The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens
Abstract
:1. Introduction
2. Results and Discussion
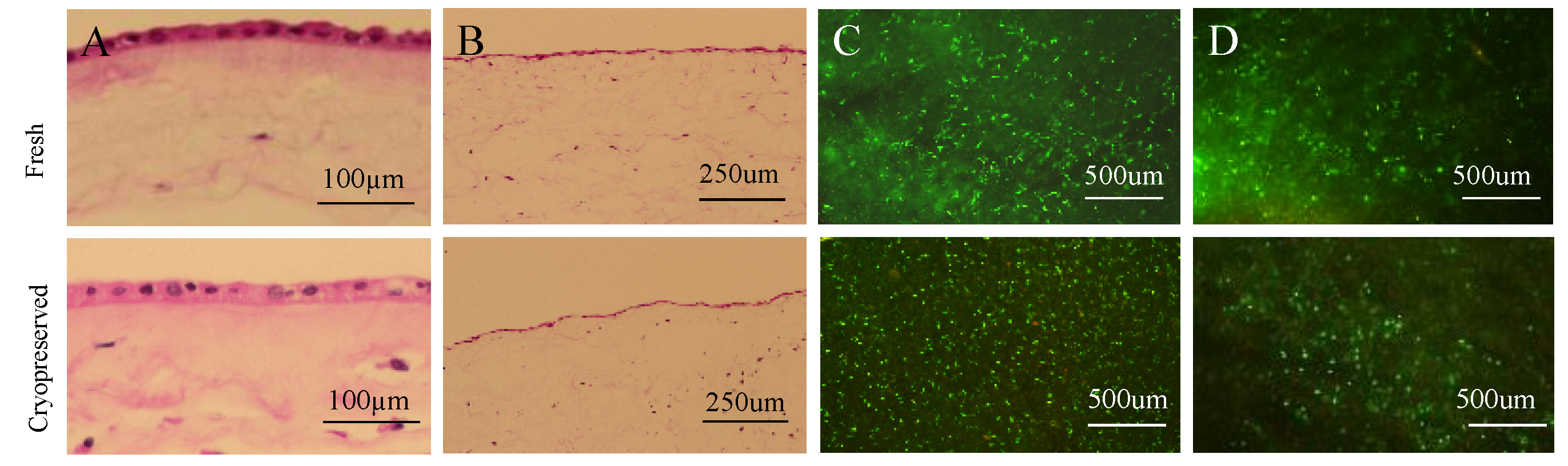
2.1. Cryopreserved Placental Tissues Retain the Native Structure and Cell Viability of Fresh Tissues
2.2. Antibacterial Activity of CVUT against ESKAPE
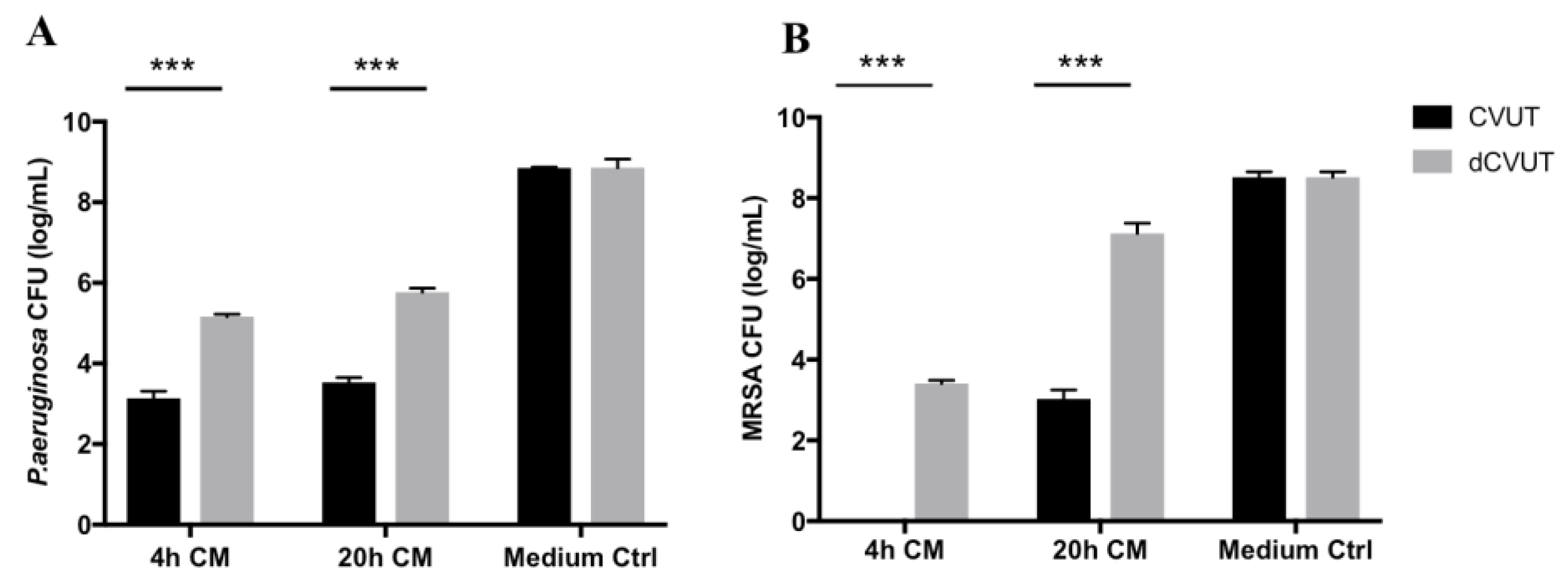
2.3. Viable Cells in CVUT Contribute to Antibacterial Activity
2.4. Antimicrobial Peptides in CVUT Conditioned Medium
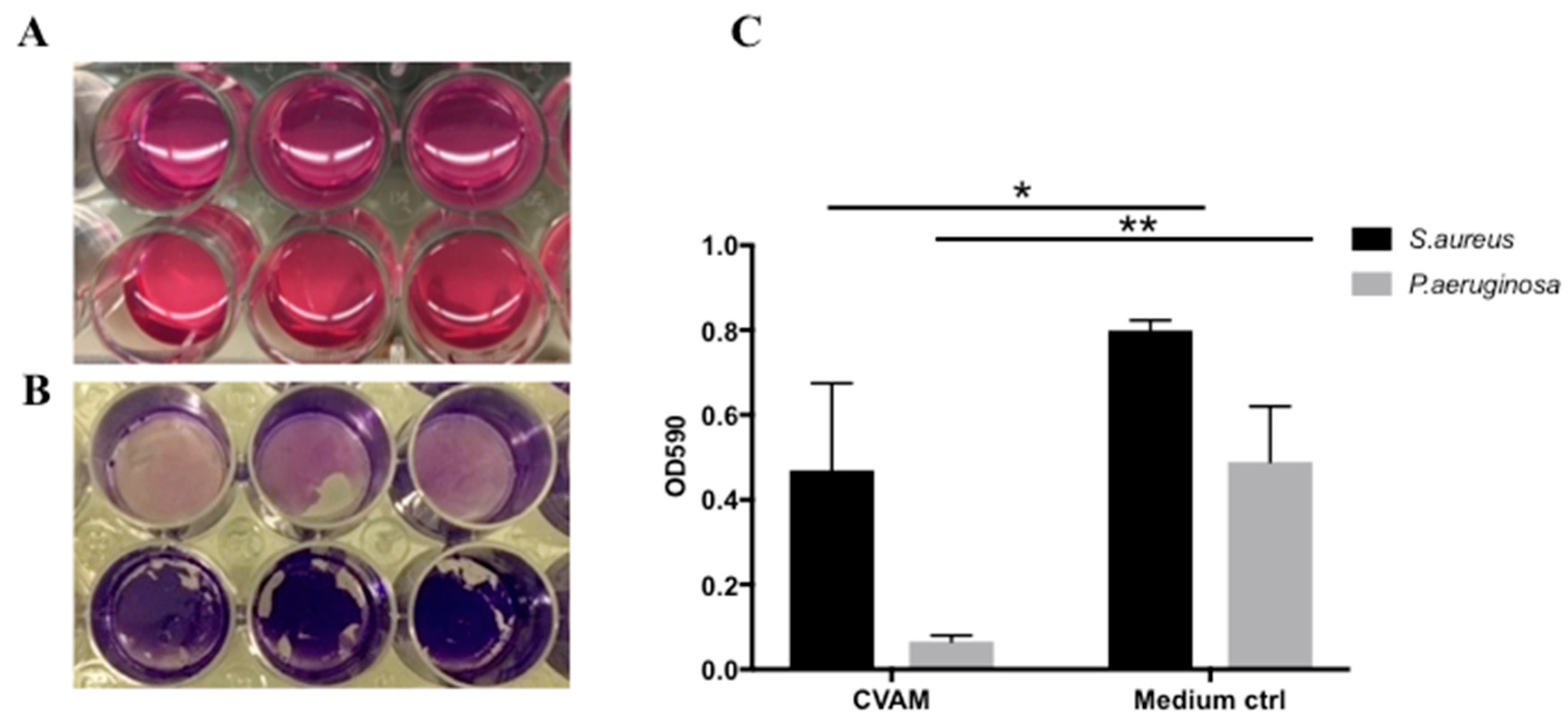
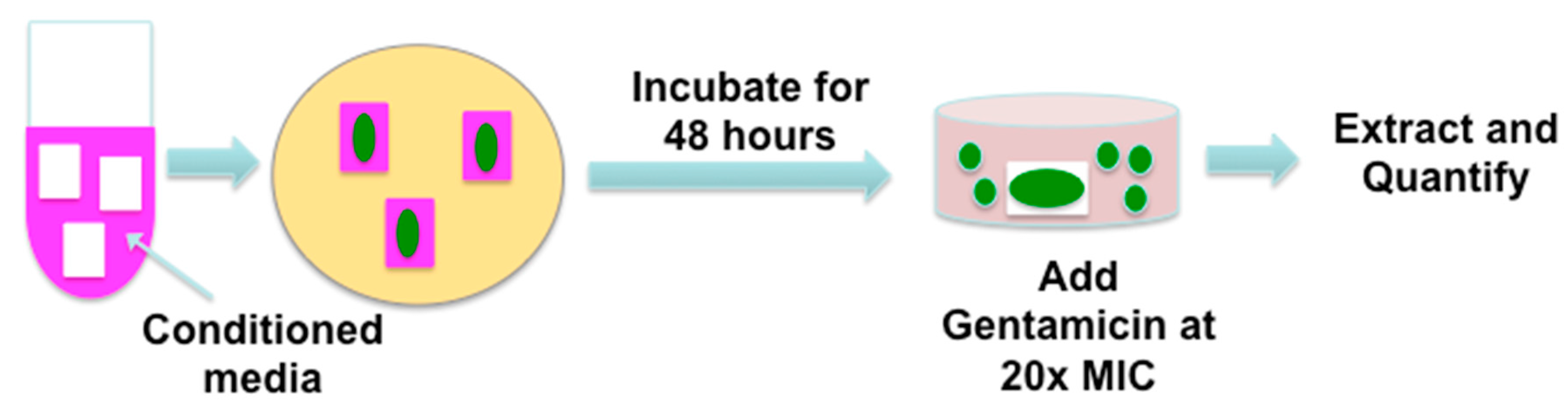
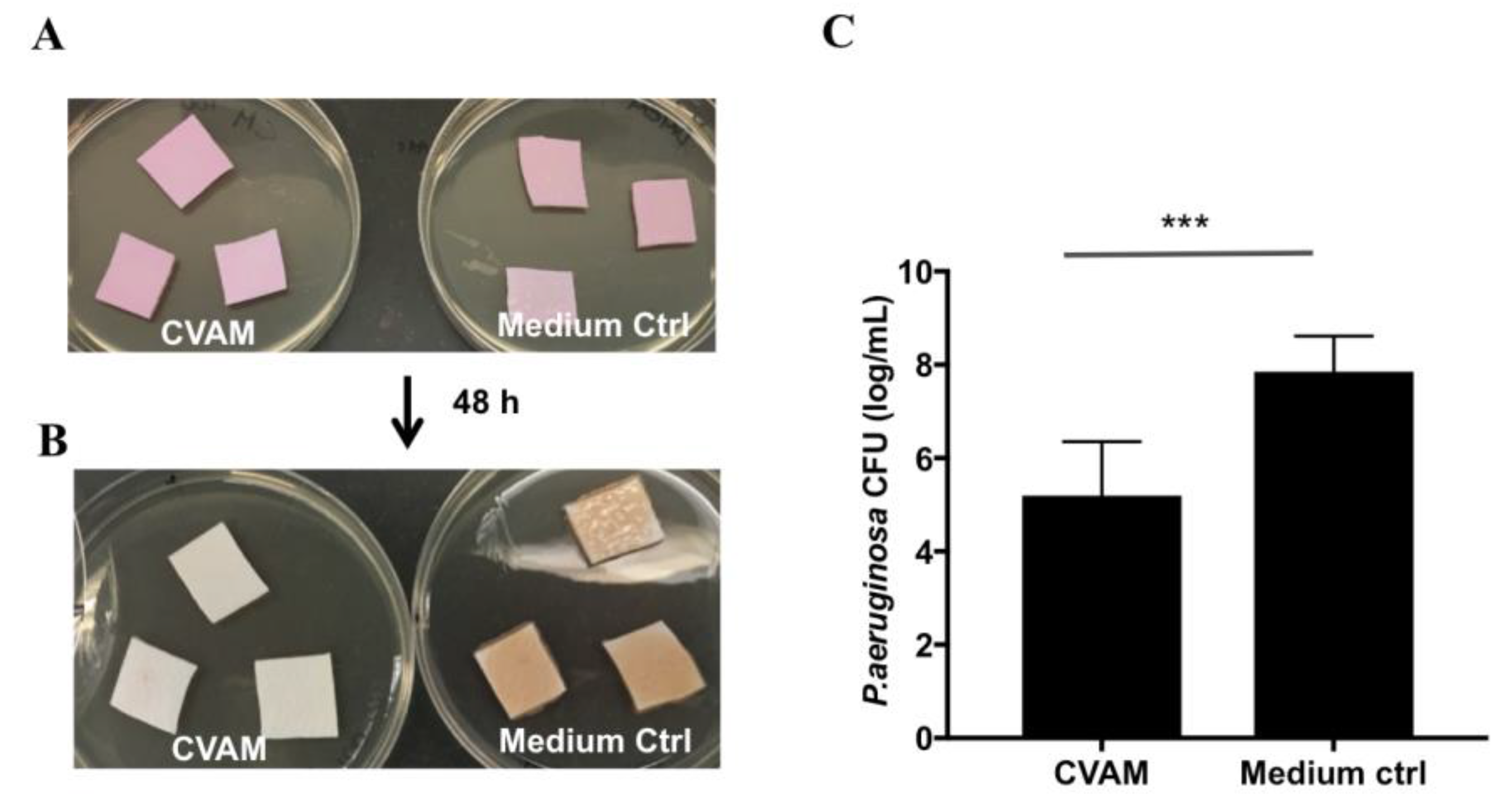
2.5. Effect of CVAM on S. aureus and P. aeruginosa Biofilm Formation
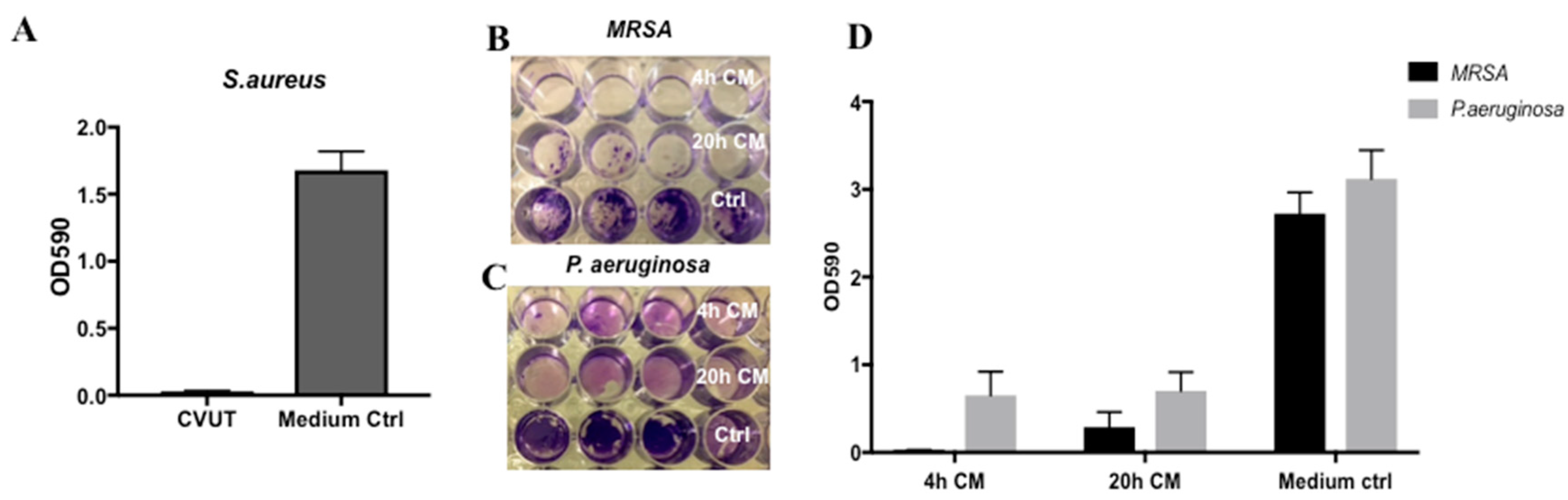
2.6. Effect of CVUT on S. aureus and P. aeruginosa Biofilm Formation
2.7. Effect of CVAM on Biofilm Formation in Porcine Dermal Tissue
2.8. Effect of CVUT on Biofilm Formation in the Porcine Dermal Tissue Explant Model
3. Materials and Methods
3.1. Preparation of CVAM and CVUT Samples and Conditioned Medium
3.2. Bacterial Cultures and Preparation of Inoculums
3.3. Liquid Antibacterial Activity Assay
3.4. Detection of Human Beta-Defensins (HBDs) and Secretory Leukocyte Protease Inhibitor (SLPI) Using ELISA
