The Tribology of Explanted Hip Resurfacings Following Early Fracture of the Femur
Abstract
:1. Introduction
| Topic | ASR | BHR | Durom |
|---|---|---|---|
| Manufacture method | Cas-HIP 1/SA 2 cup | Cast | Wrought |
| Radial clearance (μm) | 50 | 100 | 75 |
| Arc of cover (°) | 148–160 | 158–166 | Up to 166 |
| 7 year revision rate: | – | – | – |
| AOA 3 | 23.9 | 5.1 | 9.0 |
| NJR4 | 24.07 | 5.61 | 9.45 |
| Lead Author | Year | Device | Number of Hips | Fracture Number (Incidence) | Mean Time to Fracture |
|---|---|---|---|---|---|
| Amstutz [24] | 2004 | Conserve Plus 2 | 400 | 3 (0.75%) | Not recorded |
| Treacy [21] | 2005 | BHR 1 | 144 | 1 (0.7%) | 36 weeks |
| Cossey [25] | 2005 | BHR 1 | 407 | 7 (1.72%) | 6 weeks |
| Shimmin [26] | 2005 | BHR 1 | 3497 | 50 (1.46%) | 15.4 weeks |
| Marker [28] | 2007 | Conserve Plus 2 | 550 | 14 (2.55%) | 16 weeks |
| Steffen [27] | 2008 | BHR 1 | 610 | 12 (1.97%) | Not recorded |
| Beaulé [29] | 2004 | Conserve Plus 2 | 119 | 1 (0.84%) | 2 weeks |
2. Results and Discussion
2.1. Wear Comparison
