Using Si/MoS2 Core-Shell Nanopillar Arrays Enhances SERS Signal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Ordered Si NP Arrays
2.2. Fabrication of Ordered Si/MoS2 Core-Shell NP Arrays
2.3. Preparation of R6G Molecules
2.4. Characterizations
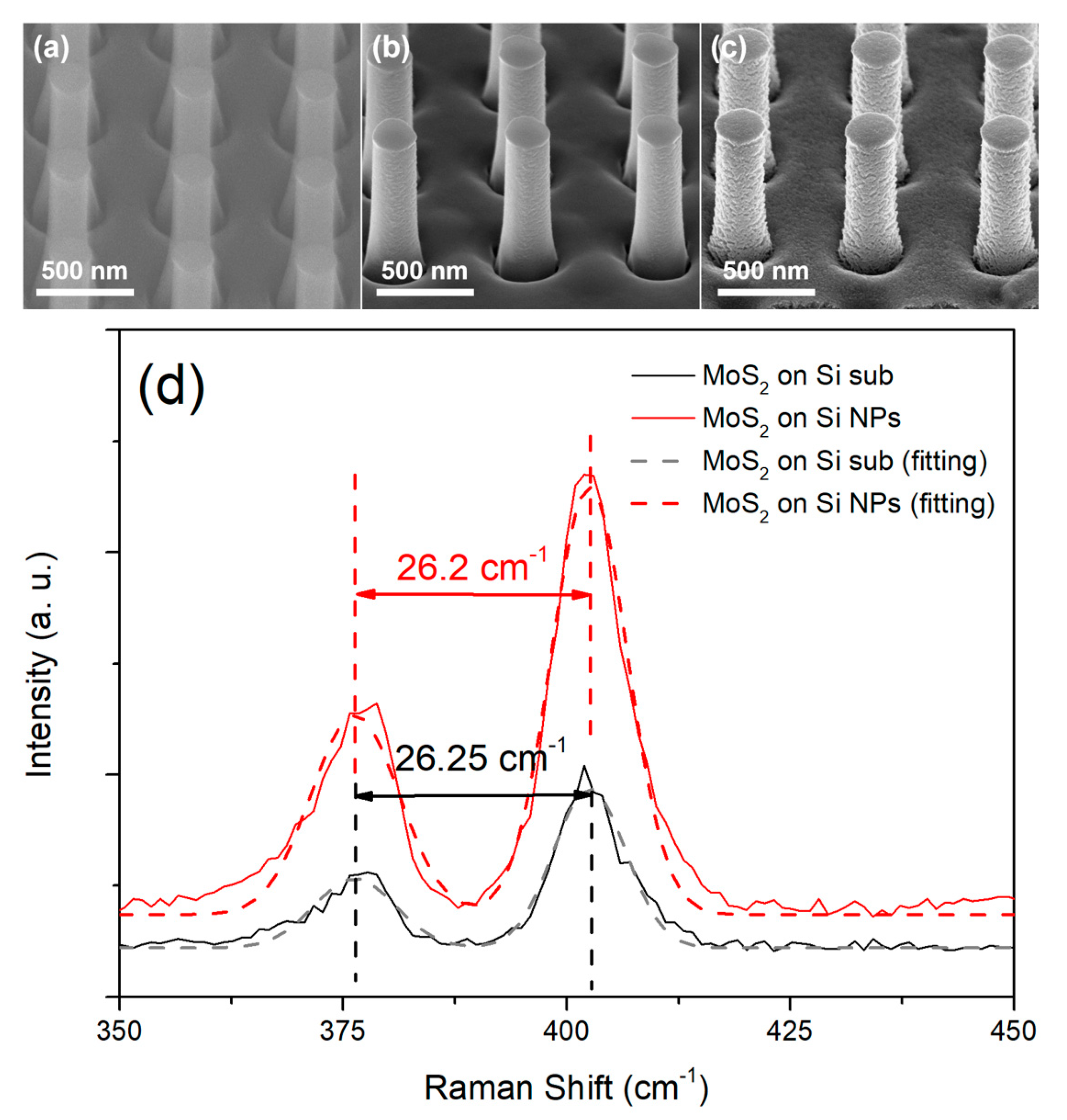
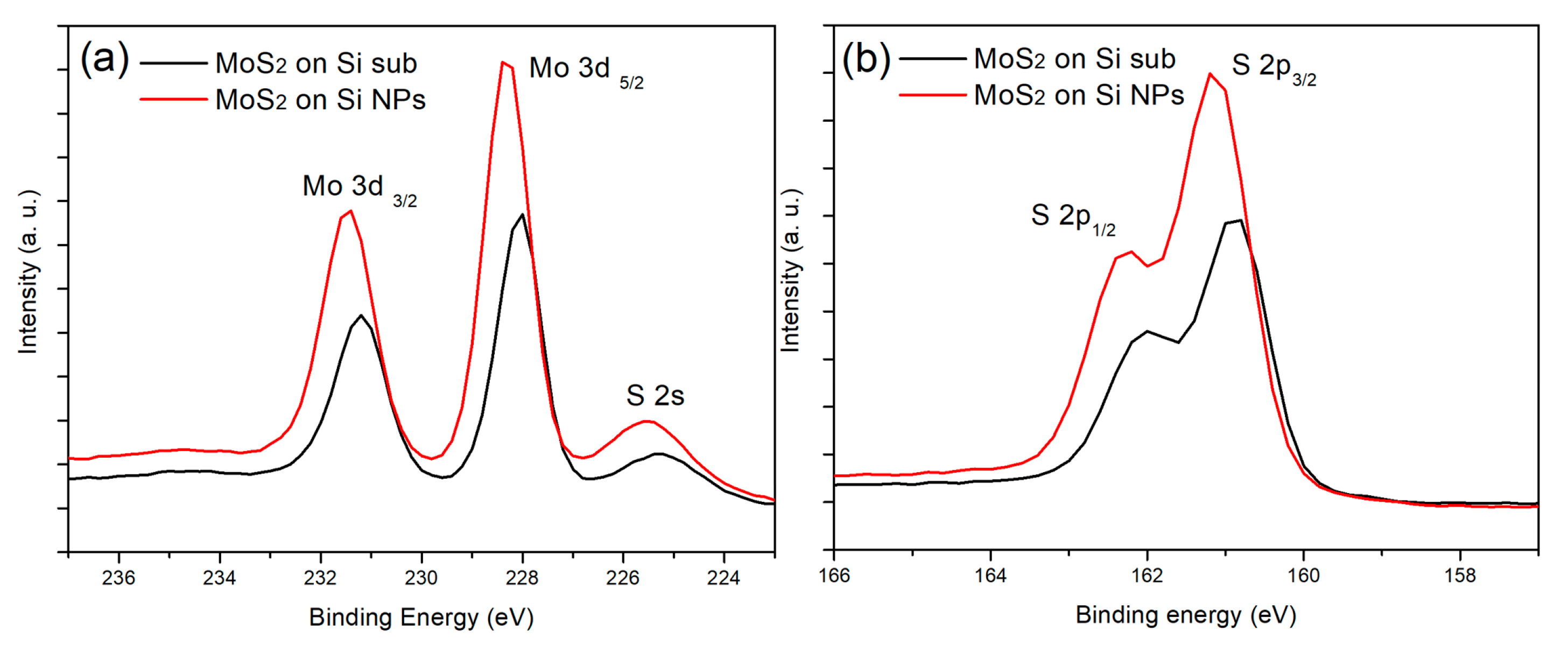
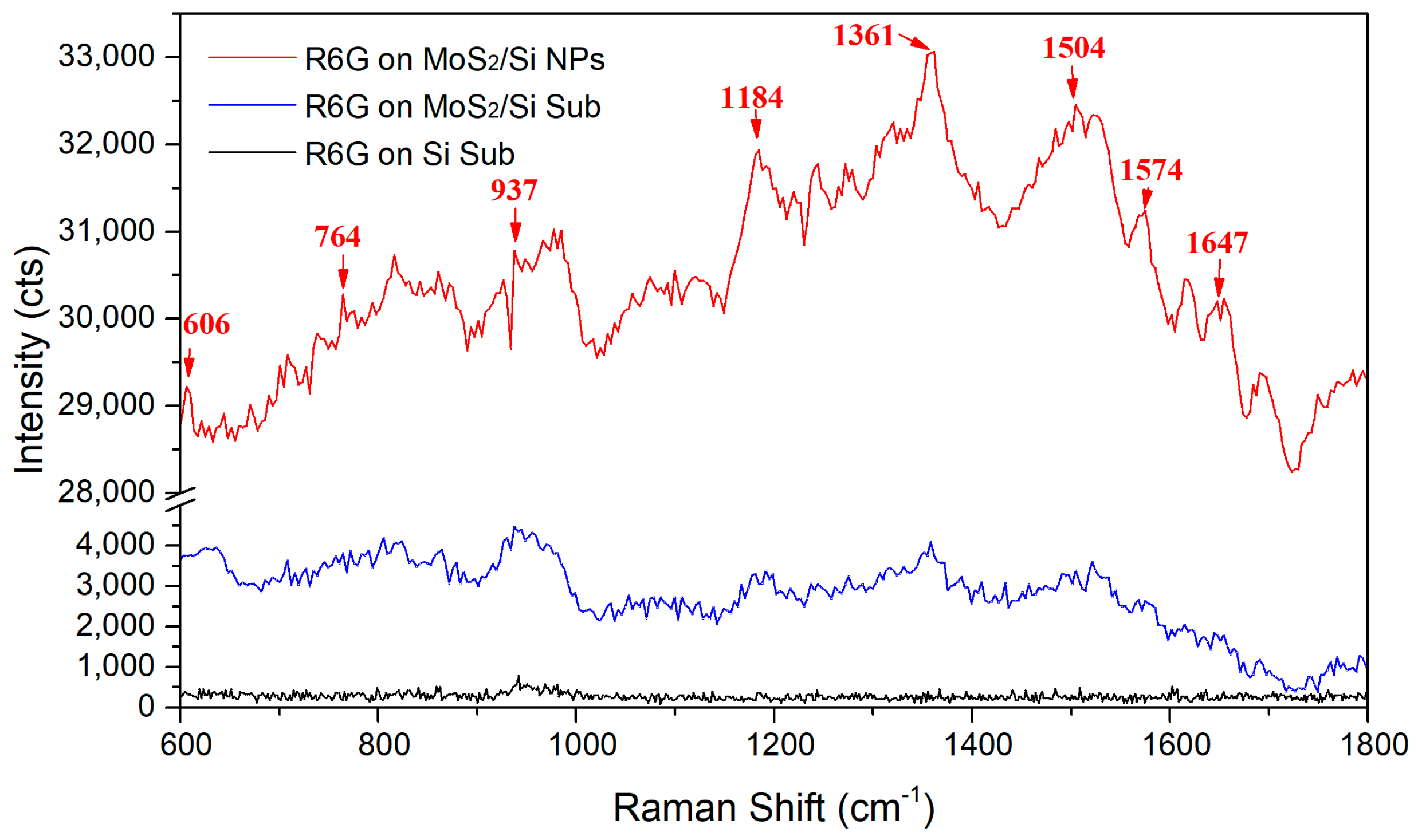
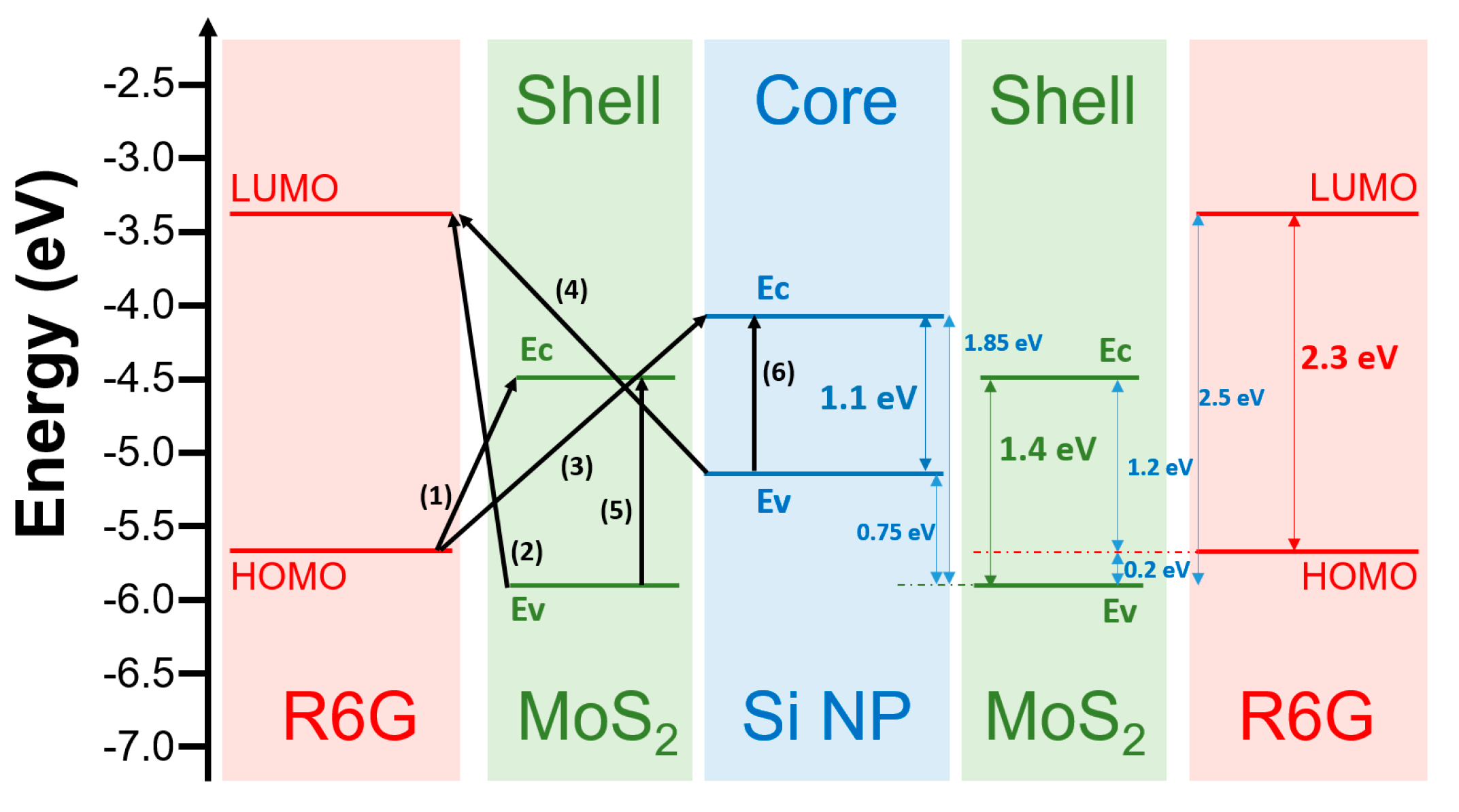
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.-Y.; Liu, X.-M.; Duan, S.; Xu, X.; Ren, B.; Lin, S.-H.; Tian, Z.-Q. Chemical Enhancement Effects in SERS Spectra: A Quantum Chemical Study of Pyridine Interacting with Copper, Silver, Gold and Platinum Metals. J. Phys. Chem. C 2008, 112, 4195–4204. [Google Scholar] [CrossRef]
- Yamada, H.; Nagata, H.; Toba, K.; Nakao, Y. Charge-transfer band and sers mechanism for the pyridine-Ag system. Surf. Sci. 1987, 182, 269–286. [Google Scholar] [CrossRef]
- Mosier-Boss, P.A. Review of SERS Substrates for Chemical Sensing. Nanomaterials 2017, 7, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schatz, G.C.; Young, M.A.; Duyne, R.P.V. Topics in Applied Physics. In Surface-Enhanced in Applied Physics; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 19. [Google Scholar]
- Freeman, R.G.; Grabar, K.C.; Allison, K.J.; Bright, R.M.; Davis, J.A.; Guthrie, A.P.; Hommer, M.B.; Jackson, M.A.; Smith, P.C.; Walter, D.G.; et al. Self-Assembled Metal Colloid Monolayers: An Approach to SERS Substrates. Science 1995, 267, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.P.; Chu, H.Y.; Abell, J.; Tripp, R.A.; Zhao, Y. Flexible and mechanical strain resistant large area SERS active substrates. Nanoscale 2012, 4, 3410–3414. [Google Scholar] [CrossRef]
