zheng, J.W.; Lin, H.Q.; Wang, Y.N.; Zheng, X.L.; Duan, X.P.; Yuan, Y.Z. Efficient low-temperature selective hydrogenation of esters on bimetallic Au–Ag/SBA-15 catalyst. J. Catal. 2013, 297, 110–118. [Google Scholar] [CrossRef]
- Cheng, S.; Mao, D.S.; Guo, X.M.; Yu, J. Synthesis of methyl glycolate from the hydrogenation of dimethyl oxalate on Ag/SiO2 catalyst: The effects of Ag contents and promoters. React. Kinet. Mech. Catal. 2019, 126, 1067–1079. [Google Scholar] [CrossRef]
- He, Z.; Lin, H.Q.; He, P.; Yuan, Y.Z. Effect of boric oxide doping on the stability and activity of a Cu–SiO2 catalyst for vapor-phase hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2011, 277, 54–63. [Google Scholar] [CrossRef]
- Song, Y.B.; Zhang, J.; Lv, J.; Zhao, Y.J.; Ma, X.B. Hydrogenation of dimethyl oxalate over copper-based catalysts: Acid–base properties and reaction paths. Ind. Eng. Chem. Res. 2015, 54, 9699–9707. [Google Scholar] [CrossRef]
- Yin, A.Y.; Guo, X.Y.; Dai, W.L.; Fan, K.N. The nature of active copper species in Cu-HMS catalyst for hydrogenation of dimethyl oxalate to ethylene glycol: New insights on the synergetic effect between Cu0 and Cu+. J. Phys. Chem. C 2009, 113, 11003–11013. [Google Scholar] [CrossRef]
- Kaddouri, A.; Gronchi, P.; Centola, P.; Del Rosso, R. On the preparation of Ni–La supported on silica by sol-gel process via propionates. J. Therm. Anal. Calorim. 2000, 62, 609–619. [Google Scholar] [CrossRef]
- Wang, W.; Song, M. Preparation of high nickel-containing MCM-41-type mesoporous silica via a modified direct synthesis method. Mater. Res. Bull. 2005, 40, 1737–1744. [Google Scholar] [CrossRef]
- Hengne, A.M.; Malawadkar, A.V.; Biradar, N.S.; Rode, C.V. Surface synergism of an Ag–Ni/ZrO2 nanocomposite for the catalytic transfer hydrogenation of bio-derived platform molecules. RSC Adv. 2014, 4, 9730–9736. [Google Scholar] [CrossRef]