| Issue | Fracture | AVN/Infection | ARMD |
|---|---|---|---|
| Radius (mm) | 24.31 | 21.87 | 23.28 |
| Inclination (°) | 43.8 | 45.6 | 51.0 |
| Anteversion (°) | 12.9 | 16.8 | 23.2 |
| Duration (months) | 3.7 | 44.4 | 30.9 |
| Wear volume (mm3) | 6.53 | 1.55 | 19.22 |
| Wear rate (mm3/year) | 23.74 | 0.37 | 8.29 |
| Blood Cr (μg/L) | 5.7 | 2.3 | 22.3 |
| Blood Co (μg/L) | 3.9 | 1.8 | 40.6 |
| Serum Cr (μg/L) | 5.7 | 2.9 | 29.2 |
| Serum Co (μg/L) | 4.2 | 2.3 | 38.2 |
| Unworn λ | 4.0 | 3.0 | 3.3 |
| Worn λ | 2.8 | 2.0 | 2.0 |
2.2. Roughness Analysis
| Failure mode | PV (μm) | RMS (μm) | Rsk | |||
|---|---|---|---|---|---|---|
| Unworn | Worn | Unworn | Worn | Unworn | Worn | |
| Fracture | 0.286 | 0.934 | 0.012 | 0.049 | −2.225 | −4.758 |
| AVN/Infection | 0.243 | 0.604 | 0.019 | 0.032 | 0.243 | −2.869 |
| ARMD | 0.285 | 1.158 | 0.016 | 0.062 | −1.075 | −3.639 |
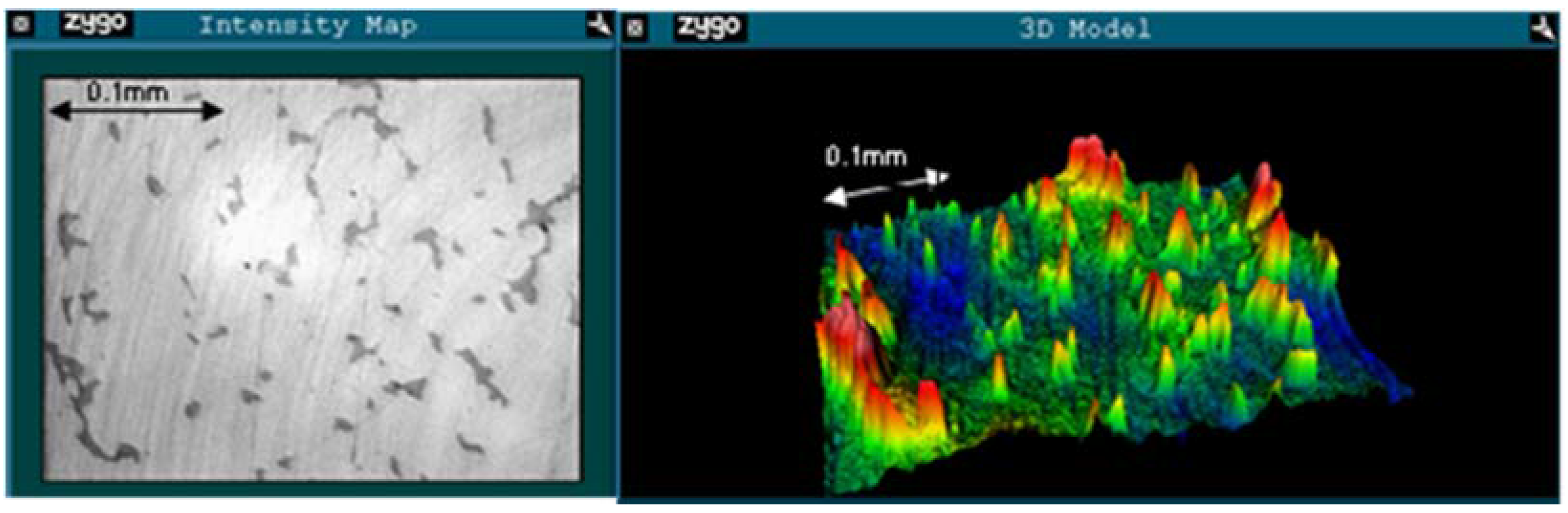
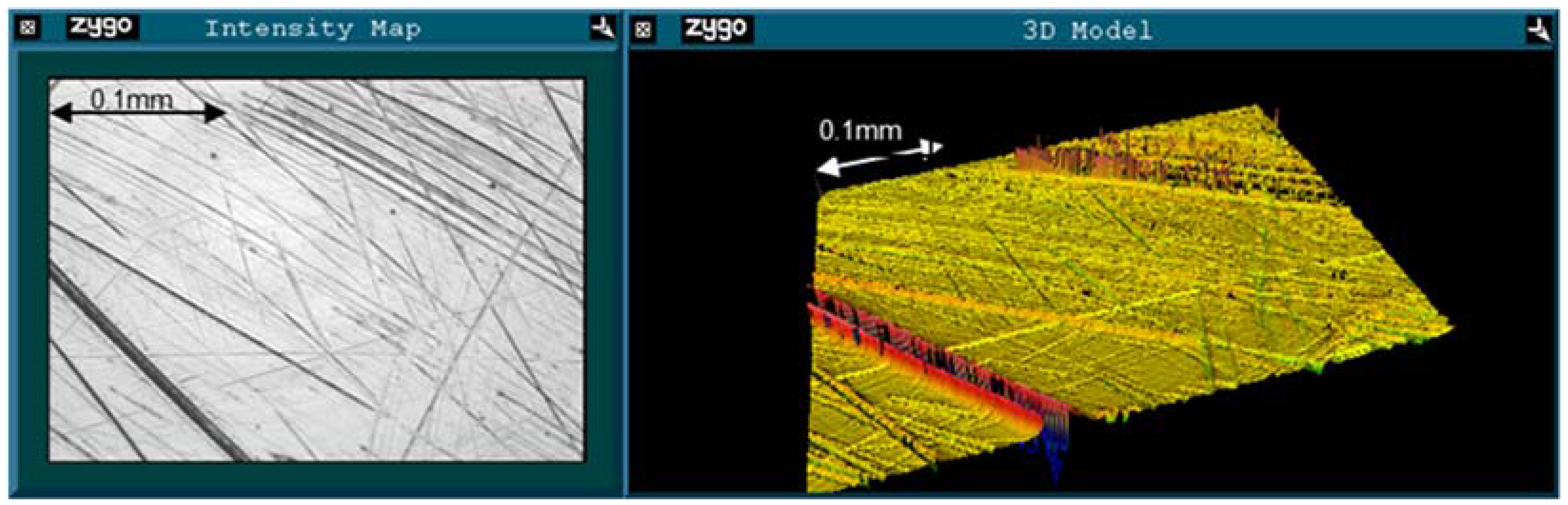
3. Experimental Section
3.1. Materials
| Diagnosis | Gender | Model | Duration (Months) | Inclination (°) | Anteversion (°) | Diameter (mm) |
