Specialized Living Wound Dressing Based on the Self-Assembly Approach of Tissue Engineering
Abstract
:1. Introduction
2. Results
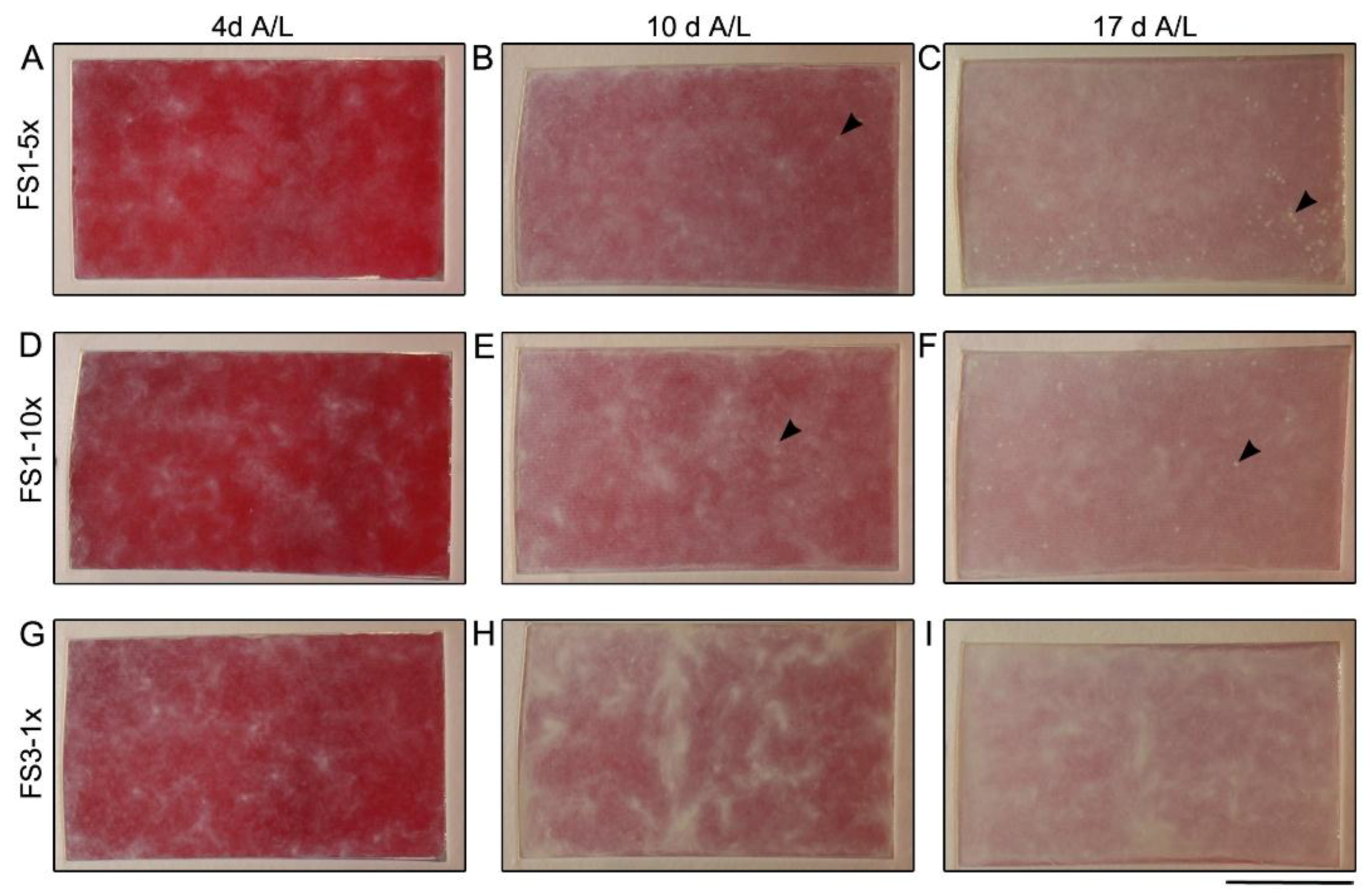
2.1. Macroscopic Appearance and Histological Analysis of Tissues Cultured in Vitro
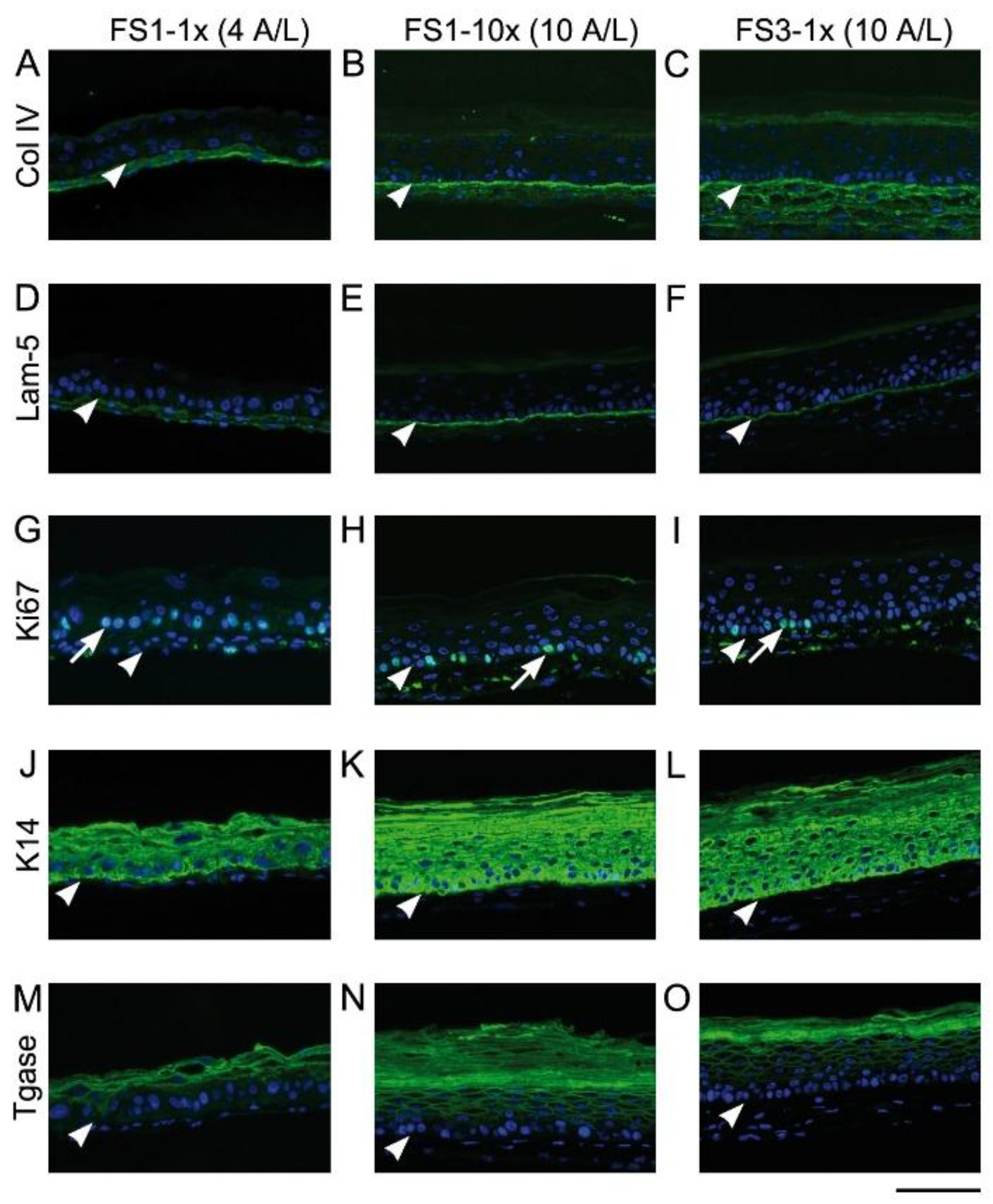
2.2. Immunofluorescence Analysis
2.3. Tissue Contraction
3. Discussion
4. Materials and Methods
4.1. Cell Populations
4.2. Cell Isolation and Culture
4.3. Tissue-Engineered Skin Production
4.4. Contraction Kinetics on Agar Substrate
4.5. Grafting on Athymic Mice
4.6. Histological and Immunofluorescence Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Krug, K.; Brogan, M.S. Physical therapy in wound care: A cost–effectiveness analysis. Med. Baltim. 2015, 94. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, N.; Franks, P.; Moffatt, C.; Connolly, M.; Oldroyd, M.; Brown, P.; Greenhalgh, R.; McCollum, C. Community leg ulcer clinics: Cost-effectiveness. Health Trends 1993, 25, 146–148. [Google Scholar] [PubMed]
- Nelson, E.A.; Adderley, U. Venous leg ulcers. 2016. Available online: https://www.ncbi.nlm.nih.gov/pubmed/26771825 (accessed on 15 January 2016).