3.5. Biofilm Formation on Synthetic Surfaces
3.6. Porcine Dermal Tissue Procurement
3.7. Biofilm Formation in Ex Vivo Porcine Dermal Tissues
3.8. Cell Viability Testing
3.9. Histological Analyses
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Bohn, G.; Hanft, J.; Harkless, L.; Kim, P.; Lavery, L.; Schultz, G.; Wolcott, R. Wound biofilm: Current perspectives and strategies on biofilm disruption and treatments. Wounds 2017, 29, S1–S17. [Google Scholar] [PubMed]
- Hall-Stoodley, L.; Stoodley, P. Biofilm formation and dispersal and the transmission of human pathogens. Trends Microbiol. 2005, 13, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Leid, J.G.; Willson, C.J.; Shirtliff, M.E.; Hassett, D.J.; Parsek, M.R.; Jeffers, A.K. The exopolysaccharide alginate protects pseudomonas aeruginosa biofilm bacteria from ifn-gamma-mediated macrophage killing. J. Immunol. 2005, 175, 7512–7518. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Vuotto, C.; Donelli, G.; Lipsky, B.A. Biofilms and wounds: An identification algorithm and potential treatment options. Adv. Wound Care 2015, 4, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and wounds: An overview of the evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Rumbaugh, K.P.; James, G.; Schultz, G.; Phillips, P.; Yang, Q.; Watters, C.; Stewart, P.S.; Dowd, S.E. Biofilm maturity studies indicate sharp debridement opens a time- dependent therapeutic window. J. Wound Care 2010, 19, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hoffman, T.; Johnson, A.; Duan-Arnold, Y.; Danikovitch, A.; Kohn, J. Human cryopreserved viable amniotic membrane inhibits the growth of bacteria associated with chronic wounds. J. Diabet. Foot Complicat. 2016, 8, 23–30. [Google Scholar]
- Lavery, L.A.; Fulmer, J.; Shebetka, K.A.; Regulski, M.; Vayser, D.; Fried, D.; Kashefsky, H.; Owings, T.M.; Nadarajah, J.; Grafix Diabetic Foot Ulcer Study Group. The efficacy and safety of grafix((r)) for the treatment of chronic diabetic foot ulcers: Results of a multi-centre, controlled, randomised, blinded, clinical trial. Int. Wound J. 2014, 11, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.K.; Go, Y.Y.; Kim, S.H.; Chae, S.W.; Song, J.J. Antimicrobial and antibiofilm effects of human amniotic/chorionic membrane extract on streptococcus pneumoniae. Front. Microbiol. 