- Chen, L.F.; Guo, P.J.; Qiao, M.H.; Yan, S.R.; Li, H.X.; Shen, W.; Xu, H.L.; Fan, K.N. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol. J. Catal. 2008, 257, 172–180. [Google Scholar] [CrossRef]
- Yen, C.W.; Lin, M.L.; Wang, A.Q.; Chen, S.A.; Chen, J.M.; Mou, C.Y. CO oxidation catalyzed by Au−Ag bimetallic nanoparticles supported in mesoporous silica. J. Phys. Chem. C 2009, 113, 17831–17839. [Google Scholar] [CrossRef]
- Rodríguez-González, B.; Sánchez-Iglesias, A.; Giersig, M.; Liz-Marzán, L.M. AuAg bimetallic nanoparticles: Formation, silica-coating and selective etching. Faraday Discuss. 2004, 125, 133–144. [Google Scholar] [CrossRef]
- Evanoff, D.D.J.; Chumanov, G. Synthesis and optical properties of silver nanoparticles and arrays. ChemPhysChem 2005, 6, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Zu, X.T.; Zhu, S.; Zhang, C.F.; Wang, L.M. Effect of annealing on the optical absorption of Ni nanoparticles in MgO single crystals. Nucl. Instrum. Methods Phys. Res. 2006, 250, 229–232. [Google Scholar] [CrossRef]
- Creighton, J.A.; Eadon, D.G. Ultraviolet–visible absorption spectra of the colloidal metallic elements. J. Chem. Soc. Faraday Trans. 1991, 87, 3881–3891. [Google Scholar] [CrossRef]
- Tsuji, M.; Hikino, S.; Matsunaga, M.; Sano, Y.; Hashizume, T.; Kawazumi, H. Rapid synthesis of Ag@Ni core–shell nanoparticles using a microwave-polyol method. Mater. Lett. 2010, 64, 1793–1797. [Google Scholar] [CrossRef]
- Chen, D.H.; Wang, S.R. Protective agent-free synthesis of Ni–Ag core–shell nanoparticles. Mater. Chem. Phys. 2006, 100, 468–471. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Bowmaker, G.A.; Metson, J.B. Oxidation of a polycrystalline silver foil by reaction with ozone. Appl. Surf. Sci. 2001, 183, 191–204. [Google Scholar] [CrossRef]