- Abdelsalam, M.E.; Bartlett, P.N.; Baumberg, J.J.; Cintra, S.; Kelf, T.A.; Russell, A.E. Electrochemical SERS at a structured gold surface. Electrochem. Commun. 2005, 7, 740–744. [Google Scholar] [CrossRef] [Green Version]
- Dridi, H.; Haji, L.; Moadhen, A. Studies of SERS efficiency of gold coated porous silicon formed on rough silicon backside. Appl. Surf. Sci. 2017, 426, 1190–1197. [Google Scholar] [CrossRef]
- Xia, L.; Chen, M.; Zhao, X.; Zhang, Z.; Xia, J.; Xu, H.; Sun, M. Visualized method of chemical enhancement mechanism on SERS and TERS. J. Raman Spectrosc. 2014, 45, 533–540. [Google Scholar] [CrossRef]
- Chen, R.; Jensen, L. Interpreting the chemical mechanism in SERS using a Raman bond model. J. Chem. Phys. 2020, 152, 024126. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Frontiera, R.R.; Henry, A.-I.; Ringe, E.; Duyne, R.P.V. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Hvolbæk, B.; Janssens, T.V.W.; Clausen, B.S.; Falsig, H.; Christensen, C.H.; Nørskov, J.K. Catalytic Activity of Au nanoparticles. Nano Today 2007, 2, 14–18. [Google Scholar] [CrossRef]
- Guerrini, L.; Jurasekova, Z.; Domingo, C.; Pérez-Méndez, M.; Leyton, P.; Campos-Vallette, M.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Importance of Metal–Adsorbate Interactions for the Surface-enhanced Raman Scattering of Molecules Adsorbed on Plasmonic Nanoparticles. Plasmonic 2007, 2, 147–156. [Google Scholar] [CrossRef]