2017, 8, 1948. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Sarkar, R.; Saha, P.; Maity, A.; Sarkar, T.; Das, D.; Chakraborty, P.D.; Bandyopadhyay, S.; Ghosh, C.K.; Karmakar, S.; et al. Effect of human placental extract in the management of biofilm mediated drug resistance—A focus on wound management. Microb. Pathog. 2017, 111, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Pettit, R.K.; Weber, C.A.; Pettit, G.R. Application of a high throughput alamar blue biofilm susceptibility assay to staphylococcus aureus biofilms. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.L.; Yang, Q.; Davis, S.; Sampson, E.M.; Azeke, J.I.; Hamad, A.; Schultz, G.S. Antimicrobial dressing efficacy against mature pseudomonas aeruginosa biofilm on porcine skin explants. Int. Wound J. 2015, 12, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Duan-Arnold, Y.; Gyurdieva, A.; Johnson, A.; Uveges, T.E.; Jacobstein, D.A.; Danilkovitch, A. Retention of endogenous viable cells enhances the anti-inflammatory activity of cryopreserved amnion. Adv. Wound Care 2015, 4, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Hoffman, T.; Singh-Varma, A.; Duan-Arnold, Y.; Moorman, M.; Danilkovitch, A.; Kohn, J. Antimicrobial