- Yin, A.Y.; Wen, C.; Guo, X.Y.; Dai, W.L.; Fan, K.N. Influence of Ni species on the structural evolution of Cu/SiO2 catalyst for the chemoselective hydrogenation of dimethyl oxalate. J. Catal. 2011, 280, 77–88. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Zhao, S.; Geng, Y.C.; Shen, Y.L.; Yue, H.R.; Lv, J.; Wang, S.P.; Ma, X.B. Ni-containing Cu/SiO2 catalyst for the chemoselective synthesis of ethanol via hydrogenation of dimethyl oxalate. Catal. Today 2016, 276, 28–35. [Google Scholar] [CrossRef]
- Lee, C.C.; Cheng, Y.Y.; Chang, H.Y.; Chen, D.H. Synthesis and electromagnetic wave absorption property of Ni–Ag alloy nanoparticles. J. Alloys Compd. 2009, 480, 674–680. [Google Scholar] [CrossRef]
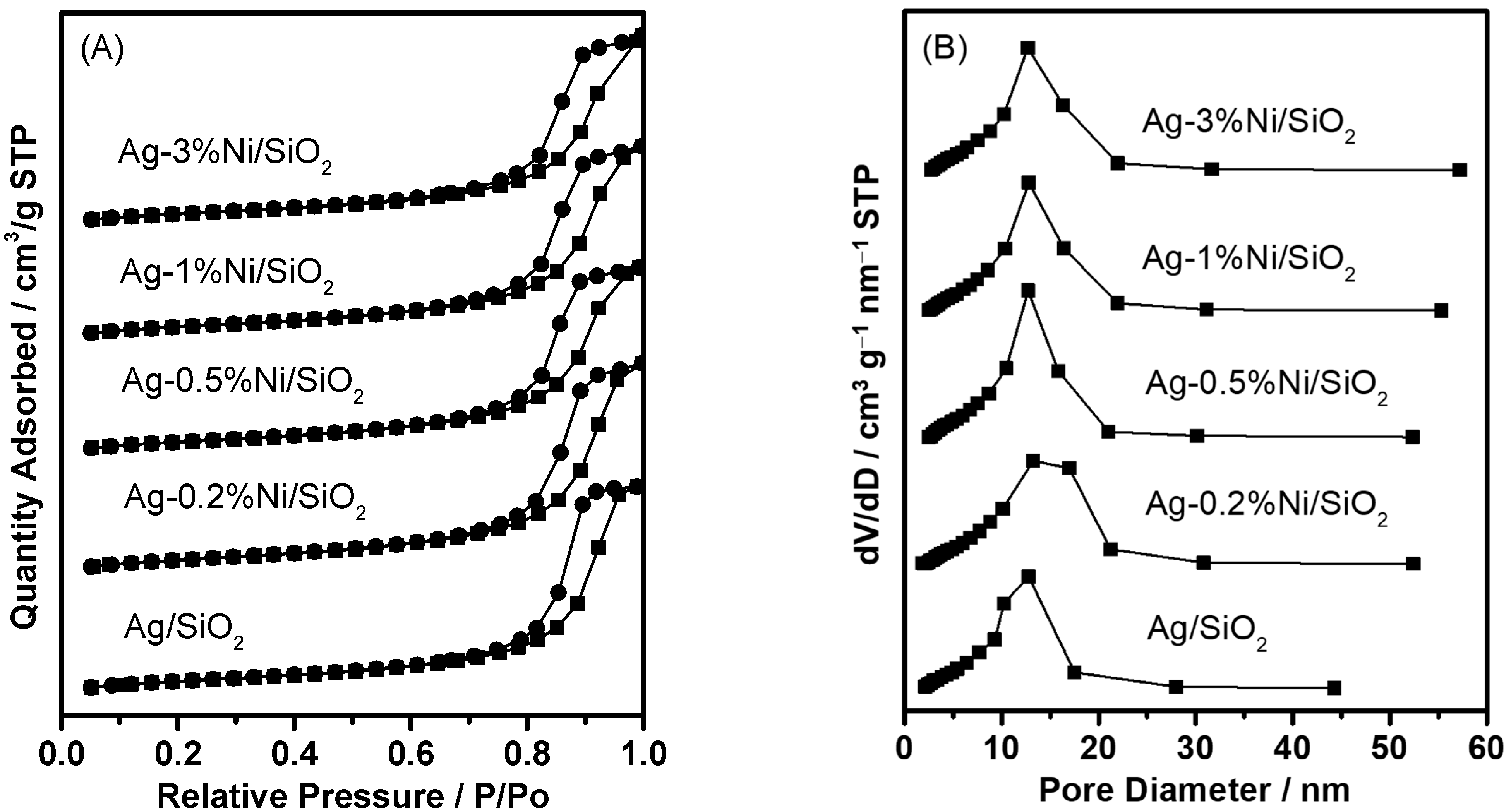
