Enhanced Electroluminescence via a Nanohybrid Material Consisting of Aromatic Ligand-Modified InP Quantum Dots and an Electron-Blocking Polymer as the Single Active Layer in Quantum Dot–LEDs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Synthesis of 4-Phenylbutan-1-Thiol (PBSH)
2.2.1. Synthesis of S-(4-Phenylbutyl) Ethanethiate (PBET)
2.2.2. Synthesis of PBSH
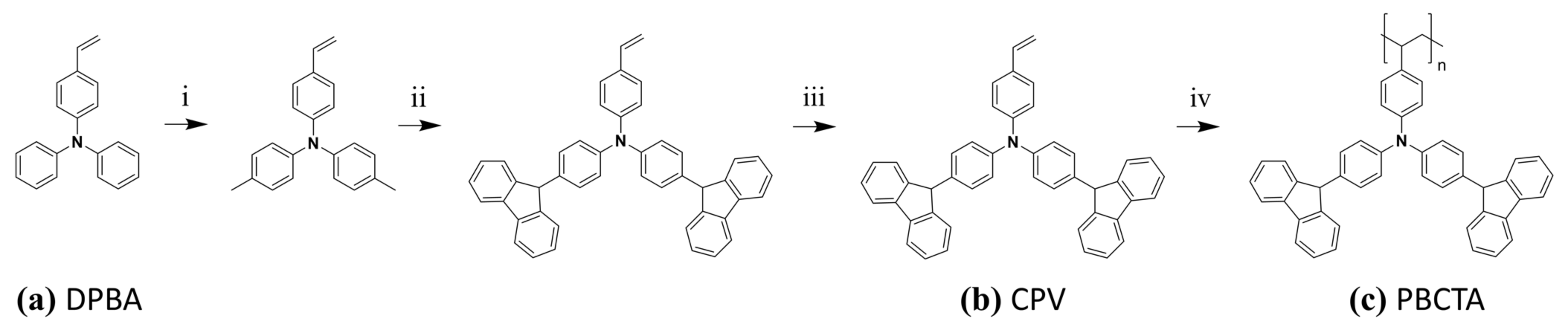
2.3. Synthesis of Poly-[N,N-Bis [4-(carbazolyl)phenyl]-4-vinylaniline] (PBCTA)
2.4. Synthesis of Zinc Octanoate (Zn(OctOAc)2)
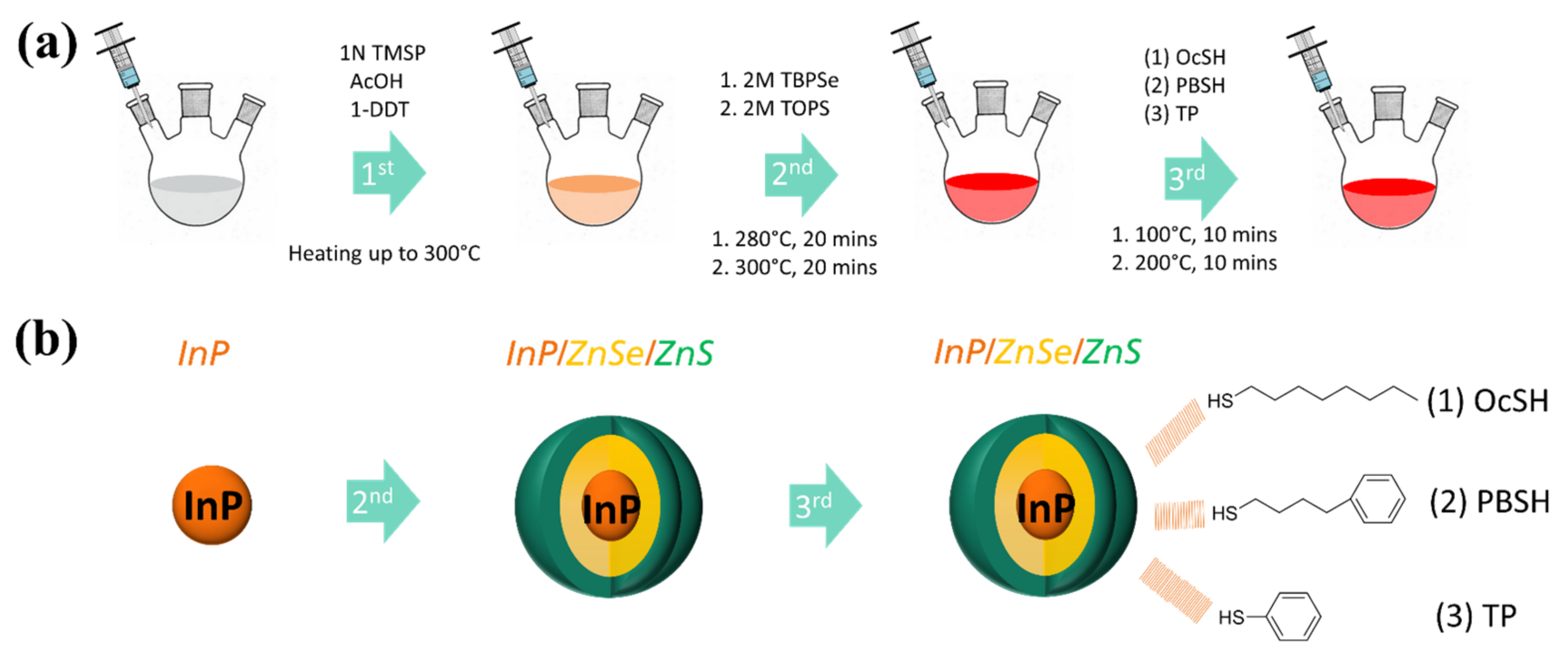
2.5. Synthesis of InP/ZnSe/ZnS Quantum Dots with Different Thiol Ligands
2.6. Inverted Structure of Quantum Dot Light-Emitting Diode (QD–LED) Fabrication
2.7. Conventional Structure QD–LED Fabrication
2.8. Characterization
3. Results and Discussion
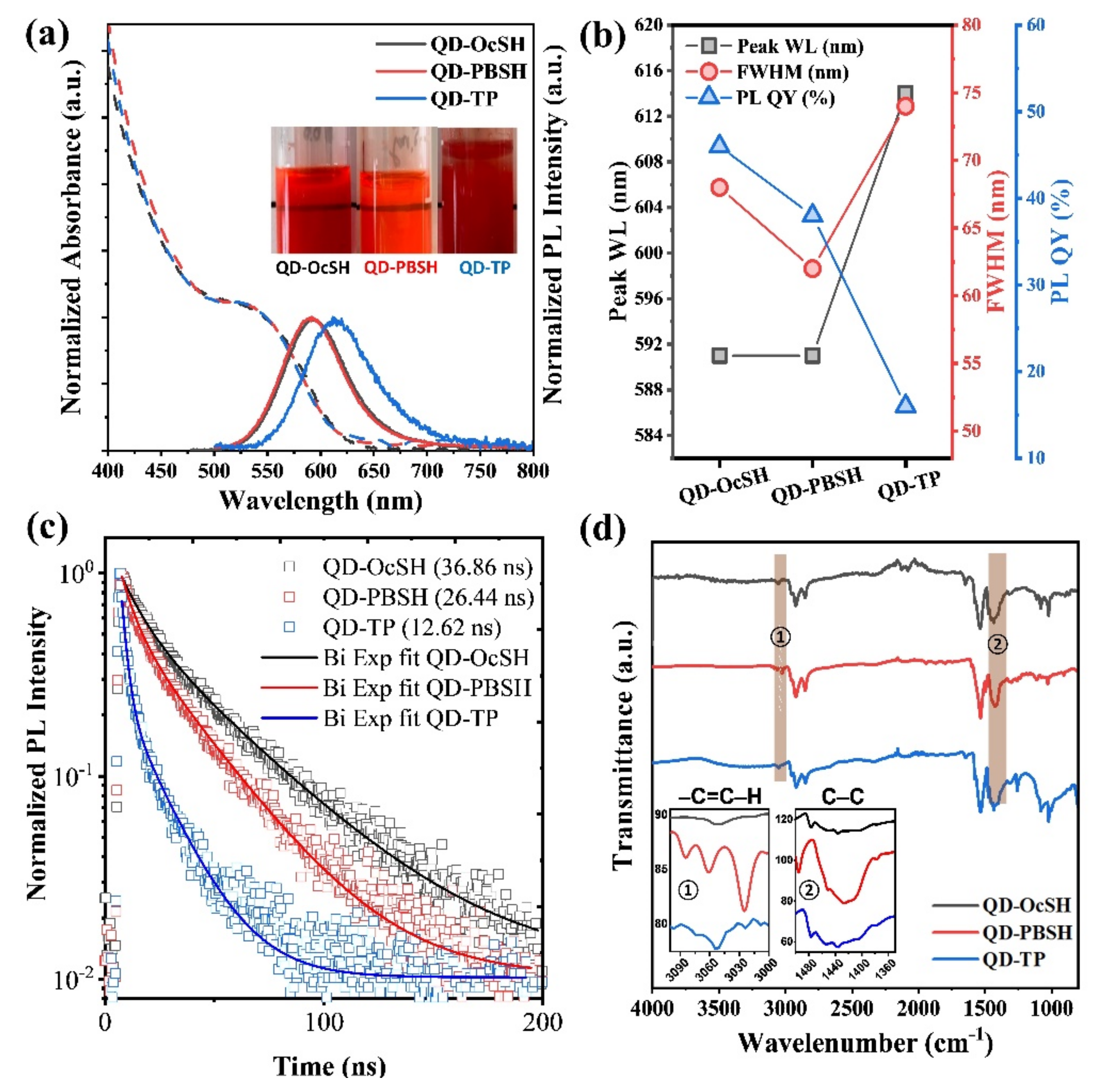
3.1. Optical and Surface Properties of QD–OcSH, QD–PBSH and QD–TP
3.2. The QD–LED Fabrications of QD–OcSH, QD–PBSH and QD–TP
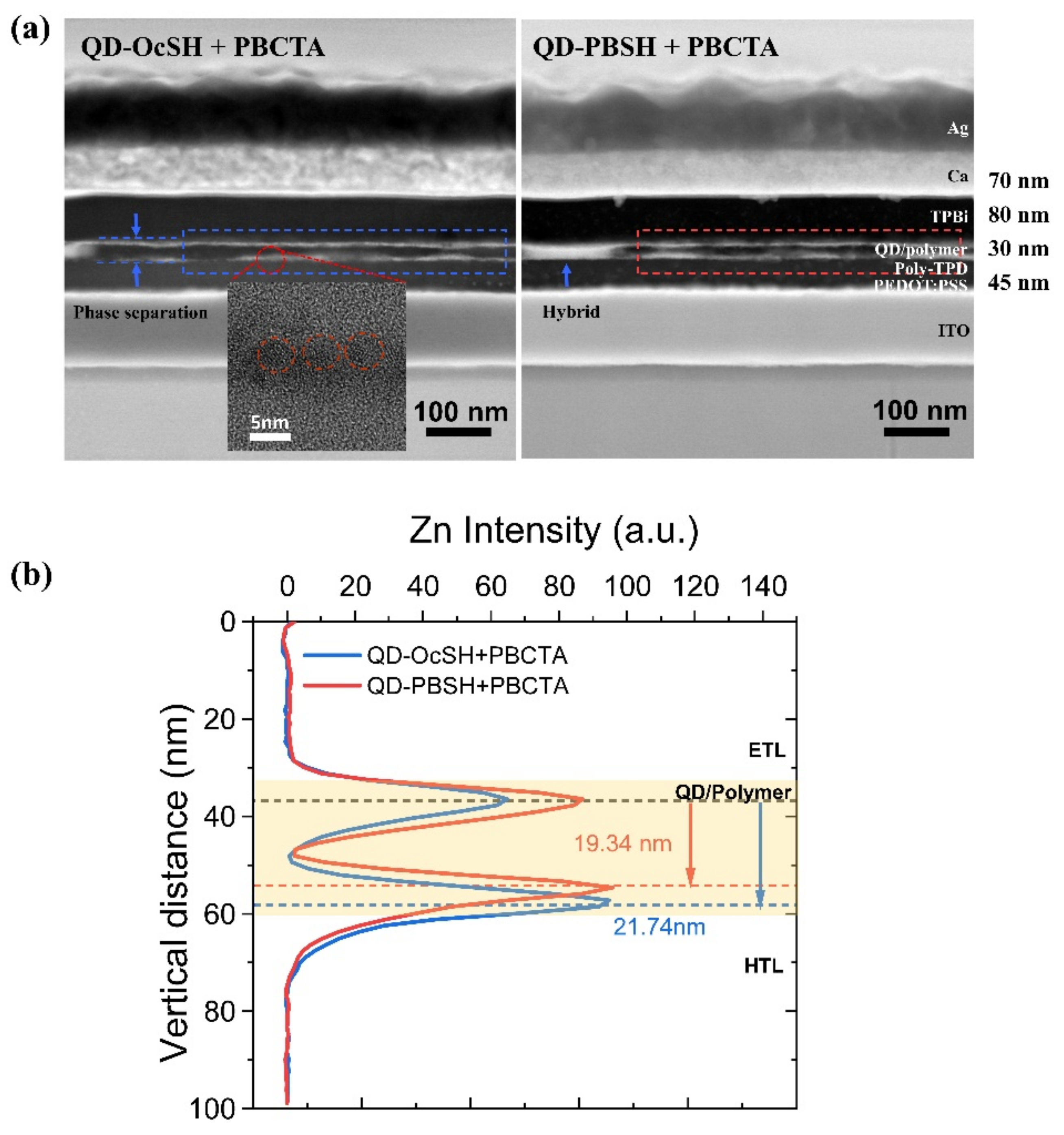
3.3. The QD–LED Fabrications of QD/Polymer Hybrids, QD–PBSH/PBCTA and QD–OcSH/PBCTA
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.; Ham, S.; Jang, H.; Min, J.H.; Chung, H.; Lee, J.; Kim, D.; Jang, E. Bright and uniform green light emitting InP/ZnSe/ZnS quantum dots for wide color gamut displays. ACS Appl. Nano Mater. 2019, 2, 1496–1504. [Google Scholar] [CrossRef]
- Tamang, S.; Lincheneau, C.; Hermans, Y.; Jeong, S.; Reiss, P. Chemistry of InP nanocrystal syntheses. Chem. Mater. 2016, 28, 2491–2506. [Google Scholar] [CrossRef]
- Kang, H.; Kim, S.; Oh, J.H.; Yoon, H.C.; Jo, J.H.; Yang, H.; Do, Y.R. Color–by–blue QD–emissive LCD Enabled by replacing RGB color filters with narrow–band GR InP/ZnSeS/ZnS QD films. Adv. Opt. Mater. 2018, 6, 1701239. [Google Scholar] [CrossRef]