|---|---|---|---|---|---|---|
| Fracture | Female | ASR | 2 | 46.9 | 20.6 | 44.517 |
| Fracture | Male | ASR | 2 | 35.7 | 28.9 | 48.504 |
| Fracture | Female | Durom | 2 | 39.4 | 5.8 | 41.986 |
| Fracture | Male | Durom | 2 | 45.6 | 1.3 | 53.981 |
| Fracture | Female | BHR | 2.5 | 56.9 | 10.8 | 45.812 |
| Fracture | Male | Durom | 4 | 40.7 | 10.2 | 49.982 |
| Fracture | Male | ASR | 6 | 49.0 | 0.0 | 48.510 |
| Fracture | Male | Durom | 6 | 38.7 | 17.7 | 51.761 |
| Fracture | Male | ASR | 7 | 41.5 | 20.5 | 52.527 |
| AVN | Male | ASR | 38 | 38.0 | 17.0 | 46.499 |
| AVN | Male | ASR | 54 | 60.3 | 18.7 | 46.500 |
| AVN | Female | BHR | 72 | 40.2 | 18.0 | 37.841 |
| Infection | Female | BHR | 28 | 43.0 | 14.1 | 45.848 |
| Infection | Female | BHR | 30 | 46.7 | 16.0 | 42.006 |
3.2. Methods
3.2.1. Clinical Data
3.2.2. Wear Measurement
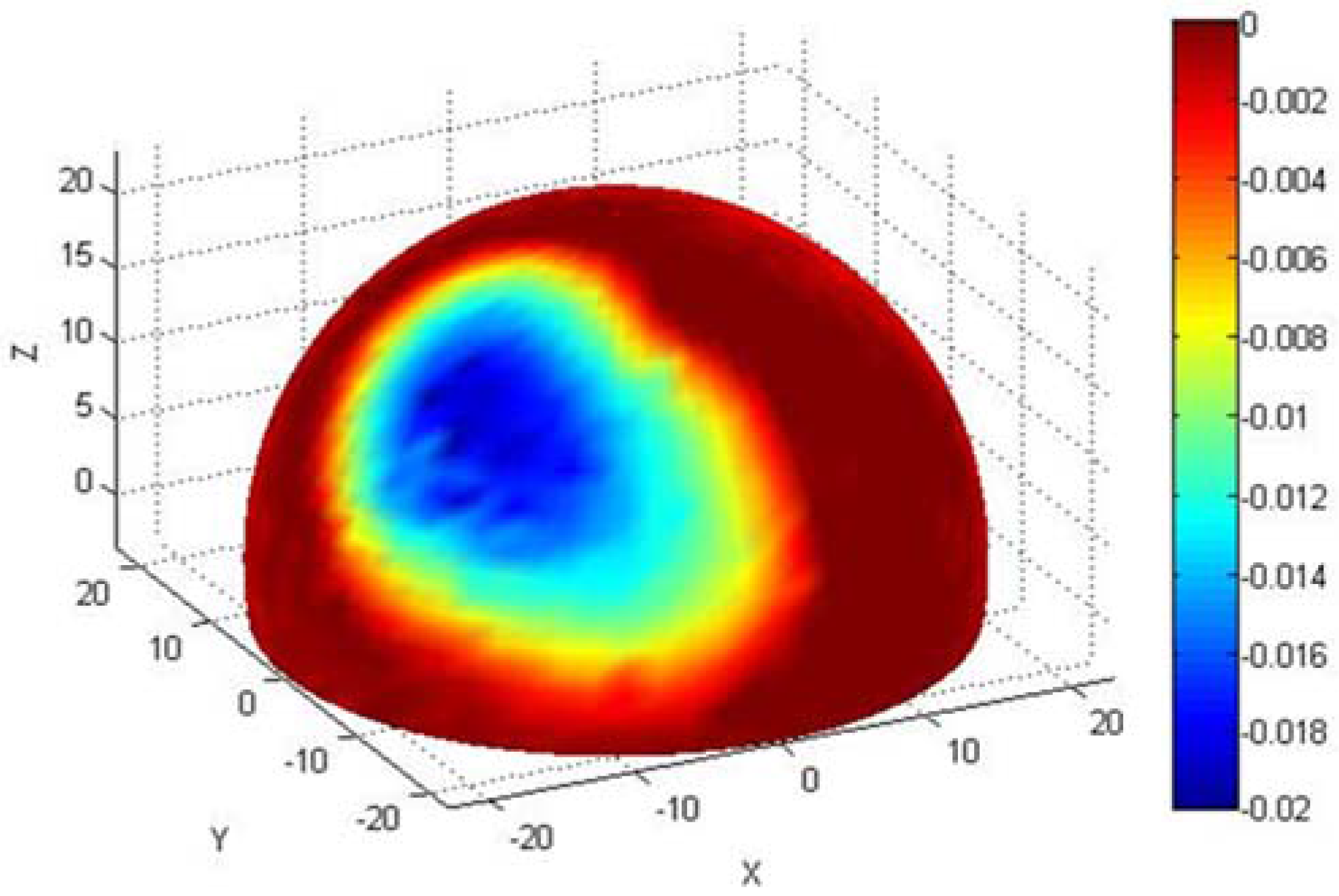
3.2.3. Roughness Measurement
- Peak to Valley (PV). The distance between the highest and lowest points on the surface. This gives the maximum size of defects in the scan area.
