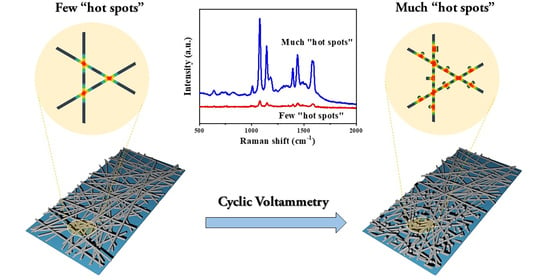
Enhancing the Activity of Silver Nanowire Membranes by Electrochemical Cyclic Voltammetry as Highly Sensitive Flexible SERS Substrate for On-Site Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Preparation of Silver Nanowires
2.4. Preparation of CV Treated Silver Nanowire Membranes
2.5. SERS Performance of CV Treated Silver Nanowire Membranes
2.6. Simulations
3. Results and Discussion
3.1. Characterization of CV-Treated Silver Nanowire Membrane
3.2. Effect of Electrochemical Conditions on the Silver Nanowires Membrane
3.3. SERS Performance of the Electrochemically Treated Silver Nanowire Membrane
3.4. SERS Detection of Simulated Aqueous Samples
3.5. Swabbing Extraction-Based SERS Detection of Simulated Solid Samples
3.6. DDA Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, J.F.; Anema, J.R.; Wandlowski, T.; Tian, Z.Q. Dielectric shell isolated and graphene shell isolated nanoparticle enhanced Raman spectroscopies and their applications. Chem. Soc. Rev. 2015, 44, 8399–8409. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, R.M.; Goodacre, R. Characterisation and identification of bacteria using SERS. Chem. Soc. Rev. 2008, 37, 931–936. [Google Scholar] [CrossRef]
- Qian, X.M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Campion, A.; Kambhampati, P. Surface-enhanced Raman scattering. Chem. Soc. Rev. 1998, 27, 241–250. [Google Scholar] [CrossRef]
- Lai, Y.; Pan, W.; Zhang, D.; Zhan, J. Silver nanoplates prepared by modified galvanic displacement for surface-enhanced Raman spectroscopy. Nanoscale 2011, 3, 2134–2137. [Google Scholar] [CrossRef] [PubMed]
- Sawai, Y.; Takimoto, B.; Nabika, H.; Murakoshi, K. Observation of a Small Number of Molecules at a Metal Nanogap Arrayed on a Solid Surface Using Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 2007, 129, 1658–1662. [Google Scholar] [CrossRef] [PubMed]
- Stockman, M.; Pandey, L.; George, T. Inhomogeneous localization of polar eigenmodes in fractals. Phys. Rev. B 1996, 53, 2183–2186. [Google Scholar] [CrossRef] [Green Version]
- Dieringer, J.A.; Lettan, R.B.; Scheidt, K.A.; Van Duyne, R.P. A Frequency Domain Existence Proof of Single-Molecule Surface-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2007, 129, 16249–16256. [Google Scholar] [CrossRef]
- Haes, A.J.; Zou, S.; Schatz, G.C.; Van Duyne, R.P. Nanoscale Optical Biosensor: Short Range Distance Dependence of the Localized Surface Plasmon Resonance of Noble Metal Nanoparticles. J. Phys. Chem. B 2004, 108, 6961–6968. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced Raman spectroscopy: A brief retrospective. J. Raman Spectrosc. 2005, 36, 485–496. [Google Scholar] [CrossRef]
- Zeng, F.; Duan, W.; Zhu, B.; Mu, T.; Zhu, L.; Guo, J.; Ma, X. Paper-Based Versatile Surface-Enhanced Raman Spectroscopy Chip with Smartphone-Based Raman Analyzer for Point-of-Care Application. Anal. Chem. 2019, 91, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, W.; Zhan, J. A positively charged silver nanowire membrane for rapid on-site swabbing extraction and detection of trace inorganic explosives using a portable Raman spectrometer. Nano Res. 2016, 9, 2487–2497. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Chang, Y.; Gao, C.; Chen, J.; Zhang, X.; Zhan, J. A 3D spongy flexible nanosheet array for on-site recyclable swabbing extraction and subsequent SERS analysis of thiram. Microchim. Acta 2019, 186, 458. [Google Scholar] [CrossRef]