- Zvaigzne, M.; Alexandrov, A.; Tkach, A.; Lypenko, D.; Nabiev, I.; Samokhvalov, P. Optimizing the PMMA electron–blocking layer of quantum dot light–emitting diodes. Nanomaterials 2021, 11, 2014. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-H.; Cho, O.; Kim, T.; Chung, D.-Y.; Kim, T.; Chung, H.; Jang, H.; Lee, J.; Kim, D.; Jang, E. Highly efficient and stable InP/ZnSe/ZnS quantum dot light–emitting diodes. Nature 2019, 575, 634–638. [Google Scholar] [CrossRef]
- Juang, F.S.; Lee, C.C.; Chen, J.Y.; Lin, Y.H.; Zhang, D.W. Lifetime study for solution processed organic light emitting diodes. IOP Conf. Ser. Mater. Sci. Eng. 2019, 600, 012018. [Google Scholar] [CrossRef] [Green Version]
- Park, M.; Roh, J.; Lim, J.; Lee, H.; Lee, D. Double metal oxide electron transport layers for colloidal quantum dot light–emitting diodes. Nanomaterials 2020, 10, 726. [Google Scholar] [CrossRef] [Green Version]
- Ikai, M.; Tokito, S.; Sakamoto, Y.; Suzuki, T.; Taga, Y. Highly efficient phosphorescence from organic light–emitting devices with an exciton–block layer. Appl. Phys. Lett. 2001, 79, 156–158. [Google Scholar] [CrossRef]
- Bozyigit, D.; Wood, V. Challenges and solutions for high–efficiency quantum dot–based LEDs. MRS Bull. 2013, 38, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Bozyigit, D.; Yarema, O.; Wood, V. Origins of low quantum efficiencies in quantum dot LEDs. Adv. Funct. Mater. 2013, 23, 3024–3029. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S.; Ding, G. Facile and highly effective synthesis of controllable lattice sulfur-doped graphene quantum dots via hydrothermal treatment of durian. ACS Appl. Mater. Interfaces 2018, 14, 5750–5759. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hahm, D.; Kim, K.; Rhee, S.; Lee, M.; Kim, S.; Chang, J.H.; Park, H.W.; Lim, J.; Lee, M.; et al. High-resolution patterning of colloidal quantum dots via non-destructive, light-driven ligand crosslinking. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Bae, W.; Park, Y.; Lim, J.; Lee, D. Controlling the influence of Auger recombination on the performance of quantum–dot light–emitting. Nat. Commun. 2013, 4, 2661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, B.N.; Ghosh, Y.; Brovelli, S.; Laocharoensuk, R.; Klimov, V.I.; Hollingsworth, J.A.; Htoon, H. “Giant” CdSe/CdS core/shell nanocrystal quantum dots as efficient electroluminescent materials: Strong influence of shell thickness on light–emitting diode performance. Nano Lett. 2012, 12, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Chae, H. Efficiency enhancement of all–solution–processed inverted–structure green quantum dot light–emitting diodes via partial ligand exchange with thiophenol derivatives having negative dipole moment. Adv. Opt. Mater. 2020, 8, 1901314. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, Z.; Jin, Y.; Niu, Y.; Cao, H.; Liang, X.; Chen, L.; Wang, J.; Peng, X. Solution–processed, high–performance light–emitting diodes based on quantum dots. Nature 2014, 515, 96–99. [Google Scholar] [CrossRef]
- Rahmati, M.; Dayneko, S.; Pahlevani, M.; Shi, Y. Highly efficient quantum dot light–emitting diodes by inserting multiple poly(methyl methacrylate) as electron–blocking layers. Adv. Funct. Mater. 2019, 29, 1906742. [Google Scholar] [CrossRef]
- Fu, Y.; Jiang, W.; Kim, D.; Lee, W.; Chae, H. Highly efficient and fully solution–processed inverted light–emitting diodes with charge control interlayers. ACS Appl. Mater. Interfaces 2018, 10, 17295–17300. [Google Scholar] [CrossRef]
- Bae, W.K.; Lim, J.; Zorn, M.; Kwak, J.; Park, Y.-S.; Lee, D.; Lee, S.; Char, K.; Zentel, R.; Lee, C. Reduced efficiency roll–off in light–emitting diodes enabled by quantum dot–conducting polymer nanohybrids. J. Mater. Chem. C 2014, 2, 4974–4979. [Google Scholar] [CrossRef]