- Dery, S.; Berg, I.; Kim, S.; Cossaro, A.; Verdini, A.; Floreano, L.; Toste, F.D.; Gross, E. Strong Metal–Adsorbate Interactions Increase the Reactivity and Decrease the Orientational Order of OH-Functionalized N-Heterocyclic Carbene Monolayers. Langmuir 2020, 36, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Ling, X.; Xiao, J.; Dresselhaus, M.S.; Kong, J.; Xu, H.; Liu, Z.; Zhang, J. Surface enhanced Raman spectroscopy on a flat graphene surface. Proc. Natl. Acad. Sci. USA 2012, 109, 9281–9286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, H.; Li, Z.; Gao, S.; Chen, P.; Zhang, C.; Jiang, S.; Li, H. Large-area MoS2 thin layers directly synthesized on Pyramid-Si substrate for surface-enhanced Raman scattering. RSC Adv. 2015, 5, 83899–83905. [Google Scholar] [CrossRef]
- Dai, Z.; Xiao, X.; Wu, W.; Zhang, Y.; Liao, L.; Guo, S.; Jiang, C. Plasmon-driven reaction controlled by the number of graphene layers and localized surface plasmon distribution during optical excitation. Light-Sci. Appl. 2015, 4, e342–e348. [Google Scholar] [CrossRef]
- Ling, X.; Fang, W.J.; Lee, Y.H.; Araujo, P.T.; Zhang, X.; Rodriguez-Nieva, J.F.; Lin, Y.X.; Zhang, J.; Kong, J.; Dresselhaus, M.S. Raman Enhancement Effect on Two-Dimensional Layered Materials: Graphene, h-BN and MoS2. Nano Lett. 2014, 14, 3033–3040. [Google Scholar] [CrossRef]
- Zhang, X.; Suo, H.; Zhang, R.; Niu, S.; Zhao, X.Q.; Zheng, J.; Guo, C. Photocatalytic activity of 3D flower-like MoS2 hemispheres. Mater. Res. Bull. 2018, 100, 249–253. [Google Scholar] [CrossRef]
