Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol
Abstract
:1. Introduction
2. Results and Discussions
2.1. Effect of 4-HR on PLGA Film Properties
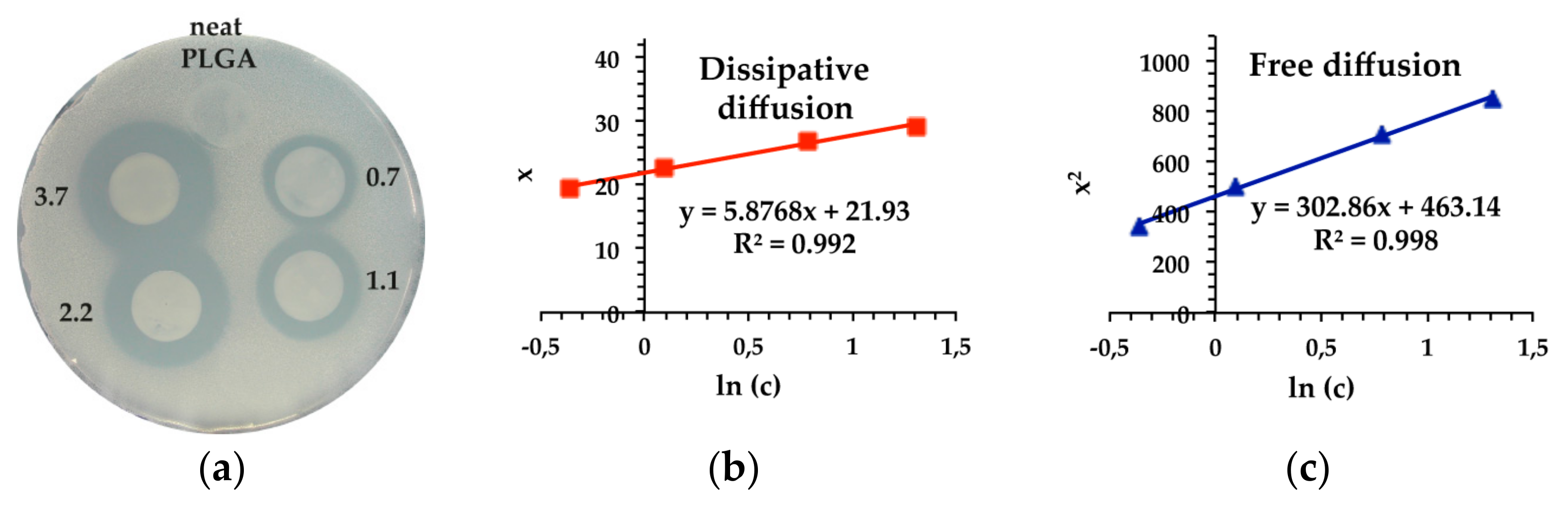
2.2. Antimicrobial Performance of 4-HR-Loaded PLGA Films
3. Materials and Methods
3.1. Materials
3.2. Film Preparation
3.3. Film Thickness and Density
3.4. Microorganisms and Inoculum Preparation
3.5. Antimicrobial Activity Screening by Agar Overlay Assay
3.6. Determination of MIC Values
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial polymers in solution and on surfaces: Overview and functional principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.A.M.; Zaat, S.A.J.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [PubMed]
- Aytac, S.A.; Taban, B.M. Food-borne microbial disease and control: Food-borne infections and intoxications. In Food Processing: Strategies for Quality Assessment; Malik, A., Erginkaya, Z., Ahmad, S., Erten, H., Eds.; Springer: New York, NY, USA, 2014; pp. 191–224. [Google Scholar]
- Huang, K.S.; Yang, C.H.; Huang, S.L.; Chen, C.Y.; Lu, Y.Y.; Lin, Y.S. Recent advances in antimicrobial polymers: A mini-review. Int. J. Mol. Sci. 2016, 17, 1578. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.R.E.; Fonseca, A.C.; Mendonca, P.V.; Branco, R.; Serra, A.C.; Morais, P.V.; Coelho, J.F.J. Recent developments in antimicrobial polymers: A review. Materials 2016, 9, 599. [Google Scholar] [CrossRef] [PubMed]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-based microparticles for producing sustained-release drug formulations and strategies for improving drug loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Manawithehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef]
- Makadia, H.; Siegel, S.J. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA based drug delivery systems: Promising carriers for wound healing activity. Wound Rep. Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, G.; Brey, E.M. Dual delivery of chlorhexidine and platelet-derived growth factor-BB for enhanced wound healing and infection control. Acta Biomater. 2013, 9, 4976–4984. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lee, J.H.; Gwon, G.; Kim, S.I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 36377. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.G.; Martins, I.M.; Fernandes, I.; Barreiro, M.F.; Rodrigues, A.E. Microencapsulation of red and white thyme oil in poly(lactic-co-glycolic) acid: Assessment of encapsulation efficiency and antimicrobial capacity of the produced microcapsules. Can. J. Chem. Eng. 2016, 94, 469–475. [Google Scholar] [CrossRef]
- Sun, D.; Li, N.; Zhang, W.; Yang, E.; Mou, Z.; Zhao, Z.; Liu, H.; Wang, W. Quercetin-loaded PLGA nanoparticles: A highly effective antibacterial agent in vitro and anti-infection application in vivo. J. Nanopart. Res. 2016, 18, 3. [Google Scholar] [CrossRef]
- Xing, Z.C.; Koo, T.H.; Kim, Y.J.; Kwon, O.H.; Kang, I.K. Surface modification of PLGA nanofibrous biocomposites using flavonoids for biomedical applications. J. Adhes. Sci. Technol. 2012, 27, 1382–1392. [Google Scholar] [CrossRef]
- Esfandyari-Manesh, M.; Ghaedi, Z.; Asemi, M.; Khanavi, M.; Manayi, A.; Jamalifar, H.; Atyabi, F.; Dinarvand, R. Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J. Pharm. Res. 2013, 7, 290–295. [Google Scholar] [CrossRef]