- Fokina, A.; Lee, Y.; Chang, J.H.; Park, M.; Sung, Y.; Bae, W.K.; Char, K.; Lee, C.; Zentel, R. The role of emission layer morphology on the enhanced performance of light-emitting diodes based on quantum dot-semiconducting polymer hybrids. Adv. Mater. Interfaces 2016, 3, 1–9. [Google Scholar] [CrossRef]
- Madec, M.-B.; Butterworth, S.; Taboada, P.; Heenan, R.; Geoghegan, M.; Yeates, S.G. The influence of directed π–π interactions in solution on the thin film organic semiconductor device properties of small molecule polymer blends. Soft Matter 2011, 7, 7065–7070. [Google Scholar] [CrossRef]
- Mas–Torrent, M.; Rovira, C. Role of molecular order and solid–state structure in organic field–effect transistors. Chem. Rev. 2011, 111, 4833–4856. [Google Scholar] [CrossRef]
- Zou, Y.; Ban, M.; Cui, W.; Huang, Q.; Wu, C.; Liu, J.; Wu, H.; Song, T.; Sun, B. A general solvent selection strategy for solution processed quantum dots targeting high performance light–emitting Diode. Adv. Funct. Mater. 2017, 27, 1603325. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO-nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Thesen, M.W.; Höfer, B.; Debeaux, M.; Janietz, S.; Wedel, A.; Anna, K.; Johannes, H.H.; Krueger, H. Hole–transporting host–Polymer series consisting of triphenylamine basic structures for phosphorescent polymer light–Emitting diodes. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3417–3430. [Google Scholar] [CrossRef]
- Kim, Y.; Ippen, C.; Greco, T.; Lee, J.; Oh, M.S.; Han, C.J.; Wedel, A.; Kim, J. Increased shell thickness in indium phosphide multishell quantum dots leading to efficiency and stability enhancement in light–emitting diodes. Opt. Mater. Express 2014, 4, 1436–1443. [Google Scholar] [CrossRef]