peptides secreted from human cryopreserved viable amniotic membrane contribute to its antibacterial activity. Sci. Rep. 2017, 7, 13722. [Google Scholar] [CrossRef] [PubMed]
- Franc, S.; Rousseau, J.C.; Garrone, R.; van der Rest, M.; Moradi-Ameli, M. Microfibrillar composition of umbilical cord matrix: Characterization of fibrillin, collagen vi and intact collagen v. Placenta 1998, 19, 95–104. [Google Scholar] [CrossRef]
- Sanchez, C.J., Jr.; Mende, K.; Beckius, M.L.; Akers, K.S.; Romano, D.R.; Wenke, J.C.; Murray, C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect. Dis. 2013, 13, 47. [Google Scholar] [CrossRef] [PubMed]


| ESKAPE | ATCC# | Gram Stain | Growth Reduction in CVAM (log) | Growth Reduction in CVUT (log) |
|---|---|---|---|---|
| Enterococcus faecium | 51559™ | Positive | 0.6 ± 0.3 * | 0.1 ± 0.1 |
| Staphylococcus aureus | 25923™ | Positive | 2.6 ± 1.5 | 7.5 ± 0.5 |
| Klebsiella pneumoniae | 700603™ | Negative | 5.1 ± 1.7 | 0.7 ± 0.1 |
| Acinetobacter baumannii | 49466™ | Negative | 5.1 ± 0.9 | 0.7 ± 0.4 |
| Pseudomonas aeruginosa | 15692™ | Negative | 6.6 ± 1.8 | 5.0 ± 1.2 |