- Iannitelli, A.; Grande, R.; Di Stefano, A.; Di Giulio, M.; Sozio, P.; Bessa, L.J.; Laserra, S.; Paolini, C.; Protasi, F.; Cellini, L. Potential antibacterial activity of carvacrol-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles against microbial biofilm. Int. J. Mol. Sci. 2011, 12, 5039–5051. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.; Moreira, R.G.; Castell-Perez, E. Poly(dl-lactide-co-glycolide) (PLGA) nanoparticles with entrapped trans-cinnamaldehyde and eugenol for antimicrobial delivery applications. J. Food Sci. 2011, 76, N16–N24. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.C.; Hill, L.E.; Zambiazi, R.C.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Nanoencapsulation of hydrophobic phytochemicals using poly(dl-lactide-co-glycolide) (PLGA) for antioxidant and antimicrobial delivery applications: Guabiroba fruit (Campomanesia xanthocarpa O. Berg) study. LWT Food Sci. Technol. 2015, 63, 100–107. [Google Scholar] [CrossRef]
- Said, S.S.; Aloufy, A.K.; El-Halfawy, O.M.; Boraei, N.A.; El-Khordagui, L.K. Antimicrobial PLGA ultrafine fibers: Interaction with wound bacteria. Eur. J. Pharm. Biopharm. 2011, 79, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Domingues, F.; Duarte, A.P. Biological properties of plant-derived alkylresorcinols: Mini-review. Mini-Rev. Med. Chem. 2016, 16, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.K. Hexylresorcinol: Providing skin benefits by modulating multiple molecular targets. In Cosmeceuticals and Active Cosmetics; Sivamani, R.K., Jagdeo, J.R., Elsner, P., Maibach, H.I., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 71–81. [Google Scholar]
- Ahn, J.; Kim, S.G.; Kim, M.K.; Kim, D.W.; Lee, J.H.; Seok, H.; Choi, J.Y. Topical delivery of 4-hexylresorcinol promotes wound healing via tumor necrosis factor-α suppression. Burns 2016, 42, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Kweon, H.Y.; Kim, S.G.; Choi, J.Y. Inhibition of foreign body giant cell formation by 4-hexylresorcinol through suppression of diacylglycerol kinase delta gene expression. Biomaterials 2014, 35, 8576–8584. [Google Scholar] [CrossRef] [PubMed]
- EFSA ANS Panel. Scientific opinion on the re-evaluation of 4-hexylresorcinol (E 586) as a food additive. EFSA J. 2014, 12, 3643. [Google Scholar] [CrossRef]
- Leonard, V. Hexyl resorcinol. The development and clinical application of a synthetic compound possessing the experimental requirements of an ideal internal urinary antiseptic. J. Urol. 1924, 12, 585–610. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Dafale, N.A.; Semwal, U.P.; Rajput, R.K.; Singh, G.N. Selection of appropriate analytical tools to determine the potency and bioactivity of antibiotics and antibiotic resistance. J. Pharm. Anal. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Hockett, K.L.; Baltrus, D.A. Use of the soft-agar overlay technique to screen for bacterially produced inhibitory compounds. J. Vis. Exp. 2017, 119, e55064. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Bonev, B.; Hooper, J.; Parisot, J. Principles of assessing bacterial susceptibility to antibiotics using the agar diffusion method. J. Antimicrob. Chemother. 2008, 61, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kwon, J.; Khang, G.; Lee, D. Reduction of inflammatory responses and enhancement of extracellular matrix formation by vanillin-incorporated poly(lactic-co-glycolic acid) scaffolds. Tissue Eng. Part A 2012, 18, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V. Food packaging permeability behaviour: A report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Lu, L.; Garcia, C.A.; Mikos, A.G. In vitro degradation of thin poly(dl-lactic-co-glycolic acid) films. J. Biomed. Mater. Res. 1999, 46, 236–244. [Google Scholar] [CrossRef]