- Mashford, B.S.; Stevenson, M.; Popovic, Z.; Hamilton, C.; Zhou, Z.; Breen, C.; Steckel, J.; Bulovic, V.; Bawendi, M.; Coe–Sullivan, S.; et al. High–efficiency quantum–dot light–emitting devices with enhanced charge injection. Nat. Photon. 2013, 7, 407–412. [Google Scholar] [CrossRef]
- Cros–Gagneux, A.; Delpech, F.; Nayral, C.; Cornejo, A.; Coppel, Y.; Chaudret, B. Surface chemistry of InP quantum dots: A comprehensive study. J. Am. Chem. Soc. 2010, 132, 18147–18157. [Google Scholar] [CrossRef]
- Sowers, K.L.; Swartz, B.; Krauss, T.D. Chemical mechanisms of semiconductor nanocrystal synthesis. Chem. Mater. 2013, 25, 1351–1362. [Google Scholar] [CrossRef]
- Shen, H.; Cao, W.; Shewmon, N.T.; Yang, C.; Li, L.S.; Xue, J. High–efficiency, Low turn–on voltage blue–violet quantum–dot–based light–emitting diodes. Nano Lett. 2015, 15, 1211–1216. [Google Scholar] [CrossRef]
- Ji, C.; Lu, M.; Wu, H.; Zhang, X.; Shen, X.; Wang, X.; Zhang, Y.; Wang, Y.; Yu, W.W. 1,2–Ethanedithiol treatment for AgIn5S8/ZnS quantum dot light–emitting diodes with high brightness. ACS Appl. Mater. Interfaces 2017, 9, 8187–8193. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Lee, W.; Chae, H. Energy–band alignment and charge balance of electron transport layer with quinary Zn–Mg–Ga–Cl–O nanoparticles in InP–based quantum dot light emitting diodes. IEEE Electron Device Lett. 2019, 40, 1872–1875. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qin, H.; Jiang, M.; Lin, L.; Fu, T.; Dai, X.; Zhang, Z.; Niu, Y.; Cao, H.; Jin, Y.; et al. Entropic ligands for nanocrystals: From unexpected solution properties to outstanding processability. Nano Lett. 2016, 16, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Badia, A.; Singh, S.; Demers, L.; Cuccia, L.; Brown, G.R.; Lennox, R.B. Self–assembled monolayers on gold nanoparticles. Chem. A Eur. J. 1996, 2, 359–363. [Google Scholar] [CrossRef]
