The UV LED assisting DHR-2 investigation was carried out before the mechanical property analysis of the neat SLR and SLR/Ag-TNP nanocomposites, in order to examine the effects of incorporated Ag-TNP curing behaviors with the SLA resin matrix. The DHR-2 analysis outcome reveals that the SLR/Ag-TNP nanocomposites own an elevated viscosity compared to neat SLR, and the viscosity shows an increase as the loading percentage of Ag-TNP increases from 0.5% to 1.2%
w/
w (refer to
Figure 5a). Moreover, the DHR-2 operational with a UV LED co-conspirator instrument can be traced as the variations in storage modulus of the nanocomposite resin in the course of the curing route by a fast sampling process.
Figure 5b demonstrates the curing behaviors of neat SLR and SLR contains with different loading %
w/
w of Ag-TNP. The following protocol was strictly maintained for DHR-2 analysis of tested samples, and the resulting outcomes were experiential in the following trend, which means that, prior to the UV light being switched ON (at time <30 s), the storage modulus of all samples stands for the homogeneous storage modulus, meaning that there were no notable storage modulus changes in the uncured resin. However, once the UV light was switched ON (at time = 30 s), the outstanding increasing trend of storage modulus was renowned, signifying that the cross-linking reaction has proceeded remarkably. After UV curing (at time ≈ 50 s), the cross-linking and curing responses were observed as a saturation level, and the storage modulus was kept in a maximum state and remained steady. The excellent storage modulus was achieved at the loading concentration of 1.0 %
w/
w Ag-TNP (refer to the violet line in insert
Figure 5b). The resulting graph (refer to Figure 7b) revealed that the UV light may evade the well-dispersed minuscule Ag-TNP very quickly, especially at 1.0 %
w/
w of loading concentration with an SLR matrix. Nevertheless, the accumulation of Ag-TNP would obstruct the light and inhibit the photopolymerization in the SLR system [
63,
64]. Consequently, as the incorporation of Ag-TNP hybrids reaches 1.2%
w/
w, significant protracted escalation of polymerization and an apparent decline of curing rate was distinguished. For the UV-instigated photopolymerization and interconnection, the nanoparticles fillers affect the gel time in two contradictory features: (a) the reinforcing effect of nanoparticles may endorse the interconnection between the monomers and oligomers to enhance the modulus of resin matrix, hence reducing the gel period; (b) the nanofillers may obstruct the light, hindering the photopolymerization, and thus extend the gel period [
65].
Figure 5b depicts that the accumulation of Ag-TNP lengthens the gel time a little bit, though the highest storage modulus was observed since it was subjected to the strengthening effect (refer to the purple line in
Figure 5b).
3.2.1. Mechanical Properties
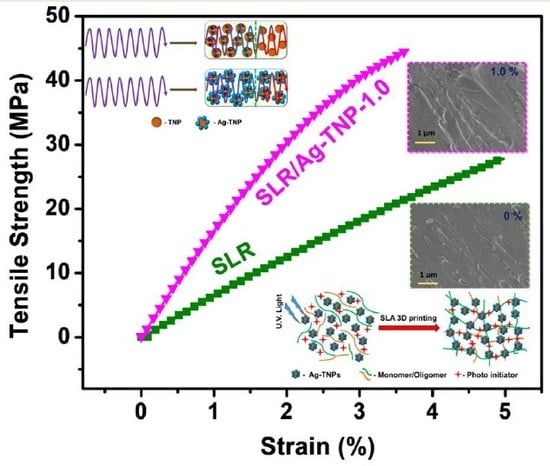
The mechanical behaviors of the SLR/Ag-TNP and neat SLR, performed with the assistance of the universal testing machine and the observed results, were discussed in this subsection. The tensile and flexural stress–strain curves of the stated dog-bone shaped 3D printed samples of neat SLR, and SLR/Ag-TNP nanocomposites were displayed with
Figure 6a,b. For a better understanding of the effects of incorporated Ag-TNP with an SLR matrix, mechanical properties enhanced the complete data set of tensile strength and a tensile modulus graph as depicted in
Figure 6c,d. The tensile strength of the SLR/Ag-TNP nanocomposites revealed an increasing trend, with increasing loading concentration of Ag-TNP. Especially at the loading content of 1.0%
w/
w, Ag-TNP shows remarkable tensile strength enhancement compared with neat SLR. The enhanced tensile strength of SLR/Ag-TNP was noted as 44.7 MPa at the 1.0 %
w/