- Vay, K. Analysis and Control of the Manufacturing Process and the Release Properties of PLGA Microparticles for Sustained Delivery of a Poorly Water-Soluble Drug. Ph.D. Thesis, Ludwig Maximilians University of Munich, München, Germany, 2012. [Google Scholar]
- Blasi, P.; D’Souza, S.S.; Selmin, F.; DeLuca, P.P. Plasticizing effect of water on poly(lactide-co-glycolide). J. Control. Release 2005, 108, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.R.; Moss, M.O. Food Microbiology, 3rd ed.; RSC Publishing: Cambridge, UK, 2008; pp. 182–300. [Google Scholar]
- Yang, W.G.; Ha, J.H.; Kim, S.G.; Chae, W.S. Spectroscopic determination of alkyl resorcinol concentration in hydroxyapatite composite. J. Anal. Sci. Technol. 2016, 7, 9. [Google Scholar] [CrossRef]
- Mosangi, D.; Pillai, S.K.; Moyo, L.; Ray, S.S. Inorganic layered double hydroxides as a 4-hexylresorcinol delivery system for topical applications. RSC Adv. 2016, 6, 77709. [Google Scholar] [CrossRef]
- Chapman, P.J.; Ribbons, D.W. Metabolism of resorcinylic compounds by bacteria: Alternative pathways for resorcinol catabolism in Pseudomonas putida. J. Bacteriol. 1976, 125, 985–998. [Google Scholar] [PubMed]
- Jo, Y.Y.; Kweon, H.Y.; Kim, D.W.; Kim, M.K.; Kim, S.G.; Kim, J.Y.; Chae, W.S.; Hong, S.P.; Park, Y.H.; Lee, S.Y.; et al. Accelerated biodegradation of silk sutures through matrix metalloproteinase activation by incorporating 4-hexylresorcinol. Sci. Rep. 2017, 7, 42441. [Google Scholar] [CrossRef] [PubMed]
- Stasiuk, M.; Kozubek, A. Biological activity of phenolic lipids. Cell. Mol. Life Sci. 2010, 67, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Nazir, M.; Shaiq Ali, M.; Hussain, H.; Lee, Y.S.; Riaz, N.; Jabbar, A. Antimicrobial natural products: An update on future antibiotic drug candidates. Nat. Prod. Rep. 2010, 27, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Trombetta, R.P.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cell Mater. 2015, 30, 232–247. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yu, S.; Kollert, M.; Mtimet, M.; Roth, S.V.; Gedde, U.W.; Johansson, E.; Olsson, R.T.; Hedenqvist, M.S. Highly absorbing antimicrobial biofoams based on wheat gluten and its biohybrids. ACS Sustain. Chem. Eng. 2016, 4, 2395–2404. [Google Scholar] [CrossRef]
- Musil, J. Flexible antibacterial coatings. Molecules 2017, 22, 813. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Scanion, M.P. Elimination of volatile chemicals in disinfectant evaluation procedures by freeze drying. Lett. Appl. Microbiol. 2000, 31, 223–227. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.T.; Davidson, P.M. Growth characteristics of selected fungi on polyvinyl chloride film. Appl. Environ. Microbiol. 1986, 51, 673–676. [Google Scholar] [PubMed]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Cutter, C.N.; Willett, J.L.; Siragusa, G.R. Improved antimicrobial activity of nisin-incorporated polymer films by formulation change and addition of food grade chelator. Lett. Appl. Microbiol. 2001, 33, 325–328. [Google Scholar] [CrossRef] [PubMed]
- One-Way ANOVA with Post-Hoc Tukey HSD Test Calculator for Comparing Multiple Treatments. Available online: http://www.astatsa.com/OneWay_Anova_with_TukeyHSD/ (accessed on 14 December 2017).