- Ashby, R.L.; Gabe, R.; Ali, S.; Adderley, U.; Bland, J.M.; Cullum, N.A.; Dumville, J.C.; Iglesias, C.P.; Kang’ombe, A.R.; Soares, M.O.; et al. Clinical and cost-effectiveness of compression hosiery versus compression bandages in treatment of venous leg ulcers (venous leg ulcer study iv, venus iv): A randomised controlled trial. Lancet 2014, 383, 871–879. [Google Scholar] [CrossRef]
- Cornwall, J.V.; Dore, C.J.; Lewis, J.D. Leg ulcers: Epidemiology and aetiology. Br. J. Surg. 1986, 73, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Skene, A.I.; Smith, J.M.; Dore, C.J.; Charlett, A.; Lewis, J.D. Venous leg ulcers: A prognostic index to predict time to healing. BMJ 1992, 305, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Moffatt, C.J.; Connolly, M.; Bosanquet, N.; Oldroyd, M.I.; Greenhalgh, R.M.; McCollum, C.N. Factors associated with healing leg ulceration with high compression. Age Ageing 1995, 24, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Voineskos, S.H.; Ayeni, O.A.; McKnight, L.; Thoma, A. Systematic review of skin graft donor-site dressings. Plast. Reconstr. Surg. 2009, 124, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Rizzuto, A.; Rossi, A.; Perri, P.; Barbetta, A.; Abdalla, K.; Caroleo, S.; Longo, C.; Amantea, B.; Sammarco, G.; et al. Skin grafting for the treatment of chronic leg ulcers—A systematic review in evidence-based medicine. Int. Wound J. 2016. [Google Scholar] [CrossRef]
- Falanga, V.; Sabolinski, M. A bilayered living skin construct (apligraf) accelerates complete closure of hard-to-heal venous ulcers. Wound Repair Regen. 1999, 7, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Spiekstra, S.W.; Breetveld, M.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. 2007, 15, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Muffler, S.; Stark, H.J.; Amoros, M.; Falkowska-Hansen, B.; Boehnke, K.; Buhring, H.J.; Marme, A.; Bickenbach, J.R.; Boukamp, P. A stable niche supports long-term maintenance of human epidermal stem cells in organotypic cultures. Stem Cells 2008, 26, 2506–2515. [Google Scholar] [CrossRef] [PubMed]
- El Ghalbzouri, A.; Ponec, M. Diffusible factors released by fibroblasts support epidermal morphogenesis and deposition of basement membrane components. Wound Repair Regen. 2004, 12, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Maas-Szabowski, N.; Shimotoyodome, A.; Fusenig, N.E. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J. Cell Sci. 1999, 112, 1843–1853. [Google Scholar] [PubMed]
- Falanga, V.; Margolis, D.; Alvarez, O.; Auletta, M.; Maggiacomo, F.; Altman, M.; Jensen, J.; Sabolinski, M.; Hardin-Young, J. Rapid healing of venous ulcers and lack of clinical rejection with an allogeneic cultured human skin equivalent. Human skin equivalent investigators group. Arch. Dermatol. 1998, 134, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Uccioli, L.; Group, T.A.S.I.S. A clinical investigation on the characteristics and outcomes of treating chronic lower extremity wounds using the tissuetech autograft system. Int. J. Low. Extrem. Wounds 2003, 2, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Nelson, E.A.; Al-Hity, A. Skin grafting for venous leg ulcers. Cochrane Database Syst. Rev. 2013, CD001737. [Google Scholar] [CrossRef]