- He, D.; Hu, B.; Yao, Q.F.; Wang, K.; Yu, S.H. Large-Scale Synthesis of Flexible Free-Standing SERS Substrates with High Sensitivity: Electrospun PVA Nanofibers Embedded with Controlled Alignment of Silver Nanoparticles. ACS Nano 2009, 3, 3993–4002. [Google Scholar] [CrossRef]
- Zhang, L.; Gong, X.; Bao, Y.; Zhao, Y.; Xi, M.; Jiang, C.; Fong, H. Electrospun Nanofibrous Membranes Surface-Decorated with Silver Nanoparticles as Flexible and Active/Sensitive Substrates for Surface-Enhanced Raman Scattering. Langmuir 2012, 28, 14433–14440. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, D.D.; Yang, K.; Zhang, X.; Zhu, Y.G. Perspective on Surface-Enhanced Raman Spectroscopic Investigation of Microbial World. Anal. Chem. 2019, 91, 15345–15354. [Google Scholar] [CrossRef]
- Wang, P.; Wu, L.; Lu, Z.C.; Li, Q.; Yin, W.M.; Ding, F.; Han, H.Y. Gecko-Inspired Nanotentacle Surface-Enhanced Raman Spectroscopy Substrate for Sampling and Reliable Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2017, 89, 2424–2431. [Google Scholar] [CrossRef]
- Xu, W.G.; Ling, X.; Xiao, J.Q.; Dresselhausb, M.S.; Kong, J.; Xu, H.X.; Liu, Z.F.; Zhang, J. Surface enhanced Raman spectroscopy on a flat graphene surface. Proc. Natl. Acad. Sci. USA 2012, 109, 9281–9286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.B.; Yin, J.; Zheng, Y.M.; Liu, Q.; Cheng, X.X.; Luo, F.H. Self-Assembly of Au Nanoparticles on PMMA Template as Flexible, Transparent, and Highly Active SERS Substrates. Anal. Chem. 2014, 86, 6262–6267. [Google Scholar] [CrossRef]
- Zhang, C.L.; Lv, K.P.; Huang, H.T.; Cong, H.P.; Yu, S.H. Co-assembly of Au nanorods with Ag nanowires within polymer nanofiber matrix for enhanced SERS property by electrospinning. Nanoscale 2012, 4, 5348–5355. [Google Scholar] [CrossRef]
- Polavarapu, L.; Liz-Marzan, L.M. Towards low-cost flexible substrates for nanoplasmonic sensing. Phys. Chem. Chem. Phys. 2013, 15, 5288–5300. [Google Scholar] [CrossRef]
- Geng, Y.; Ali, M.A.; Clulow, A.J.; Fan, S.; Burn, P.L.; Gentle, I.R.; Meredith, P.; Shaw, P.E. Unambiguous detection of nitrated explosive vapours by fluorescence quenching of dendrimer films. Nat. Commun. 2015, 6, 8240. [Google Scholar] [CrossRef] [Green Version]
- Tao, A.; Kim, F.; Hess, C.; Goldberger, J.; He, R.; Sun, Y.; Xia, Y.; Yang, P. Langmuir-Blodgett Silver Nanowire Monolayers for Molecular Sensing Using Surface-Enhanced Raman Spectroscopy. Nano Lett. 2003, 3, 1229–1233. [Google Scholar] [CrossRef]
- De, S.; Higgins, T.M.; Lyons, P.E.; Doherty, E.M.; Nirmalraj, P.N.; Blau, W.J.; Boland, J.J.; Coleman, J.N. Silver Nanowire Networks as Flexible, Transparent, Conducting Films: Extremely High DC to Optical Conductivity Ratios. ACS Nano 2009, 3, 1767–1774. [Google Scholar] [CrossRef]
- Aroca, R.F.; Goulet, P.J.; dos Santos, D.S.; Alvarez-Puebla, R.A.; Oliveira, O.N. Silver Nanowire Layer-by-Layer Films as Substrates for Surface-Enhanced Raman Scattering. Anal. Chem. 2005, 77, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Madaria, A.R.; Kumar, A.; Ishikawa, F.N.; Zhou, C. Uniform, Highly Conductive, and Patterned Transparent Films of a Percolating Silver Nanowire Network on Rigid and Flexible Substrates Using a Dry Transfer Technique. Nano Res. 2010, 3, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Li, L.; Yang, M.; Jiang, X.; Zhao, Q.; Zhan, J. A disordered silver nanowires membrane for extraction and surface-enhanced Raman spectroscopy detection. Analyst 2014, 139, 2525–2530. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Zhang, S.; Tian, X.; Xu, H. Highly tunable propagating surface plasmons on supported silver nanowires. Proc. Natl. Acad. Sci. USA 2013, 110, 4494–4499. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Yang, W.; Zhang, S.; Chen, J. High surface roughness gold nanoparticle/centimeter level silver nanowire heterostructure detectors for SERS application. Sens. Actuators A 2018, 279, 457–461. [Google Scholar] [CrossRef]