- Lee, T.; Shimura, K.; Kim, D. Surface modification effects on defect–related photoluminescence in colloidal CdS quantum dots. Phys. Chem. Chem. Phys. 2018, 20, 11954–11958. [Google Scholar] [CrossRef] [Green Version]
- Thuy, U.T.D.; Reiss, P.; Liem, N.Q. Luminescence properties of In(Zn)P alloy core/ZnS shell quantum dots. Appl. Phys. Lett. 2010, 97, 193104. [Google Scholar] [CrossRef]
- Man, M.T.; Lee, H.S. Clarifying photoluminescence decay dynamics of self–assembled quantum dots. Sci. Rep. 2019, 9, 4613. [Google Scholar] [CrossRef] [Green Version]
- Shriner, R.L.; Hermann, C.K.; Morrill, T.C.; Curtin, D.Y.; Fuson, R.C. The Systematic Identification of Organic Compounds; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003; ISBN 978–0–471–21503–540. [Google Scholar]
- Shariffudin, S.S.; Salina, M.; Herman, S.H.; Rusop, M. Effect of film thickness on structural, electrical, and optical properties of sol–gel deposited layer–by–layer ZnO nanoparticles. Trans. Electr. Electron. Mater. 2012, 13, 102–105. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, J.A.; Maldonado, A.; Juarez, H.; Pacio, M. Synthesis of colloidal ZnO nanoparticles and deposit of thin films by spin coating technique. J. Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Ryu, S.Y.; Duong, A.T.; Lee, S. Impact of 1,2–ethanedithiol treatment on luminescence and charge–transport characteristics in colloidal quantum–dot LEDs. Nanotechnology 2019, 30, 505202. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Fu, Y.; Kim, J.; Lee, K.H.; Kim, H.; Yang, H.; Chae, H. Improved electroluminescence of quantum dot light–emitting diodes enabled by a partial ligand exchange with benzenethiol. Nanotechnology 2016, 27, 245203. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Subramanyam, G.; Dai, L.; Check, M.; Campbell, A.; Naik, R.; Grote, J.; Wang, Y. Highly efficient quantum-dot light-emitting diodes with DNA−CTMA as a combined hole-transporting and electron-blocking layer. ACS Nano 2009, 3, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, P.; Zhang, W.; Wang, K.; Sun, X.W. Development of InP quantum dot-based light-emitting diodes. ACS Energy Lett. 2020, 5, 1095–1106. [Google Scholar] [CrossRef]