w incorporation of as-prepared Ag-TNP, which shows a 60.8% enhancement compared with neat SLR (27.8 MPa) (refer to the red line in
Figure 6c). In addition, the increased tensile modulus was also being observed with the increasing Ag-TNP loading with the SLR matrix (refer to the blue line in
Figure 6c). In addition,
Figure 6e demonstrates the elongation at break analysis of the neat SLR and SLR/nanofillers nanocomposite, which illustrates that the elongation at break reduces with escalating of the nanofillers (Ag-TNPs) content. The integrated nanofillers may confine the SLR chain segmental mobility to improve the inflexibility of the polymer matrix and make the SLR system more brittle, and then lastly directs to the destitute elongation at break [
66]. Concurrently, the ductility of the SLR nanocomposites was abridged while inserting the reinforcing nanofillers into the SLR system, which would appreciably diminish the stress transfer [
25,
61] and make the SLR system more fragile with inferior ductility caused by the more complicated chain mobility by the rigidity nature of the incorporated nanofillers [
62,
67]. However, the incorporated nanofillers (Ag-TNPs) render striking enrichment in mechanical properties, owing to their incredible mechanical properties [
68,
69]. The ductility of 3D printed SLR/Ag-TNP-1.2 (2.9) was reduced 40.8% less than the 3D printed neat SLR (4.9). The increasing mechanical properties were observed with the incorporation of increasing Ag-TNP, but the unexpected decrement was noted when the loading concentration goes beyond 1.0%
w/
w of Ag-TNP. This crucial phenomenon was maybe the possible agglomeration of Ag-TNP within the SLR matrix when the incorporation goes to a higher level. However, the better mechanical enhancement was achieved at lower content assimilation of Ag-TNP (1.0%
w/
w), due to the homogenous dispersion of incorporated Ag-TNP. Moreover, the low energy bandgap, high crystalline structure, and elevated dispersion of Ag-TNP within the SLR matrix, which helps with the rapid generation of electron–hole pairs and could enhance photopolymerization by a strong bonding with the monomers and oligomers present in the urethane–acrylate photo resin. Hence, the as-prepared Ag-TNP being able to induce the catalytic activity of photopolymerization along with the nanoscale-sized Ag-TNP that can achieve excellent dispersion within the SLR matrix were the key reasons for the mechanical properties enhancement.
The flexural strength and flexural modulus correlation graph of neat SLR and SLR/Ag-TNP were displayed in
Figure 6d. The resulting graph showed that, as the loading %
w/
w of Ag-TNP increases, the flexural strength and flexural modulus increase progressively until 1.0%
w/
w, although the sudden decrement was noted when the loading content increased beyond the 1.0%
w/
w concentration of Ag-TNP (refer to redline in
Figure 6d). The flexural strength and flexural modulus of the 3D printed samples of neat SLR were observed as the lowest compared to all the SLR/Ag-TNP nanocomposites. Overall, the sample SLR/Ag-TNP-1.0 shows the adequate flexural strength and flexural modulus among all the other samples with an increment of 71.8% and 59%, respectively, compared to that of neat 3D printed SLR. The elevated loading of Ag-TNP (higher than 1.0%
w/
w) would pile into the SLR system, which caused the lower cross-linking density existing in SLA nanocomposites, directing to a weakening of mechanical behaviors of SLR/Ag-TNP nanocomposites, which results in the diminution of flexural and tensile properties [
70]. In order to understand the mechanical properties enhancement in a better way, the flexural strength, tensile strength, % elongation at break, and Young’s modulus of the neat SLR and SLR/Ag-TNP nanocomposites were endowed with
Table 1 and
Table 2, subsequent to the effects with
Figure 6c,d, respectively.