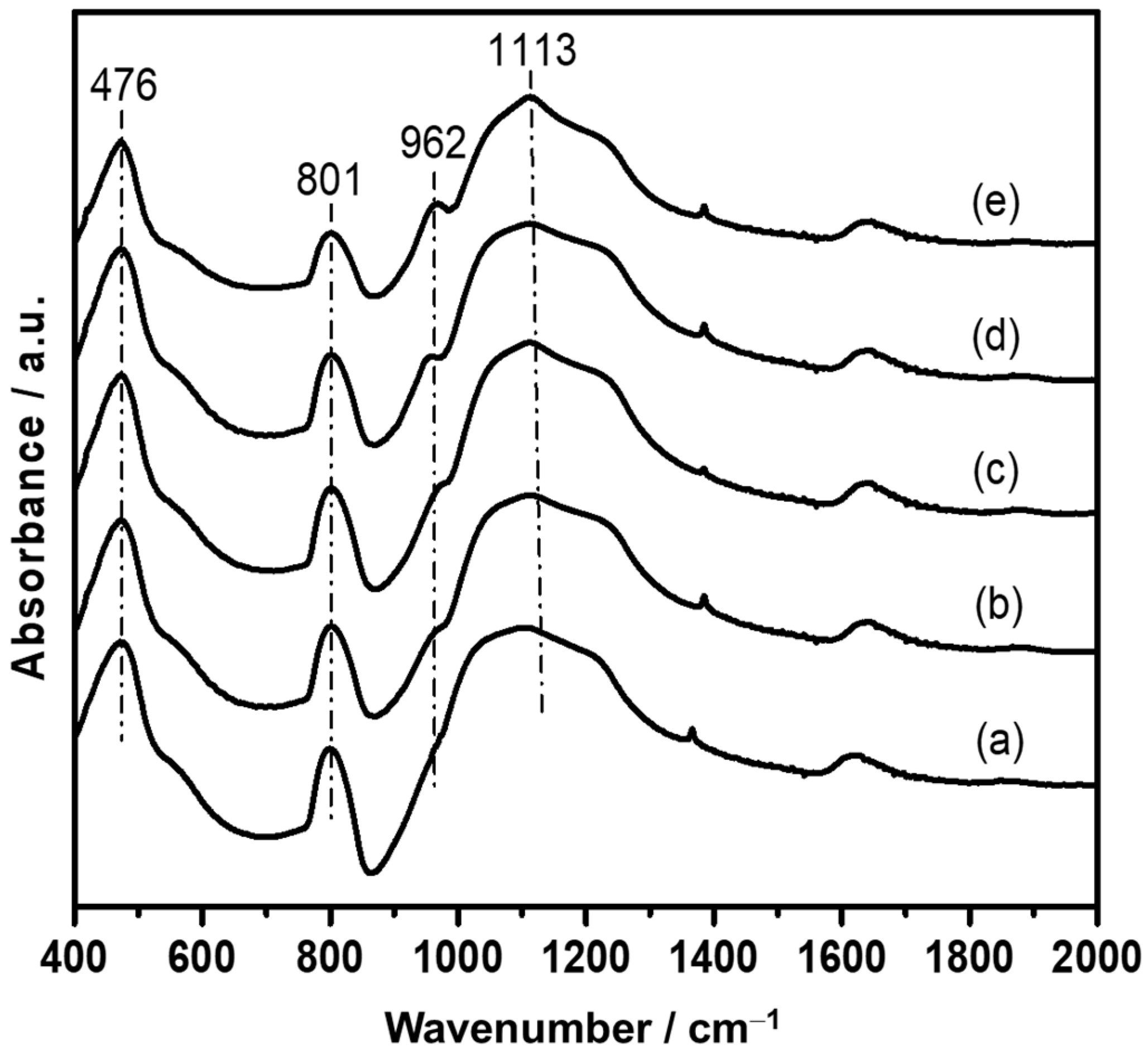
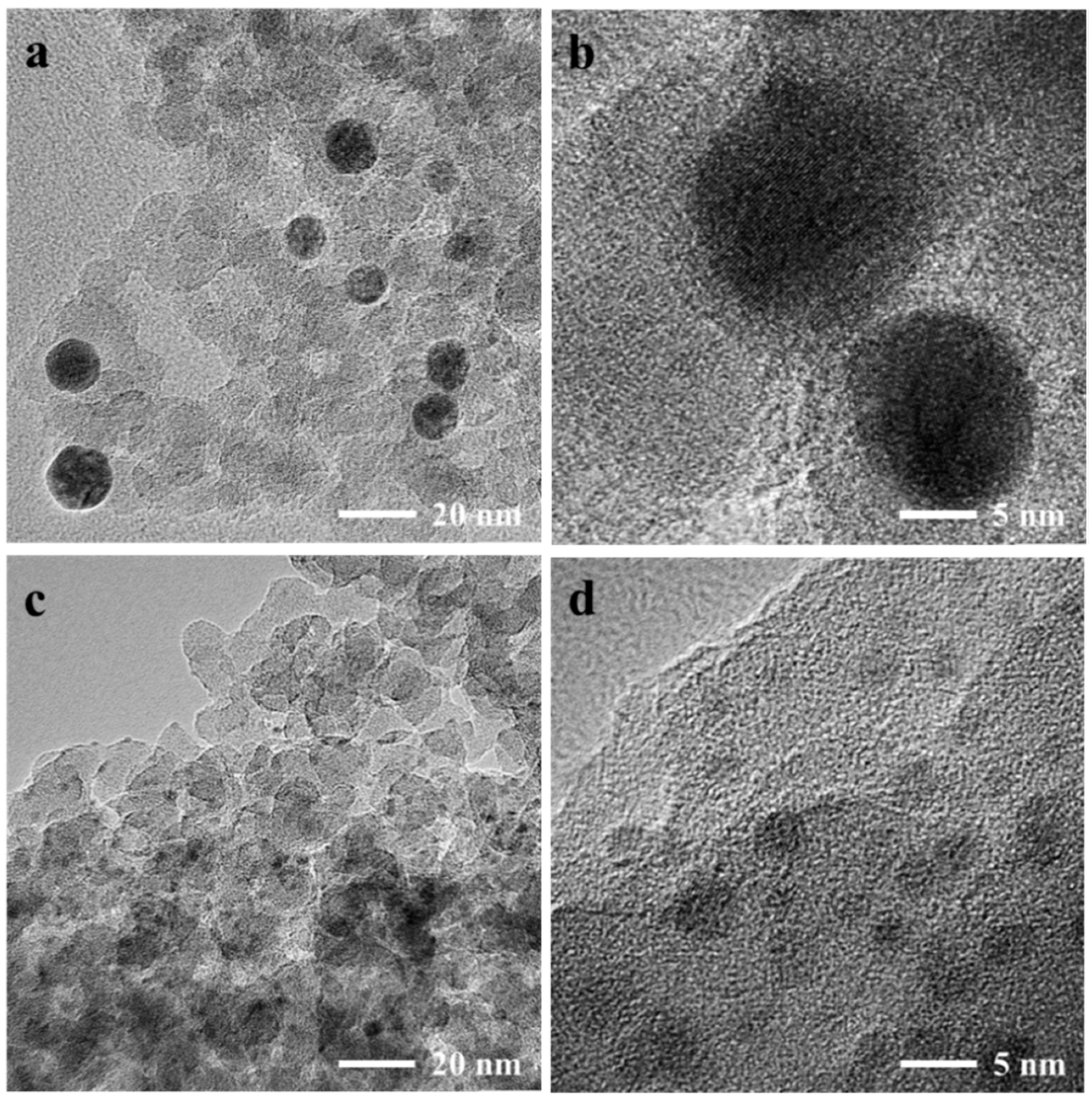
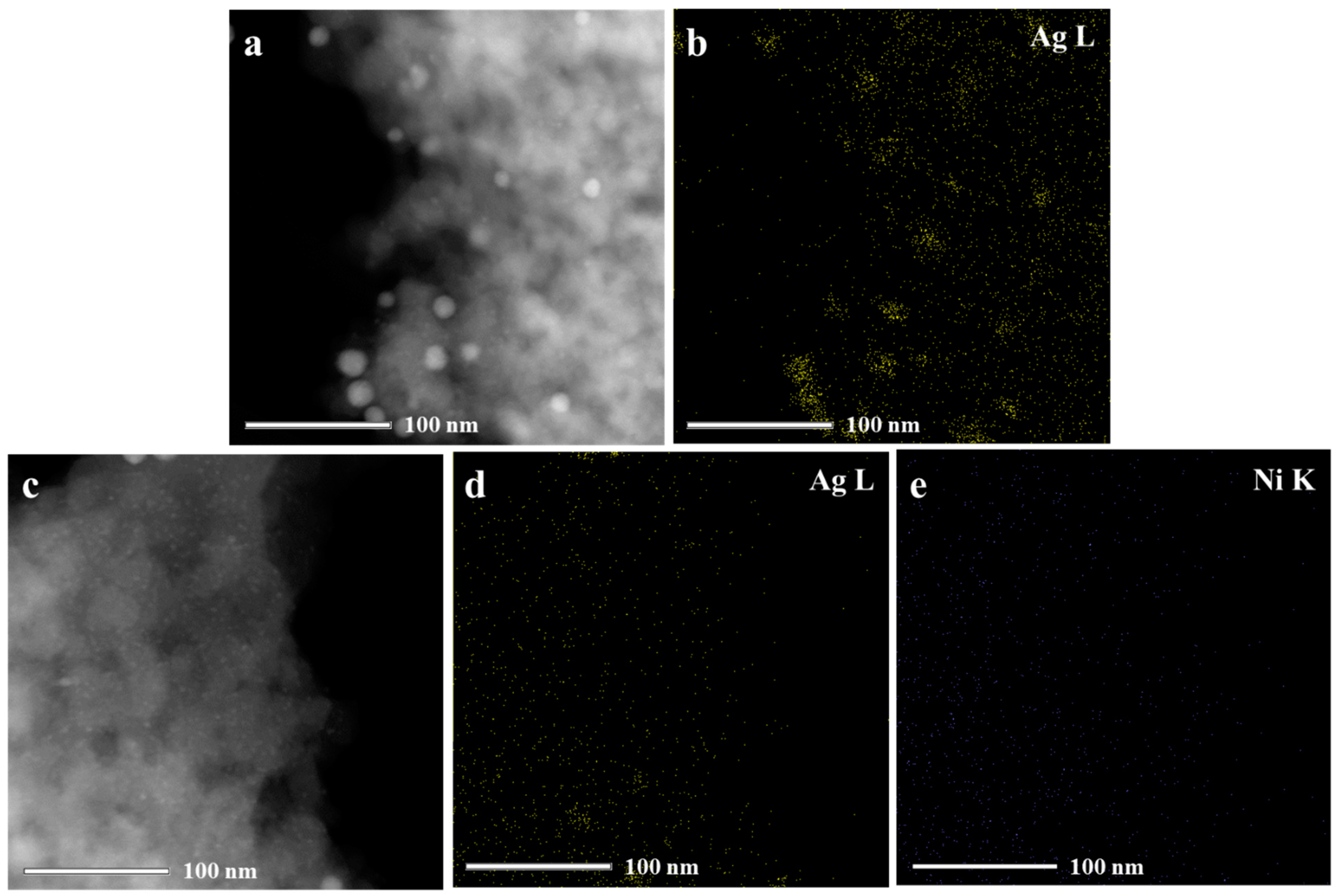

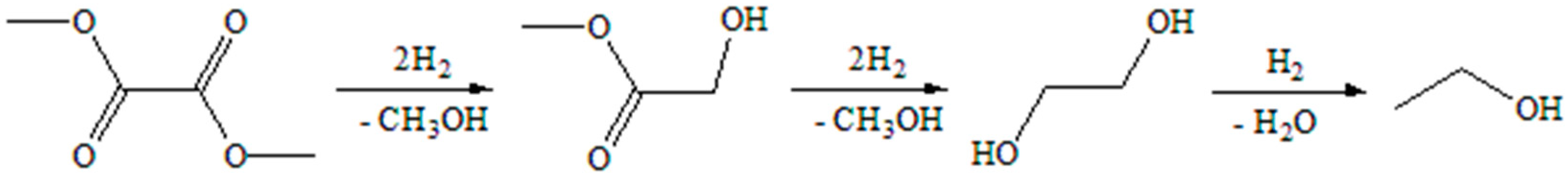
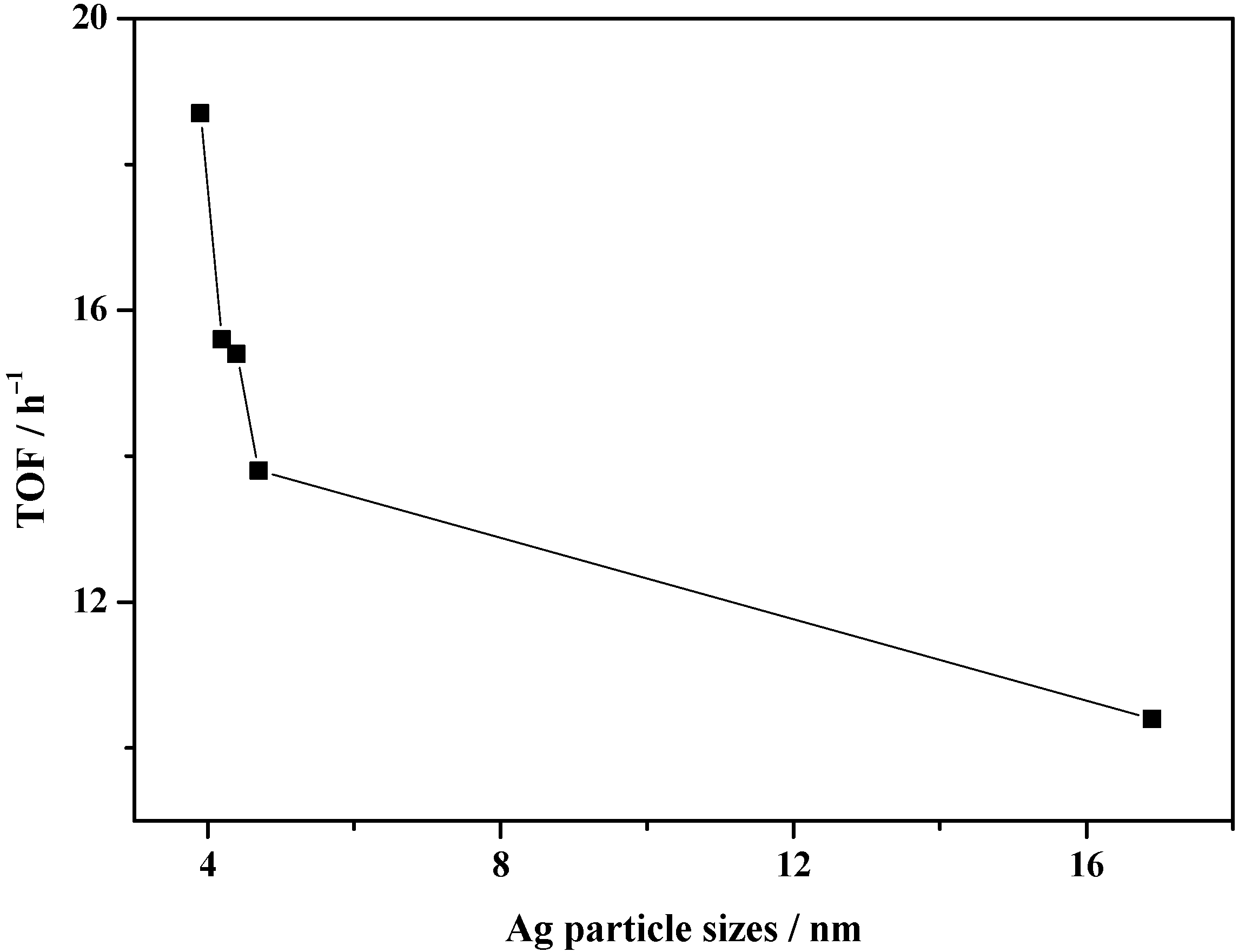

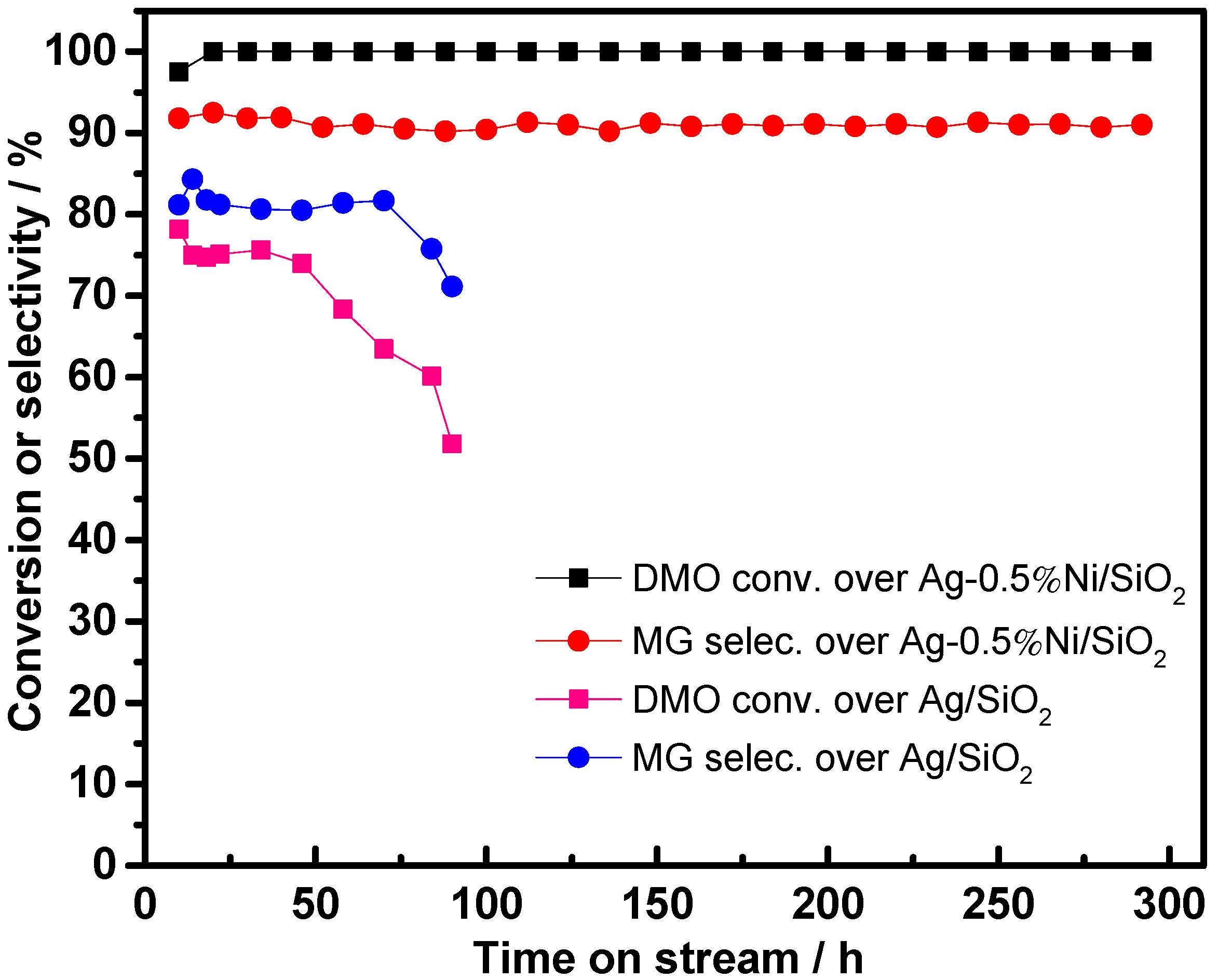
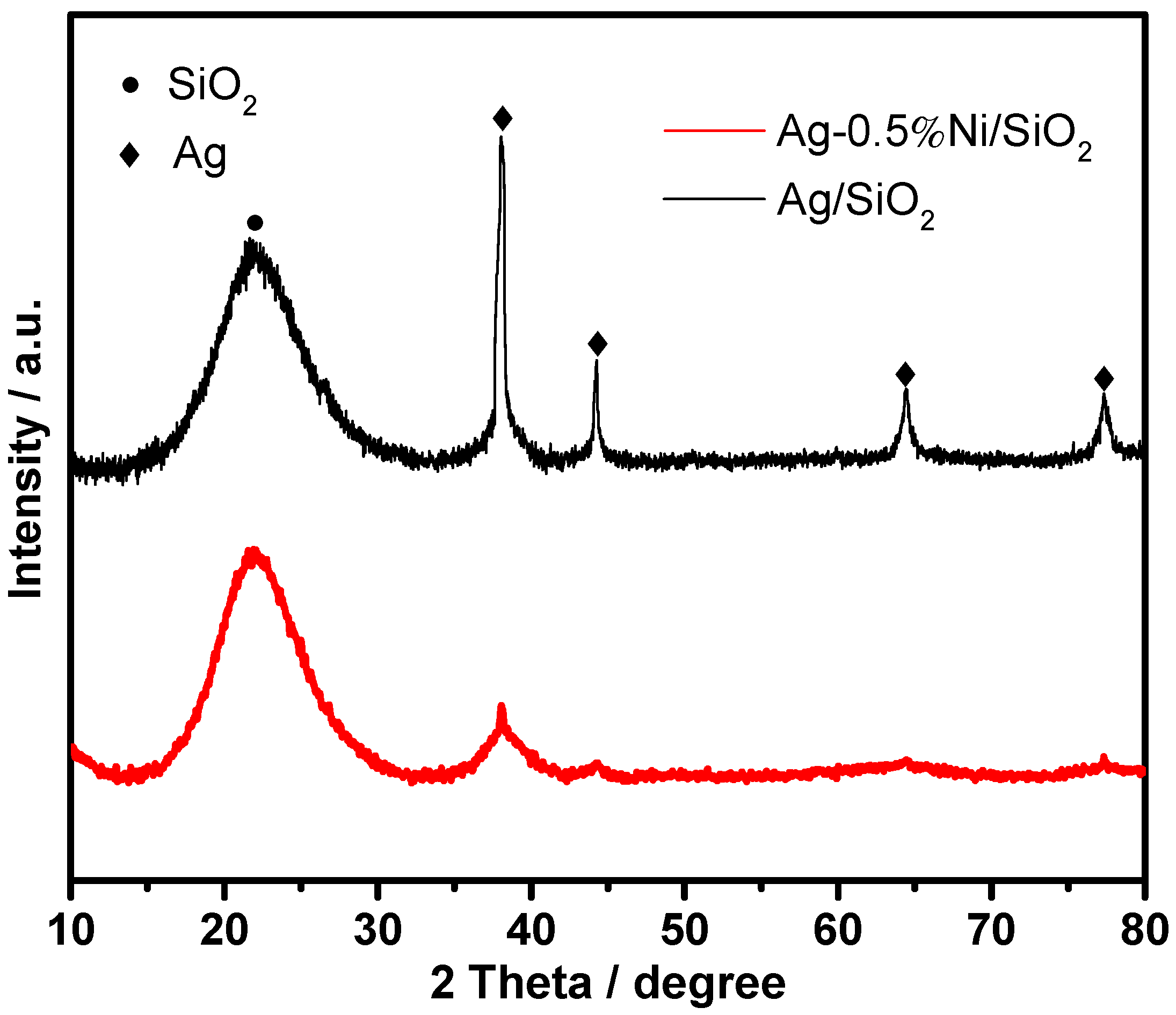
| Catalyst | SBETa (m2∙g) | Dporeb (nm) | Vporec (cm3∙g) | Ag Loading (wt.%) | Ni Loading (wt.%) | ||
|---|---|---|---|---|---|---|---|
| Theoretical | Actual d | Theoretical | Actual d | ||||
| Ag/SiO2 | 179.4 | 10.6 | 0.65 | 10 | 9.8 | 0 | - |
| Ag-0.2%Ni/SiO2 | 171.4 | 12.6 | 0.64 | 10 | 9.8 | 0.2 | 0.17 |
| Ag-0.5%Ni/SiO2 | 161.1 | 12.0 | 0.60 | 10 | 9.9 | 0.5 | 0.46 |
| Ag-1%Ni/SiO2 | 164.6 | 12.4 | 0.61 | 10 | 9.7 | 1.0 | 0.89 |
| Ag-3%Ni/SiO2 | 153.9 | 12.6 | 0.60 | 10 | 9.8 | 3.0 | 2.85 |
| Catalyst | Binding Energy (eV) | Surface Elemental Concentration (at.%) | ||
|---|---|---|---|---|