- Shi, H.Y.; Hu, B.; Yu, X.C.; Zhao, R.L.; Ren, X.F.; Liu, S.L.; Liu, J.W.; Feng, M.; Xu, A.W.; Yu, S.H. Ordering of Disordered Nanowires: Spontaneous Formation of Highly Aligned, Ultralong Ag Nanowire Films at Oil—Water—Air Interface. Adv. Funct. Mater. 2010, 20, 958–964. [Google Scholar] [CrossRef]
- Kudelski, A. Raman studies of rhodamine 6G and crystal violet sub-monolayers on electrochemically roughened silver substrates: Do dye molecules adsorb preferentially on highly SERS-active sites? Chem. Phys. Lett. 2005, 414, 271–275. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yu, C.C.; Sheu, S.F. Low concentration rhodamine 6G observed by surface-enhanced Raman scattering on optimally electrochemically roughened silver substrates. J. Mater. Chem. 2006, 16, 3546–3551. [Google Scholar] [CrossRef]
- Yang, C.; Gu, H.W.; Lin, W.; Yuen, M.M.; Wong, C.P.; Xiong, M.Y.; Gao, B. Silver Nanowires: From Scalable Synthesis to Recyclable Foldable Electronics. Adv. Mater. 2011, 23, 3052. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Liu, B.; Hu, C.L.; Chen, S.Y.; Liu, X.Q.; Liu, J.Y.; Chen, F.; Chen, J.; Xie, F.Y. A facile method in removal of PVP ligands from silver nanowires for high performance and reusable SERS substrate. Spectrochim. Acta Part A 2020, 228, 117733. [Google Scholar] [CrossRef]
- Xiao, L.L.; Zhang, M.; Liu, Z.; Bian, W.W.; Zhang, X.L.; Zhan, J.H. Hydrophobic silver nanowire membrane for swabbing extraction and in situ SERS detection of polycyclic aromatic hydrocarbons on toys. Anal. Methods 2017, 9, 1816–1824. [Google Scholar] [CrossRef]
- Draine, B.T.; Flatau, P.J. Discrete dipole approximation for scattering calculations. J. Opt. Soc. Am. A 1994, 11, 1491–1499. [Google Scholar] [CrossRef]
- Palik, E.D. Handbook of Optical Constants of Solids; Academic Press: Pittsburgh, PA, USA, 1998; Volume 3. [Google Scholar]
- Yang, K.H.; Liu, Y.C.; Yu, C.C. Enhancements in intensity and stability of surface-enhanced Raman scattering on optimally electrochemically roughened silver substrates. J. Mater. Chem. 2008, 18, 4849–4855. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, J.S. Improved surfaced-enhanced Raman scattering based on electrochemically roughened silver substrates modified through argon plasma treatment. Electrochim. Acta 2008, 53, 5615–5620. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yu, C.C.; Yang, K.H.; Chen, J.S. Optimally electrochemically roughened silver substrates prepared in gold complexes-containing solutions for trace molecules detection. J. Electroanal. Chem. 2007, 611, 217–224. [Google Scholar] [CrossRef]
- Lai, Y.; Wang, C.; Shao, H. Thioctic Acid-Modified Silver Nanoplates on Copper Foil for Low Interference Detection of Fluoranthene by Surface-Enhanced Raman Spectroscopy. ACS Appl. Nano Mater. 2020, 3, 1800–1807. [Google Scholar] [CrossRef]
- Wang, H.; Levin, C.S.; Halas, N.J. Nanosphere Arrays with Controlled Sub-10-nm Gaps as Surface-Enhanced Raman Spectroscopy Substrates. J. Am. Chem. Soc. 2005, 127, 14992–14993. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Luo, W.; Liu, Q.; Hao, N.; Zhu, Y.; Liu, M.; Wang, L.; Yang, H.; Chen, X. Simultaneous In Situ Extraction and Fabrication of Surface-Enhanced Raman Scattering Substrate for Reliable Detection of Thiram Residue. Anal. Chem. 2018, 90, 13647–13654. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Mai, Z.; Yang, Y.; Zhu, B.; Xu, X.; Lu, L.; Zou, X. Determination of multi-residue for malachite green, gentian violet and their metabolites in aquatic products by high-performance liquid chromatography coupled with molecularly imprinted solid-phase extraction. J. Chromatogr. A 2009, 1216, 2275–2281. [Google Scholar] [CrossRef]