- Vattikuti, S.V.P.; Byon, C.; Reddy, C.V.; Ravikumar, R.V.S.S.N. Improved photocatalytic activity of MoS2 nanosheets decorated with SnO2 nanoparticles. RSC Adv. 2015, 5, 86675–86684. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, K.; Hu, E.; Guo, J.; Han, C.; Hu, X. Double hollow MoS2 nano-spheres: Synthesis, tribological properties, and functional conversion from lubrication to photocatalysis. Appl. Surf. Sci. 2017, 392, 1144–1152. [Google Scholar] [CrossRef]
- Gu, L.; Zheng, K.; Zhou, Y.; Li, J.; Mo, X.; Patzke, G.R.; Chen, G. Humidity sensors based on ZnO/TiO2 core/shell nanorod arrays with enhanced sensitivity. Sens. Actuators B Chem. 2011, 159, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Heyne, M.H.; Chiappe, D.; Meersschaut, J.; Nuytten, T.; Conard, T.; Bender, H.; Huyghebaert, C.; Radu, I.P.; Caymax, M.; Marneffe, J.-F.D.; et al. Multilayer MoS2 growth by metal and metal oxide sulfurization. J. Mater. Chem. C 2016, 4, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Singh, M.; Sharma, R.K.; Reddy, G.B. Sulfurization of α-MoO3 nanostructured thin film. AIP Conf. Proc. 2015, 1675, 030051. [Google Scholar]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yin, Z.; He, Q.; Li, H.; Huang, X.; Lu, G.; Fam, D.W.H.; Tok, A.I.Y.; Zhang, Q.; Zhang, H. Fabrication of Single- and Multilayer MoS2 Film-Based Field-Effect Transistors for Sensing NO at Room Temperature. Small 2012, 8, 63–67. [Google Scholar] [CrossRef]