| Ag 3d5/2 | Ni 2p | Ag | Ni | |
| Ag/SiO2 | 367.8 | - | 0.53 | - |
| Ag-0.2%Ni/SiO2 | 368.5 | 855.3 | 0.85 | 0.26 |
| Ag-0.5%Ni/SiO2 | 368.4 | 856.1 | 0.91 | 0.32 |
| Ag-1%Ni/SiO2 | 368.1 | 856.2 | 0.80 | 0.89 |
| Ag-3%Ni/SiO2 | 368.0 | 856.4 | 0.76 | 1.02 |
| Catalyst | Conversion/% | Selectivity/% | dAg c (nm) | DAg d/% | TOFXRD e/h−1 | |
|---|---|---|---|---|---|---|
| MG | Others b | |||||
| Ag/SiO2 f | 15.7 | 91.9 | 8.1 | 16.9 | 9.0 | 10.4 |
| Ag-0.2%Ni/SiO2 | 24.7 | 95.6 | 4.4 | 4.2 | 28.0 | 15.6 |
| Ag-0.5%Ni/SiO2 | 29.6 | 100 | 0 | 3.9 | 30.2 | 18.7 |
| Ag-1%Ni/SiO2 | 23.2 | 97.6 | 2.5 | 4.4 | 26.8 | 15.4 |
| Ag-3%Ni/SiO2 | 19.5 | 96.1 | 3.9 | 4.7 | 25.1 | 13.8 |
| Catalyst | Conversion/% | Selectivity/% | MG Yield/% | ||
|---|---|---|---|---|---|
| MG | MF | EG | |||
| Ag/SiO2 | 77.8 | 89.3 | 2.2 | 8.5 | 69.5 |
| Ag-0.2%Ni/SiO2 | 96.7 | 90.7 | 3.7 | 5.6 | 87.7 |