- Root Mean Square (RMS). The square root of the mean of the height differences squared. This gives a value for deviation in the surface height and accounts for both positive and negative variation (peaks and valleys).
- Skewness (Rsk). A measure of whether the surface is dominated by peaks (positive skew) or valleys (negative skew). A surface with negative skew is indicative of a series of valleys.
- Roughness average (Ra). The arithmetic average of the absolute height deviations.
3.3. Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- National Joint Registry for England, Wales and Northern Ireland; 11th Annual Report; Pad Creative Ltd.: Hemel Hempstead, UK, 2014.
- Dowson, D.; Jin, Z.M. Metal-on-metal hip joint tribology. Proc. Inst. Mech. Eng. Part H-J. Eng. Med. 2006, 220, 107–118. [Google Scholar] [CrossRef]
- Sieber, H.; Rieker, C.; Kottig, P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J. Bone Joint Surg. Br. 1999, 81, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.H. The problem is osteolysis. Clin. Orthop. Relat. Res. 1995, 311, 46–53. [Google Scholar] [PubMed]
- Firkins, P.J.; Tipper, J.L.; Ingham, E.; Stone, M.H.; Farrar, R.; Fisher, J. Influence of simulator kinematics on the wear of metal-on-metal hip prostheses. J. Eng. Med. 2001, 215, 119–121. [Google Scholar] [CrossRef]
- Heisel, C.; Kleinhans, J.; Menge, M.; Kretzer, J. Ten different hip resurfacing systems: Biomechanical analysis of design and material properties. Int. Orthop. 2009, 33, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Udofia, I.J.; Jin, Z.M. Elastohydrodynamic lubrication analysis of metal-on-metal hip-resurfacing prostheses. J. Biomech. 2003, 36, 537–544. [Google Scholar] [CrossRef]
- Joyce, T.J.; Langton, D.J.; Jameson, S.S.; Nargol, A.V.F. Tribological analysis of failed resurfacing hip prostheses and comparison with clinical data. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2009, 223, 317–323. [Google Scholar] [CrossRef]
- Hamrock, B.J.; Dowson, D. Elastohydrodynamic lubrication of elliptical contacts for materials of low elastic modulus. I: Fully flooded conjunction. Trans. Am. Soc. Mech. Eng. J. Lubr. Technol. 1978, 100, 236–245. [Google Scholar] [CrossRef]
- Dowson, D. Tribological principles in metal-on-metal hip joint design. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 161–171. [Google Scholar] [CrossRef]
- Daniel, J.; Pynsent, P.; McMinn, D. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J. Bone Joint Surg. Br. 2004, 86, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Wienroth, M.; McCormack, P.; Joyce, T. Precaution, governance and the failure of medical implants: The ASR™ hip in the UK. Life Sci. Soc. Policy 2014, 10. [Google Scholar] [CrossRef]
- Langton, D.J.; Sprowson, A.P.; Joyce, T.J.; Reed, M.; Carluke, I.; Partington, P.; Nargol, A.V.F. Blood metal ion concentrations after hip resurfacing arthroplasty: A comparative study of articular surface replacement and Birmingham hip resurfacing arthroplasties. J. Bone Joint Surg. Br. 2009, 91, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Berton, C.; Girard, J.; Krantz, N.; Migaud, H. The Durom large diameter head acetabular component: Early results with a large-diameter metal-on-metal bearing. J. Bone Joint Surg. Br. 2010, 92, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Langton, D.J.; Jameson, S.S.; Joyce, T.J.; Webb, J.; Nargol, A.V.F. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J. Bone Joint Surg. Br. 2008, 90, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.; Nanson, C.; Springer, B.; Davies, M.; Fehring, T. Reduced articular surface of one-piece cups: A cause of runaway wear and early failure. Clin. Orthop. Relat. Res. 2010, 468, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- De Haan, R.; Pattyn, C.; Gill, H.S.; Murray, D.W.; Campbell, P.A.; de Smet, K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J. Bone Joint Surg. Br. 2008, 90, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Langton, D.J.; Sidaginamale, R.P.; Joyce, T.J.; Natu, S.; Blain, P.; Jefferson, R.D.; Rushton, S.; Nargol, A.V.F. The clinical implications of elevated blood metal ion concentrations in asymptomatic patients with MoM hip resurfacings: A cohort study. BMJ Open 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Australian_Orthopaedic_Association. National Joint Replacement Registry; Annual Report 2014; Commonwealth Government: Adelaide, Australia, 2014. [Google Scholar]