| Chemical Structure | Physico-Chemical Properties [26] |
|---|---|
 | Molar mass 194.27 g/mol Melting point 62–67 °C Log Po/w 3.88 |
| 4-HR Content (mg/mg PLGA) | Thickness (µm) | Density (g/cm3) |
|---|---|---|
| Neat PLGA | 75 ± 18 a | 0.94 ± 0.06 a,b |
| 0.03 | 81 ± 7 a | 0.83 ± 0.12 a |
| 0.06 | 83 ± 19 a | 0.81 ± 0.07 a |
| 0.1 | 66 ± 12 a | 0.99 ± 0.08 a,b |
| 0.15 | 64 ± 14 a | 1.10 ± 0.17 b |
| 0.3 | 67 ± 8 a | 1.06 ± 0.13 b |
| 0.5 | 92 ± 15 a | 1.08 ± 0.10 b |
| Indicator Strains | Free Diffusion | Dissipative Diffusion | ||
|---|---|---|---|---|
| MIC * | R2 * | MIC * | R2 * | |
| Gram-positive bacteria | ||||
| Bacillus pumilus DSM 361 | 8.3 | 0.995 | 2.6 | 0.987 |
| Kocuria rhizophila DSM 348 | 8.6 | 0.967 | 3.5 | 0.96 |
| Listeria innocua DSM 20649 | 6 | 0.978 | 1.5 | 0.966 |
| Staphylococcus carnosus DSM 20501 | 3 | 0.998 | 0.4 | 0.992 |
| Gram-negative bacteria | ||||
| Aeromonas ichthiosmia DSM 6393 | 7.5 | 0.993 | 2.4 | 0.988 |
| Escherichia coli ATCC 10798 | 6 | 0.987 | 1.2 | 0.975 |
| Pseudomonas putida DSM 291 | 28.5 | 0.999 | 12 | 1 |
| Raoultella planticola DSM 3069 | 9 | 0.995 | 2.3 | 0.994 |
| Yeasts | ||||
| Rhodotorula mucilaginosa DSM 70825 | 7.5 | 0.982 | 3 | 0.975 |
| Yarrowia lipolytica DSM 70561 | 7.5 | 0.977 | 3 | 0.972 |
| Filamentous fungi | ||||
| Botrytis cinerea DSM 4709 | 6 | 0.992 | 0.8 | 0.989 |
| Penicillium italicum DSM 2754 | 9 | 0.999 | 3 | 0.995 |
| Indicator Strains a | Liquid Growth Medium b | Plating Medium b | T (°C) | Viable Cell Count (CFU/mL) c |
|---|---|---|---|---|
| Gram-positive bacteria | ||||
| B. pumilus DSM 361 | NB | MHB | 28 | 3 × 108 |
| K. rhizophila DSM 348 | NB | MHB | 28 | 7 × 107 |
| L. innocua DSM 20649 | BHI | MHB | 37 | 9 × 108 |
| S. carnosus DSM 20501 | TSB | MHB | 37 | 3 × 108 |
| Gram-negative bacteria | ||||
| A. ichthiosmia DSM 6393 | NB | MHB | 28 | 4 × 108 |
| E. coli ATCC 10798 | TB | MHB | 37 | 8 × 108 |
| P. putida DSM 291 | NB | MHB | 28 | 8 × 107 |
| R. planticola DSM 3069 | NB | MHB | 28 | 2 × 108 |
| Yeasts | ||||
| R. mucilaginosa DSM 70825 | YM | SDB | 28 | 2 × 107 |
| Y. lipolytica DSM 70561 | YPD | SDB | 28 | 1 × 107 |
| Filamentous fungi | ||||
| B. cinerea DSM 4709 | MEP | SDB | 28 | 1 × 106 d |
| P. italicum DSM 2754 | MEP | SDB | 28 | 4 × 108 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemme, M.; Heinzel-Wieland, R. Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. J. Funct. Biomater. 2018, 9, 4. https://doi.org/10.3390/jfb9010004
Kemme M, Heinzel-Wieland R. Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. Journal of Functional Biomaterials. 2018; 9(1):4. https://doi.org/10.3390/jfb9010004
Chicago/Turabian StyleKemme, Michael, and Regina Heinzel-Wieland. 2018. "Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol" Journal of Functional Biomaterials 9, no. 1: 4. https://doi.org/10.3390/jfb9010004
APA StyleKemme, M., & Heinzel-Wieland, R. (2018). Quantitative Assessment of Antimicrobial Activity of PLGA Films Loaded with 4-Hexylresorcinol. Journal of Functional Biomaterials, 9(1), 4. https://doi.org/10.3390/jfb9010004