- Safarik, I.; Safarikova, M. Detection of low concentrations of malachite green and crystal violet in water. Water Res. 2002, 36, 196–200. [Google Scholar] [CrossRef]
- Strehle, K.R.; Clialla, D.; Rosch, P.; Henkel, T.; Kohler, M.; Popp, J. A Reproducible Surface-Enhanced Raman Spectroscopy Approach. Online SERS Measurements in a Segmented Microfluidic System. Anal. Chem. 2007, 79, 1542–1547. [Google Scholar] [CrossRef]
- Liu, B.; Han, G.; Zhang, Z.; Liu, R.; Jiang, C.; Wang, S.; Han, M.Y. Shell Thickness-Dependent Raman Enhancement for Rapid Identification and Detection of Pesticide Residues at Fruit Peels. Anal. Chem. 2012, 84, 255–261. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Y.; Kannan, P.; Zhang, L.; Lin, Z.; Zhang, J.; Chen, T.; Guo, L. Flexible and Adhesive Surface Enhance Raman Scattering Active Tape for Rapid Detection of Pesticide Residues in Fruits and Vegetables. Anal. Chem. 2016, 88, 2149–2155. [Google Scholar] [CrossRef]
- Motzer, W.E. Perchlorate: Problems, Detection, and Solutions. Environ. Forensics 2001, 2, 301–311. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, X.; Cui, J.; Shi, Y.; Jiang, X.; Liu, Z.; Zhan, J. Silver nanoplate-decorated copper wire for the on-site microextraction and detection of perchlorate using a portable Raman spectrometer. Analyst 2015, 140, 2815–2822. [Google Scholar] [CrossRef]
- Tan, E.Z.; Yin, P.G.; You, T.T.; Wang, H.; Guo, L. Three Dimensional Design of Large-Scale TiO2 Nanorods Scaffold Decorated by Silver Nanoparticles as SERS Sensor for Ultrasensitive Malachite Green Detection. ACS Appl. Mater. Interfaces 2012, 4, 3432–3437. [Google Scholar] [CrossRef]
- Li, Z.H.; Bai, J.H.; Zhang, X.; Lv, J.M.; Fan, C.S.; Zhao, Y.M.; Wu, Z.L.; Xu, H.J. Facile synthesis of Au nanoparticle-coated Fe3O4 magnetic composite nanospheres and their application in SERS detection of malachite green. Spectrochim. Acta Part A 2020, 241, 18532. [Google Scholar] [CrossRef]
- Wenzl, T.; Simon, R.; Anklam, E.; Kleiner, J. Analytical methods for polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. TrAC Trends Anal. Chem. 2006, 25, 716–725. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, X.; Qu, B.; Zhan, J. Portable kit for high-throughput analysis of polycyclic aromatic hydrocarbons using surface enhanced Raman scattering after dispersive liquid-liquid microextraction. Talanta 2017, 175, 495–500. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Lai, Y.; Zhan, J. Enhancing the Activity of Silver Nanowire Membranes by Electrochemical Cyclic Voltammetry as Highly Sensitive Flexible SERS Substrate for On-Site Analysis. Nanomaterials 2021, 11, 672. https://doi.org/10.3390/nano11030672
Zhang R, Lai Y, Zhan J. Enhancing the Activity of Silver Nanowire Membranes by Electrochemical Cyclic Voltammetry as Highly Sensitive Flexible SERS Substrate for On-Site Analysis. Nanomaterials. 2021; 11(3):672. https://doi.org/10.3390/nano11030672
Chicago/Turabian StyleZhang, Rui, Yongchao Lai, and Jinhua Zhan. 2021. "Enhancing the Activity of Silver Nanowire Membranes by Electrochemical Cyclic Voltammetry as Highly Sensitive Flexible SERS Substrate for On-Site Analysis" Nanomaterials 11, no. 3: 672. https://doi.org/10.3390/nano11030672