| Chemical | Abbreviation | Supplier | Purity |
|---|---|---|---|
| 4–phenyl–1–butene | PhBe | Sigma Aldrich | 99% |
| 1–dodecanethiol | 1–DDT | Sigma Aldrich | 98% |
| 1–octadecene, | 1–ODE | Sigma Aldrich | 90% technical grade |
| 1-octanethiol | OcSH | Sigma Aldrich | ≥98.5%, |
| 1–octanoic acid | 1–OctOAc | Sigma Aldrich | 99% |
| 2,2′,2″–(1,3,5–benzinetriyl)–tris(1–phenyl–1–H–benzimidazole, | TPBi | Lumtec | LT–E302 |
| 2,2′–azobis(2–methylpropionitrile) | AIBN | Sigma Aldrich | 98%, |
| 2–propanol | IPA | Carl Roth | 99.5% |
| 4,4′,4″–tris(carbazol–9–yl) triphenylamine | TCTA | Lumtec | |
| acetic acid glacial | AcOH | Sigma Aldrich | 99% |
| acetone | AC | Carl Roth | 99.9% |
| calcium | Ca | Balzers | |
| chloroform | CHCl3 | Sigma Aldrich | ≥99.8, anhydrous |
| ethanol | EtOH | Carl Roth | 96% |
| ethyl acetate | EA | Acros organic | 99.5% |
| indium (III) acetate | In(OAc)3 | Sigma Aldrich | 99.99% trace metals basis |
| magnesium sulfate | Mg2SO4 | Sigma Aldirch | 99.99% trace metals basis, |
| methanol | MeOH | Carl Roth | 99% |
| molybdenum oxide | MoO3 | Sigma Aldrich | 99.99% |
| n–heptane | n–HEP | Sigma Aldrich | 99% |
| poly–(N,N′–bis(4–butylphenyl)–N,N′–bis(phenyl)benzidine | PEDOT:PSS | Heraeus | CH8000 |
| poly–(N,N′–bis(4–butylphenyl)–N,N′–bis(phenyl)benzidine) | poly-TPD | Solaris Chem Inc | SOL2420H |
| silver | Ag | Umicore | Lot # C000291487 |
| tetrahyrofuran | THF | Sigma Aldrich | 99.9% |
| thioacetic acid | TA | Alfa Aesar | 96% |
| tributylphosphine | TBP | Sigma Aldrich | 97% |
| trioctylphosphine | TOP | Sigma Aldrich | 97% |