For the better perception of the mechanical improvement over the effect of Ag-TNP on the SLR/Ag-TNP nanocomposites, the crack surfaces of the tested tensile trials of neat SLR and SLR/Ag-TNP were investigated by the assistance of FESEM analysis, and the observed morphology images were presented with the
Figure 7. The fracture surfaces of neat SLR (refer to
Figure 7a) were noted as moderately even and very smooth surfaces had few linear fissures, whereas the fractured surfaces of the SLR/Ag-TNP nanocomposites (refer to
Figure 7b–e) were observed with uneven and rough surfaces having some tears without linear cracks. Performing as stress concentrators, the incorporated subsistence of Ag-TNP may power the tear to proliferate along a convoluted path and promote the configuration of numerous micro-fissures. However, when the loading of Ag-TNP increases to 1.2%
w/
w, the agglomeration of Ag-TNP was noticed remarkably, which may affect the curing followed by a cross-linking process of the SLR and persuade the development of micro-breakdowns, ensuing with a worsening of mechanical behaviors, together with a considerable lessening in tensile properties (refer to
Figure 6c and
Table 1) and flexural properties (refer to
Figure 6d and
Table 2).
The nanoindentation analysis was demonstrated to examine the micro-mechanical behaviors of the 3D printed samples of neat SLR and SLR/Ag-TNP nanocomposites on a small scale, for instance, hardness and reduced modulus.
Figure 8a represents a plot of the applied load adjacent to the indentation intensity for the SLR/Ag-TNP and neat SLR nanocomposites. In conformity with
Figure 8a, expressing that all the SLR/Ag-TNP nanocomposites’ indentation depths were recorded as reduced spectacularly compared to the neat SLR nanocomposites. The indentation depths for the SLR/Ag-TNP nanocomposites declined as the addition of Ag-TNP increased with the loading of Ag-TNP up to 1.0%
w/
w; later, the privileged indentation depth was observed when the loading goes beyond 1.0%
w/
w of Ag-TNP, which determines that the enhancement of micro-mechanical behavior for the Ag-TNP incorporated SLR nanocomposites was active at the lowest loading content of Ag-TNP. The privileged indentation depth of beyond 1.0%
w/
w loaded Ag-TNP may be the inhibition of the plastic deformation rising because of the agglomeration of nanofillers within the SLR nanocomposites.
Figure 8b demonstrates the reduced modulus, and hardness of the 3D printed samples of SLR/Ag-TNP and pure SLR nanocomposites concerning the different loading percentages of Ag-TNP to the SLR nanocomposites. As the increasing incorporation of Ag-TNP varies from 0.5 to 1.0%
w/
w, the hardness and the reduced modulus were monitored as remarkable increases. By the addition of 1.0%
w/
w of Ag-TNP into the SLR matrix, the hardness (0.225 GPa) and reduced modulus (3.86 GPa) attained the optimized standards and increased by 40.6% and 34.9%, equating to the neat SLR (0.16 GPa for hardness and 2.86 GPa for reduced modulus), respectively. However, through promoting the increasing of Ag-TNP afar 1.0%
w/
w, the hardness and reduced modulus values decrease due to the agglomeration of elevated content of Ag-TNP, which leads to the weak interaction between the SLR matrix and Ag-TNP. The experimental results of indentation depth, hardness, and reduced modulus of the SLR/Ag-TNP and pure SLR nanocomposites were supplied with
Table 3.
The thermo-mechanical behaviors of the SLR/Ag-TNP and neat SLR nanocomposites were investigated with the help of Q800 TA instruments, and the observed results were discussed in this subsection. The DMA method was founded on an easy principle; when a model is subjected to a sinusoidal oscillating stress, its reply is a sinusoidal oscillation with parallel frequency furnishing the material staying within its elastic limits. When the material reacts to the employed oscillating stress entirely elastically, the responding strain wave is in-phase (elastic response or storage), while a viscous material responds with an out-of-phase strain wave (a viscous response or loss).