- Khan, M.; Kuiper, J.H.; Edwards, D.; Robinson, E.; Richardson, J.B. Birmingham hip arthroplasty: Five to eight years of prospective multicenter results. J. Arthropl. 2009, 24, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Treacy, R.B.C.; McBryde, C.W.; Pynsent, P.B. Birmingham hip resurfacing arthroplasty: A minimum follow-up of five years. J. Bone Joint Surg. Br. 2005, 87, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Treacy, R.B.C.; McBryde, C.W.; Shears, E.; Pynsent, P.B. Birmingham hip resurfacing: A minimum follow-up of ten years. J. Bone Joint Surg. Br. 2011, 93, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Australian_Orthopaedic_Association. National Joint Replacement Registry; Annual Report 2010; Commonwealth Government: Adelaide, Australia, 2010. [Google Scholar]
- Amstutz, H.C.; Beaule, P.E.; Dorey, F.J.; le Duff, M.J.; Campbell, P.A.; Gruen, T.A. Metal-on-metal hybrid surface arthroplasty: Two to six-year follow-up study. J. Bone Joint Surg. Am. 2004, 86, 28–39. [Google Scholar] [PubMed]
- Cossey, A.J.; Back, D.L.; Shimmin, A.; Young, D.; Spriggins, A.J. The nonoperative management of periprosthetic fractures associated with the Birmingham hip resurfacing procedure. J. Arthropl. 2005, 20, 358–361. [Google Scholar] [CrossRef]
- Shimmin, A.J.; Back, D. Femoral neck fractures following Birmingham hip resurfacing: A national review of 50 cases. J. Bone Joint Surg. Br. 2005, 87, 463–464. [Google Scholar] [CrossRef] [PubMed]
- Steffen, R.T.; Pandit, H.P.; Palan, J.; Beard, D.J.; Gundle, R.; McLardy-Smith, P.; Murray, D.W.; Gill, H.S. The five-year results of the Birmingham hip resurfacing arthroplasty: An independent series. J. Bone Joint Surg. Br. 2008, 90, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Marker, D.R.; Seyler, T.M.; Jinnah, R.H.; Delanois, R.E.; Ulrich, S.D.; Mont, M.A. Femoral neck fractures after metal-on-metal total hip resurfacing: A prospective cohort study. J. Arthropl. 2007, 22, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Beaulé, P.E.; Dorey, F.J.; le Duff, M.; Gruen, T.; Amstutz, H.C. Risk factors affecting outcome of metal-on-metal surface arthroplasty of the hip. Clin. Orthop. Relat. Res. 2004, 418, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Beaule, P.; Ebramzadeh, E.; le Duff, M.; de Smet, K.; Lu, Z.; Amstutz, H. A study of implant failure in metal-on-metal surface arthroplasties. Clin. Orthop. Relat. Res. 2006, 453, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Joyce, T.J.; Langton, D.J.; Nargol, A.V.F. A study of the wear of explanted metal-on-metal resurfacing hip prostheses. Tribol. Int. 2011, 44, 517–522. [Google Scholar] [CrossRef]
- Langton, D.J.; Jameson, S.S.; Joyce, T.J.; Hallab, N.J.; Natu, S.; Nargol, A.V.F. Early failure of metal-on-metal bearings in hip resurfacing and large-diameter total hip replacement: A consequence of excess wear. J. Bone Joint Surg. Br. 2010, 92, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Angadji, A.; Royle, M.; Collins, S.N.; Shelton, J.C. Influence of cup orientation on the wear performance of metal-on-metal hip replacements. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2009, 223, 449–457. [Google Scholar] [CrossRef]