| Enterobacter aerogenes | 49469™ | Negative | 3.6 ± 1.4 | 3.3 ± 0.6 |
| VRE | 700221™ | Positive | 0.6 ± 0.1 * | 0.1 ± 0.2 |
| MRSA | BAA-1720™ | Positive | 5.8 ± 0.9 | 6.6 ± 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, Y.; Singh-Varma, A.; Hoffman, T.; Dhall, S.; Danilkovitch, A.; Kohn, J. The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens. J. Funct. Biomater. 2018, 9, 3. https://doi.org/10.3390/jfb9010003
Mao Y, Singh-Varma A, Hoffman T, Dhall S, Danilkovitch A, Kohn J. The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens. Journal of Functional Biomaterials. 2018; 9(1):3. https://doi.org/10.3390/jfb9010003
Chicago/Turabian StyleMao, Yong, Anya Singh-Varma, Tyler Hoffman, Sandeep Dhall, Alla Danilkovitch, and Joachim Kohn. 2018. "The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens" Journal of Functional Biomaterials 9, no. 1: 3. https://doi.org/10.3390/jfb9010003
APA StyleMao, Y., Singh-Varma, A., Hoffman, T., Dhall, S., Danilkovitch, A., & Kohn, J. (2018). The Effect of Cryopreserved Human Placental Tissues on Biofilm Formation of Wound-Associated Pathogens. Journal of Functional Biomaterials, 9(1), 3. https://doi.org/10.3390/jfb9010003