| tris(trimethylsilyl)phosphine | P(TMSi)3 | Vezerf Laborsynthesen GmbH | 97.5% |
| zinc acetate | Zn(OAc)2 | Sigma Aldrich | 99.99%, |
| zinc oxide zinc oxide | ZnO | Sigma Aldrich | 99.99% |
| InP/ZnSe/ZnS–QD | Peak WL (nm) (b/a) | FWHM (nm) (b/a) | PL QY (%) (b/a) |
|---|---|---|---|
| QD–OcSH | 591/591 | 68/68 | 46/46 |
| QD–PBSH | 591/592 | 67/62 | 36/38 |
| QD–TP | 593/614 | 69/74 | 36/16 |
| QD | τ1 (ns) | α1 | f1 | τ2 (ns) | α2 | f2 | (ns) |
|---|---|---|---|---|---|---|---|
| QD–OcSH | 7.75 | 0.31 | 0.09 | 35.44 | 0.68 | 0.91 | 32.95 |
| QD–PBSH | 5.97 | 0.42 | 0.13 | 27.99 | 0.58 | 0.86 | 25.02 |
| QD–TP | 2.5 | 0.81 | 0.37 | 18.1 | 0.18 | 0.62 | 12.25 |
| Film | QD–OcSH | QD–PBSH | QD–TP |
|---|---|---|---|
| Rq (nm) | 2.15 | 1.84 | 2.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.-S.; Janietz, S.; Roddatis, V.; Geßner, A.; Wedel, A.; Kim, J.; Kim, Y. Enhanced Electroluminescence via a Nanohybrid Material Consisting of Aromatic Ligand-Modified InP Quantum Dots and an Electron-Blocking Polymer as the Single Active Layer in Quantum Dot–LEDs. Nanomaterials 2022, 12, 408. https://doi.org/10.3390/nano12030408
Choi H-S, Janietz S, Roddatis V, Geßner A, Wedel A, Kim J, Kim Y. Enhanced Electroluminescence via a Nanohybrid Material Consisting of Aromatic Ligand-Modified InP Quantum Dots and an Electron-Blocking Polymer as the Single Active Layer in Quantum Dot–LEDs. Nanomaterials. 2022; 12(3):408. https://doi.org/10.3390/nano12030408
Chicago/Turabian StyleChoi, Hyung-Seok, Silvia Janietz, Vladimir Roddatis, Andre Geßner, Armin Wedel, Jiyong Kim, and Yohan Kim. 2022. "Enhanced Electroluminescence via a Nanohybrid Material Consisting of Aromatic Ligand-Modified InP Quantum Dots and an Electron-Blocking Polymer as the Single Active Layer in Quantum Dot–LEDs" Nanomaterials 12, no. 3: 408. https://doi.org/10.3390/nano12030408