| Ag-0.5%Ni/SiO2 | 100 | 92.5 | 0 | 7.5 | 92.5 |
| Ag-1%Ni/SiO2 | 92.7 | 89.3 | 2.7 | 8.0 | 82.7 |
| Ag-3%Ni/SiO2 | 87.2 | 89.1 | 5.0 | 6.0 | 77.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, S.; Meng, T.; Mao, D.; Guo, X.; Yu, J.; Ma, Z. Ni-Modified Ag/SiO2 Catalysts for Selective Hydrogenation of Dimethyl Oxalate to Methyl Glycolate. Nanomaterials 2022, 12, 407. https://doi.org/10.3390/nano12030407
Cheng S, Meng T, Mao D, Guo X, Yu J, Ma Z. Ni-Modified Ag/SiO2 Catalysts for Selective Hydrogenation of Dimethyl Oxalate to Methyl Glycolate. Nanomaterials. 2022; 12(3):407. https://doi.org/10.3390/nano12030407
Chicago/Turabian StyleCheng, Shuai, Tao Meng, Dongsen Mao, Xiaoming Guo, Jun Yu, and Zhen Ma. 2022. "Ni-Modified Ag/SiO2 Catalysts for Selective Hydrogenation of Dimethyl Oxalate to Methyl Glycolate" Nanomaterials 12, no. 3: 407. https://doi.org/10.3390/nano12030407