- Harding, K.; Sumner, M.; Cardinal, M. A prospective, multicentre, randomised controlled study of human fibroblast-derived dermal substitute (dermagraft) in patients with venous leg ulcers. Int. Wound J. 2013, 10, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Marston, W.A.; Cardinal, M. The incidence of lower-extremity amputation and bone resection in diabetic foot ulcer patients treated with a human fibroblast-derived dermal substitute. Adv. Skin Wound Care 2015, 28, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Frykberg, R.G.; Cazzell, S.M.; Arroyo-Rivera, J.; Tallis, A.; Reyzelman, A.M.; Saba, F.; Warren, L.; Stouch, B.C.; Gilbert, T.W. Evaluation of tissue engineering products for the management of neuropathic diabetic foot ulcers: An interim analysis. J. Wound Care 2016, 7, S18–S25. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.; Zaat, S.A.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- L’Heureux, N.; Stoclet, J.C.; Auger, F.A.; Lagaud, G.J.; Germain, L.; Andriantsitohaina, R. A human tissue-engineered vascular media: A new model for pharmacological studies of contractile responses. FASEB J. 2001, 15, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Michel, M.; L’Heureux, N.; Pouliot, R.; Xu, W.; Auger, F.A.; Germain, L. Characterization of a new tissue-engineered human skin equivalent with hair. In Vitro Cell. Dev. Biol. Anim. 1999, 35, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Cantin-Warren, L.; Desgagne, M.; Guignard, R.; Martel, I.; Ayoub, A.; Lavoie, A.; Gauvin, R.; Auger, F.A.; Moulin, V.J.; et al. Improved methods to produce tissue-engineered skin substitutes suitable for the permanent closure of full-thickness skin injuries. Biores Open Access 2016, 5, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A.; Bussière, A.; et al. Autologous bilayered self-assembled skin substitutes (sasss) as permanent grafts: A case series of 14 severely burned patients indicating clinical effectiveness. Eur. Cell Mater. 2018, 36, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Boa, O.; Cloutier, C.B.; Genest, H.; Labbe, R.; Rodrigue, B.; Soucy, J.; Roy, M.; Arsenault, F.; Ospina, C.E.; Dube, N.; et al. Prospective study on the treatment of lower-extremity chronic venous and mixed ulcers using tissue-engineered skin substitute made by the self-assembly approach. Adv. Skin Wound Care 2013, 26, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Gauvin, R.; Larouche, D.; Marcoux, H.; Guignard, R.; Auger, F.A.; Germain, L. Minimal contraction for tissue-engineered skin substitutes when matured at the air-liquid interface. J. Tissue Eng. Regen. Med. 2013, 7, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Martinson, M.; Martinson, N. A comparative analysis of skin substitutes used in the management of diabetic foot ulcers. J. Wound Care 2016, 25, S8–S17. [Google Scholar] [CrossRef] [PubMed]
- Blok, C.S.; Vink, L.; de Boer, E.M.; van Montfrans, C.; van den Hoogenband, H.M.; Mooij, M.C.; Gauw, S.A.; Vloemans, J.A.; Bruynzeel, I.; van Kraan, A.; et al. Autologous skin substitute for hard-to-heal ulcers: Retrospective analysis on safety, applicability, and efficacy in an outpatient and hospitalized setting. Wound Repair Regen. 2013, 21, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Sabolinski, M.L.; Alvarez, O.; Auletta, M.; Mulder, G.; Parenteau, N.L. Cultured skin as a ‘smart material’ for healing wounds: Experience in venous ulcers. Biomaterials 1996, 17, 311–320. [Google Scholar] [CrossRef]
- Michel, M.; L’Heureux, N.; Auger, F.; Germain, L. From newborn to adult: Phonotypic and functional properties of skin equivalent and human skin as a function of donor age. J. Cell. Physiol. 1997, 171, 179–189. [Google Scholar] [CrossRef]
- Chabaud, S.; Rousseau, A.; Marcoux, T.L.; Bolduc, S. Inexpensive production of near-native engineered stromas. J. Tissue Eng. Regen. Med. 2017, 11, 1377–1389. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.M.; Valderrama, R.; Navarro, S.; Imperial, S. Aprotinin inhibits unspecific degradation of collagen in rat and human pancreas. Int. J. Pancreatol. 1996, 19, 55–60. [Google Scholar] [PubMed]