- Chen, M.; Ji, B.; Dai, Z.; Du, X.; He, B.; Chen, G.; Liu, D.; Chen, S.; Lo, K.H.; Wang, S.; et al. Vertically-aligned 1T/2H-MS2 (M = Mo, W) nanosheets for surface-enhanced Raman scattering with long-term stability and large-scale uniformity. Appl. Sur. Sci. 2020, 527, 146769–146777. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Z.; Bhoyate, S.; Morey, T.; Neria, B.L.; Vasiraju, V.; Gupta, G.; Palchoudhury, S.; Kahol, P.K.; Mishra, S.R.; et al. MoS2 Decorated Carbon Nanofibers as Efficient and Durable Electrocatalyst for Hydrogen Evolution Reaction. C J. Carbon Res. 2017, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Vangelista, S.; Cinquanta, E.; Martella, C.; Alia, M.; Longo, M.; Lamperti, A.; Mantovan, R.; Basset, F.B.; Pezzoli, F.; Molle, A. Towards a uniform and large-scale deposition of MoS2 nanosheets via sulfurization of ultra-thin Mo-based solid films. Nanotechnology 2016, 27, 175703–175712. [Google Scholar] [CrossRef] [PubMed]
- Mahatha, S.K.; Menon, K.S.R. Inhomogeneous band bending on MoS2 (0001) arising from surface steps and dislocations. J. Phys. Condens. Matter 2012, 24, 305502–305507. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Alfonso, J.E.; López-Carreño, L.D. XPS and X-ray diffraction characterization of MoO3 thin films prepared by laser evaporation. Phys. Stat. Sol. (C) 2005, 2, 3726–3729. [Google Scholar] [CrossRef]
- Bai, S.; Serien, D.; Hu, A.; Sugioka, K. 3D microfluidic surface-enhanced Raman spectroscopy (SERS) chips fabricated by all-femtosecond-laser-processing for real-time sensing of toxic substances. Adv. Funct. Mater. 2018, 28, 1706262–1706271. [Google Scholar] [CrossRef]