Figure 9a,b represent the deviations of storage modulus (E′) and tan
δ as a function of temperature, respectively. In accordance with
Figure 9a, the storage modulus of 3D printed samples decreased slowly when temperature increased from 25 °C to 180 °C. The storage modulus of the SLR/Ag-TNP nanocomposites was observed as increases with increasing Ag-TNP content with the SLR up to 1.0%
w/
w. The increase in the storage modulus signifies the superior scattering and excellent interfacial interaction between the Ag-TNP and SLR matrix in the SLR/Ag-TNP nanocomposites [
71]. In addition,
Figure 9b demonstrates that, with the increasing nanofillers incorporation, the loss modulus increases gradually, with respect to the storage modulus and glass transition temperature. Later, a sudden decrement was noted when the nanofillers loading reaches over 1 wt%. The loss modulus and storage modulus of SLR/Ag-TNP nanocomposites were decreased when the incorporation of Ag-TNP concentration was more than 1.0%
w/
w, due to the elevated nanofillers loading causing reasonable agglomeration of Ag-TNP within the SLR matrix. The sample SLR/AgTNP-1.0 sustains the best storage modulus compared to all other samples with the highest value of 1953.1MPa, compared to a neat SLR value of 1495.2MPa, which was about 30.5% higher than the neat SLR. It ensured that the adding of Ag-TNP with SLR could enhance the storage modulus of 3D printed structure compared to neat SLR.
In addition to the storage modulus,
Figure 9c shows the comparative study of tan
δ curves for the SLR/Ag-TNP and neat SLR nanocomposites. It was investigated from the tan
δ curves that the glass transition temperature (T
g) of the SLR/Ag-TNP-1.0 (86.5 °C) was the highest and could be improved by an elevated difference of 7.2 °C compared to neat SLR (79.3 °C) (refer to the inset in
Figure 9c). Beyond the 1.0 wt% loading concentration, the T
g value decreases due to higher agglomeration leading to the poor dispersion of Ag-TNP within the SLR matrix. The improved T
g could be interpreted as it ensures that the SLR/Ag-TNP-1.0 nanocomposites were thermally highly stable compared to all other samples. The optimized loading content of Ag-TNP expresses a good dispersion within the SLR matrix, and superior cross-linking density directs to a more significant interruption from polymer chain association so that the glass T
g progress was noted at upper temperatures for SLR/Ag-TNP-1.0 nanocomposites. The detailed observed values of DMA analysis were given with
Table 4.
3.2.2. Thermal Properties
The thermogravimetric analysis of neat SLR and SLR/Ag-TNP nanocomposites was analyzed, and the resulted graph, as shown in
Figure 10a. In conformity with Figure 12a and
Table 5, in which the residual weight of pristine SLR was noted as the lowest among all the TGA tested samples, and an increasing trend of residual char was noted with the loading percentage of Ag-TNP into the SLR matrix. The residual char weight % in the SLR/Ag-TNP nanocomposites was considerably high compared to pure SLR (5.86), which was showing that the relatively high thermal stability of the SLR/Ag-TNP nanocomposites at high temperatures (for the SLR/Ag-TNP-1.0 sample, the residue weight % was noted as 8.01). However, the loading concentration of Ag-TNP increases beyond the level of 1.0%
w/
w, and the sudden decrement of residual char value was noted as a reason for these phenomena was maybe the higher concentration of Ag-TNP could make possible agglomeration within the SLR matrix, which leads to poor dispersion and weakening the thermal stability of SLR/Ag-TNP nanocomposites. The above-mentioned interpretation proposes that the exceptional thermal stability with a lower decomposition rate has been found in the sample SLR/Ag-TNP-1.0. In addition, the value of the mean temperature of the squalor (T
-50%, temperature) and the residue char value at 800 °C were abridged in
Table 5. For the neat SLR nanocomposites, the T
-50% was noted as 424.1, and it was significantly increased for the samples of SLR/Ag-TNP nanocomposites. The enhancement of thermal stability can be reorganized by taking into account of the tears, movement, and thermal disintegration of the polymer manacles. With the strong interactions between the incorporated Ag-TNP and SLR matrix, the movements of the polymer chains were partial for the period of the thermal degradation process, which was significantly responsible for the progress in thermal enhancement [
72].