- Williams, S.; Leslie, I.; Isaac, G.; Jin, Z.; Ingham, E.; Fisher, J. Tribology and wear of metal-on-metal hip prostheses: Influence of cup angle and head position. J. Bone Joint Surg. Am. 2008, 90, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Dowson, D.; Goldsmith, A.A.J. The effect of femoral head diameter upon lubrication and wear of metal-on-metal total hip replacements. J. Eng. Med. 2001, 215, 161–170. [Google Scholar] [CrossRef]
- Heisel, C.; Streich, N.; Krachler, M.; Jakubowitz, E.; Kretzer, J.P. Characterization of the running-in period in total hip resurfacing arthroplasty: An in vivo and in vitro metal ion analysis. J. Bone Joint Surg. Am. 2008, 90, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Saikko, V.; Ahlroos, T.; Revitzer, H.; Ryti, O.; Kuosmanen, P. The effect of acetabular cup position on wear of a large-diameter metal-on-metal prosthesis studied with a hip joint simulator. Tribol. Int. 2013, 60, 70–76. [Google Scholar] [CrossRef]
- Vassiliou, K.; Elfick, A.P.D.; Scholes, S.C.; Unsworth, A. The effect of “running-in” on the tribology and surface morphology of metal-on-metal Birmingham hip resurfacing device in simulator studies. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2006, 220, 269–277. [Google Scholar] [CrossRef]
- Joyce, T.J.; Grigg, H.; Langton, D.J.; Nargol, A.V.F. Quantification of self-polishing in vivo from explanted metal-on-metal total hip replacements. Tribol. Int. 2011, 44, 513–516. [Google Scholar] [CrossRef]
- Amstutz, H.C.; le Duff, M.J.; Campbell, P.A.; Gruen, T.A.; Wisk, L.E. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J. Bone Joint Surg. Am. 2010, 92, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.K.; Langton, D.J.; Nargol, A.V.F.; Joyce, T.J. Volumetric wear assessment of failed metal-on-metal hip resurfacing prostheses. Wear 2011, 272, 79–87. [Google Scholar] [CrossRef]
- Langton, D.J.; Joyce, T.J.; Jameson, S.S.; Lord, J.; van Orsouw, M.; Holland, J.P.; Nargol, A.V.F.; de Smet, K.A. Adverse reaction to metal debris following hip resurfacing: The influence of component type, orientation and volumetric wear. J. Bone Joint Surg. Br. 2011, 93, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Langton, D.J.; Joyce, T.J.; Mangat, N.; Lord, J.; van Orsouw, M.; Smet, K.D.; Nargol, A.V.F. Reducing metal ion release following hip resurfacing arthroplasty. Orthop. Clin. N. Am. 2011, 42, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]
- Scholes, S.C.; Unsworth, A.; Hall, R.M.; Scott, J.T. The effects of material combination and lubricant on the friction of total hip prostheses. Wear 2000, 241, 209–213. [Google Scholar] [CrossRef]
- Vendittoli, P.A.; Mottard, S.; Roy, A.G.; Dupont, C.; Lavigne, M. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J. Bone Joint Surg. Br. 2007, 89, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.M.; Stone, M.; Ingham, E.; Fisher, J. (v) Biotribology. Curr. Orthop. 2006, 20, 32–40. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lord, J.K.; Langton, D.J.; Nargol, A.V.F.; Meek, R.M.D.; Joyce, T.J. The Tribology of Explanted Hip Resurfacings Following Early Fracture of the Femur. J. Funct. Biomater. 2015, 6, 1021-1035. https://doi.org/10.3390/jfb6041021
Lord JK, Langton DJ, Nargol AVF, Meek RMD, Joyce TJ. The Tribology of Explanted Hip Resurfacings Following Early Fracture of the Femur. Journal of Functional Biomaterials. 2015; 6(4):1021-1035. https://doi.org/10.3390/jfb6041021
Chicago/Turabian StyleLord, James K., David J. Langton, Antoni V.F. Nargol, R.M. Dominic Meek, and Thomas J. Joyce. 2015. "The Tribology of Explanted Hip Resurfacings Following Early Fracture of the Femur" Journal of Functional Biomaterials 6, no. 4: 1021-1035. https://doi.org/10.3390/jfb6041021
APA StyleLord, J. K., Langton, D. J., Nargol, A. V. F., Meek, R. M. D., & Joyce, T. J. (2015). The Tribology of Explanted Hip Resurfacings Following Early Fracture of the Femur. Journal of Functional Biomaterials, 6(4), 1021-1035. https://doi.org/10.3390/jfb6041021