- Sobral, C.S.; Gragnani, A.; Cao, X.; Morgan, J.R.; Ferreira, L.M. Human keratinocytes cultured on collagen matrix used as an experimental burn model. J. Burns Wounds 2007, 7, e6. [Google Scholar] [PubMed]
- Dvorak, H.F.; Mihm, M.C., Jr.; Dvorak, A.M.; Barnes, B.A.; Galli, S.J. The microvasculature is the critical target of the immune response in vascularized skin allograft rejection. J. Invest. Dermatol. 1980, 74, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F.; Mihm, M.C., Jr.; Dvorak, A.M.; Barnes, B.A.; Manseau, E.J.; Galli, S.J. Rejection of first-set skin allografts in man. The microvasculature is the critical target of the immune response. J. Exp. Med. 1979, 150, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.E.; Hull, B.E.; Rosen, S.; Church, D.; Friedman, L.; Bell, E. Acceptance of allogeneic fibroblasts in skin equivalent transplants. Transplantation 1983, 36, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Cuono, C.B.; Langdon, R.; Birchall, N.; Barttelbort, S.; McGuire, J. Composite autologous-allogeneic skin replacement: Development and clinical application. Plast. Reconstr. Surg. 1987, 80, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Reagan, B.J.; Madden, M.R.; Huo, J.; Mathwich, M.; Staiano-Coico, L. Analysis of cellular and decellular allogeneic dermal grafts for the treatment of full-thickness wounds in a porcine model. J. Trauma 1997, 43, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Larouche, D.; Paquet, C.; Fradette, J.; Carrier, P.; Auger, F.A.; Germain, L. Regeneration of skin and cornea by tissue engineering. Methods Mol. Biol. 2009, 482, 233–256. [Google Scholar] [PubMed]
- Bisson, F.; Rochefort, E.; Lavoie, A.; Larouche, D.; Zaniolo, K.; Simard-Bisson, C.; Damour, O.; Auger, F.A.; Guerin, S.L.; Germain, L. Irradiated human dermal fibroblasts are as efficient as mouse fibroblasts as a feeder layer to improve human epidermal cell culture lifespan. Int. J. Mol. Sci. 2013, 14, 4684–4704. [Google Scholar] [CrossRef] [PubMed]
- Beaudoin Cloutier, C.; Goyer, B.; Perron, C.; Guignard, R.; Larouche, D.; Moulin, V.J.; Germain, L.; Gauvin, R.; Auger, F.A. In vivo evaluation and imaging of a bilayered self-assembled skin substitute using a decellularized dermal matrix grafted on mice. Tissue Eng. Part A 2017, 23, 313–322. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cantin-Warren, L.; Guignard, R.; Cortez Ghio, S.; Larouche, D.; Auger, F.A.; Germain, L. Specialized Living Wound Dressing Based on the Self-Assembly Approach of Tissue Engineering. J. Funct. Biomater. 2018, 9, 53. https://doi.org/10.3390/jfb9030053
Cantin-Warren L, Guignard R, Cortez Ghio S, Larouche D, Auger FA, Germain L. Specialized Living Wound Dressing Based on the Self-Assembly Approach of Tissue Engineering. Journal of Functional Biomaterials. 2018; 9(3):53. https://doi.org/10.3390/jfb9030053
Chicago/Turabian StyleCantin-Warren, Laurence, Rina Guignard, Sergio Cortez Ghio, Danielle Larouche, François A. Auger, and Lucie Germain. 2018. "Specialized Living Wound Dressing Based on the Self-Assembly Approach of Tissue Engineering" Journal of Functional Biomaterials 9, no. 3: 53. https://doi.org/10.3390/jfb9030053
APA StyleCantin-Warren, L., Guignard, R., Cortez Ghio, S., Larouche, D., Auger, F. A., & Germain, L. (2018). Specialized Living Wound Dressing Based on the Self-Assembly Approach of Tissue Engineering. Journal of Functional Biomaterials, 9(3), 53. https://doi.org/10.3390/jfb9030053