- Fan, J.-G.; Tang, X.-J.; Zhao, Y.-P. Water contact angles of vertically aligned Si nanorod arrays. Nanotechnology 2004, 15, 501–504. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. Theory of Surface-Enhanced Raman Scattering in Semiconductors. J. Phys. Chem. C 2014, 118, 11120–11130. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, H.; Lee, J.; Yu, S.H.; Hwang, E.; Lee, C.; Ahn, J.H.; Cho, J.H. Enhanced Raman Scattering of Rhodamine 6G Films on Two-Dimensional Transition Metal Dichalcogenides Correlated to Photoinduced Charge Transfer. Chem. Mater. 2016, 28, 180–187. [Google Scholar] [CrossRef]
- Muehlethaler, C.; Considine, C.R.; Menon, V.; Line, W.-C.; Lee, Y.-H.; Lombardi, J.R. Ultrahigh Raman Enhancement on Monolayer MoS2. ACS Photonics 2016, 3, 1164–1169. [Google Scholar] [CrossRef]
- Kitadai, H.; Wang, X.; Mao, N.; Huang, S.; Ling, X. Enhanced Raman Scattering on Nine 2D van der Waals Materials. J. Phys. Chem. Lett. 2019, 10, 3043–3050. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ko, T.-S.; Liu, H.-Y.; Shieh, J.; Shieh, D.; Chen, S.-H.; Chen, Y.-L.; Lin, E.-T. Using Si/MoS2 Core-Shell Nanopillar Arrays Enhances SERS Signal. Nanomaterials 2021, 11, 733. https://doi.org/10.3390/nano11030733
Ko T-S, Liu H-Y, Shieh J, Shieh D, Chen S-H, Chen Y-L, Lin E-T. Using Si/MoS2 Core-Shell Nanopillar Arrays Enhances SERS Signal. Nanomaterials. 2021; 11(3):733. https://doi.org/10.3390/nano11030733
Chicago/Turabian StyleKo, Tsung-Shine, Han-Yuan Liu, Jiann Shieh, De Shieh, Szu-Hung Chen, Yen-Lun Chen, and En-Ting Lin. 2021. "Using Si/MoS2 Core-Shell Nanopillar Arrays Enhances SERS Signal" Nanomaterials 11, no. 3: 733. https://doi.org/10.3390/nano11030733