Figure 10b represents the thermal conductivity measurements of the neat SLR and SLR/Ag-TNP nanocomposites. From
Figure 10b and
Table 5, it was investigated that the thermal conductivity of the SLR/Ag-TNP nanocomposites considerably increases when the loading percentage of Ag-TNP increases with the SLR matrix. The average thermal conductivity value of the neat SLR was noted as 0.2465 (W·m
−1·K
−1) and the thermal conductivity of the SLR/Ag-TNP nanocomposites increasing with respect to the increases in the %
w/
w of Ag-TNP, as it reaches 1.0%
w/
w of Ag-TNP nanoparticles with a value of 0.3456 (W·m
−1·K
−1) (40.2% of increment in the thermal conductivity more than the neat SLR). When increasing the loading percentage of Ag-TNP in excess of 1.0%
w/
w, within the SLR matrix, the deprived thermal conductivity was noted. The thermal conductivity initially increased and was a decreasing trend with the incorporation of Ag-TNP, which was due to the elevated loading of nanofillers possibly causing agglomeration, which leads to poor dispersion within the polymer matrix and lessens the thermal conductivity. Moreover, silver was considered as one of the excellent thermal conductors among the metals. Here, the enhancement of thermal conductivity may be due to the silver decorated TNP attaining very good dispersion within the SLR matrix at lower loading concentration, which could possibly enhance the thermal conductivity of SLR/Ag-TNP nanocomposites [
73]. To study the thermal conductivity significance of our research work, the observed thermal conductivity values were compared with other reported photocurable resin/nanocomposites thermal conductivity values. The comparative thermal conductivity was displayed in
Table 6.
The kinetic study of photo-polymerization reaction of both neat SLR resin and Ag-TNP nanofillers incorporated SLR nanocomposites analyzed with the assistance of Real-Time Fourier transform infrared spectra (RT-FTIR), and the observed results are presented in
Figure 11. The photopolymerization kinetics was resolved by the decline of absorption peak intensity around 809–811 cm
−1 from the FTIR analysis, which was attributed to the twisting vibration mode of double-bond in an acrylic group [
79]. The degree of conversion (DC) of a double-bond was calculated from Equation (3) [
80,
81]:
where A
0 is the initial peak area before the irradiation at 809.5 cm
−1, while A
t is the peak area at irradiation time t at 809.5 cm
−1 during the polymerization, respectively.
The analysis was executed on the basis of different time intervals to comprehend the photopolymerization progression. Initially, the uncured vat slurry was examined by FTIR, followed by UV curing with an irradiation power of 32 mW/cm
2 at different time intervals such as 10 s, 30 s, 60 s, 120 s, 240 s, 400 s, and 600 s. The neat SLR and SLR/Ag-TNP-1 slurry analysis is presented in
Figure 11a, and all other SLR/nanofillers analyses such as SLR/Ag-TNP-0.5, SLR/Ag-TNP-0.8, and SLR/Ag-TNP-1.2 are displayed in
Supplementary Figure S5. The analysis was employed to determine the absorbance intensity of the peak at 809.5 cm
−1, which is related to acrylate double bond. From the FTIR analysis, the percentage conversion versus time curves was plotted to understand the photopolymerization reactions based on the double bond (C=C) conversion ratio taking place in different time periods.
Figure 11b shows that the conversion rates of the photopolymerization reactions were enhanced by the addition of nanofillers content.
Figure 11b expresses that the SLR/Ag-TNP-1 nanocomposite showed a better C=C conversion rate of photopolymerization among all other SLR/nanofillers’ composites. The percentage conversion of SLR/Ag-TNP-1 was reached to 62.2% at the 600 seconds, where percent conversion of SLR was only 45.8%. In addition, to investigate the effect of radiation power on the photopolymerization kinetics, as prepared SLR/Ag-TNP-1% nanocomposites have been taken to perform under the different UV power intensities. The recorded RT-FTIR polymerization profiles of the sample with different power densities of UV-irradiation at different time intervals are illustrated in the
supplementary Figure S6. The analysis was executed on the basis of different time intervals to realize the photopolymerization evolution. Primarily, the uncured SLR/Ag-TNP-1 slurry was examined by FTIR, followed by UV curing with an irradiation power of 24 mW/cm
2 at different time intervals such as 10 s, 30 s, 60 s, 120 s, 240 s, 400 s, and 600 s. The same procedure carried out for SLR/Ag-TNP-1 with different power densities of UV irradiation of 28 mW/cm
2 and 32 mW/cm
2 in the above-mentioned time intervals. The percentage conversions of SLR/Ag-TNP-1 at 600s in the different UV exposure power densities of 24 mW/cm
2, 28 mW/cm
2 and 32 mW/cm
2 are 55.8%, 59.1%, and 62.2%, respectively.