Using Magnetic Nanoparticles for Gene Transfer to Neural Stem Cells: Stem Cell Propagation Method Influences Outcomes
Abstract
:1. Introduction
2. Results and Discussion
2.1. MNP-Mediated Gene Delivery to NSCs: Effect of Static and Oscillating Magnetic Fields
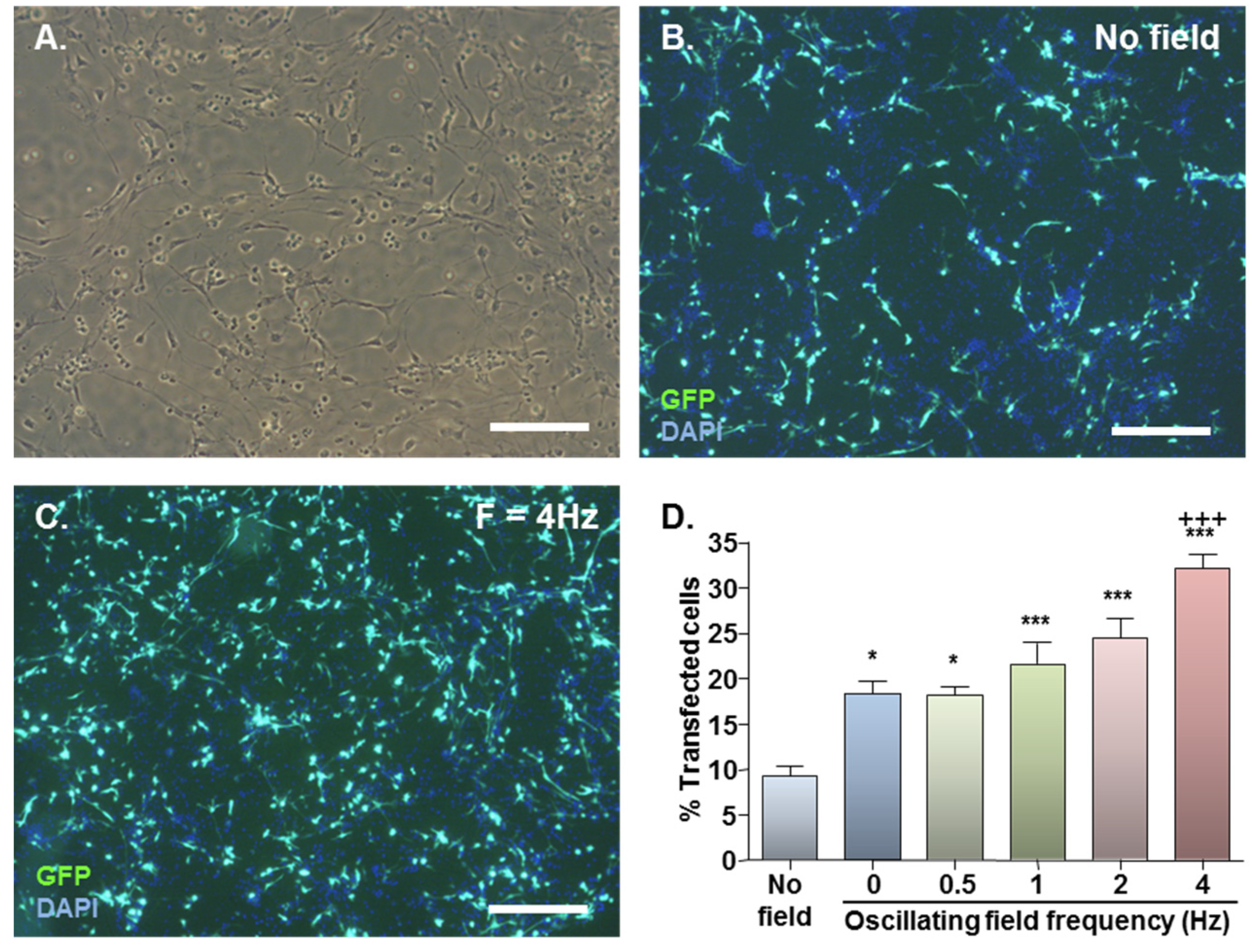
| Days in vitro | Field | GFP+ spheres (%) | Extent of GFP expression (% GFP+ spheres) | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| 7 (n = 4) | None | 33.2 ± 3.0 | 95.5 ± 2.7 | 4.5 ± 2.7 | 0.0 |
| F = 0 Hz | 57.7 ± 7.2 a | 89.1 ± 7.3 | 6.3 ± 3.6 | 4.9 ± 4.5 | |
| F = 4 Hz | 69.8 ± 1.6 c | 76.3 ± 11.0 | 16.0 ± 5.0 | 7.8 ± 6.9 | |
| 14 (n = 3) | None | 3.6 ± 0.6 | 100.0 | 0.0 | 0.0 |
| F = 0 Hz | 9.6 ± 1.4 a | 100.0 | 0.0 | 0.0 | |
| F = 4 Hz | 13.4 ± 2.3 b | 100.0 | 0.0 | 0.0 | |
2.2. Safety of MNP-Mediated Gene Delivery

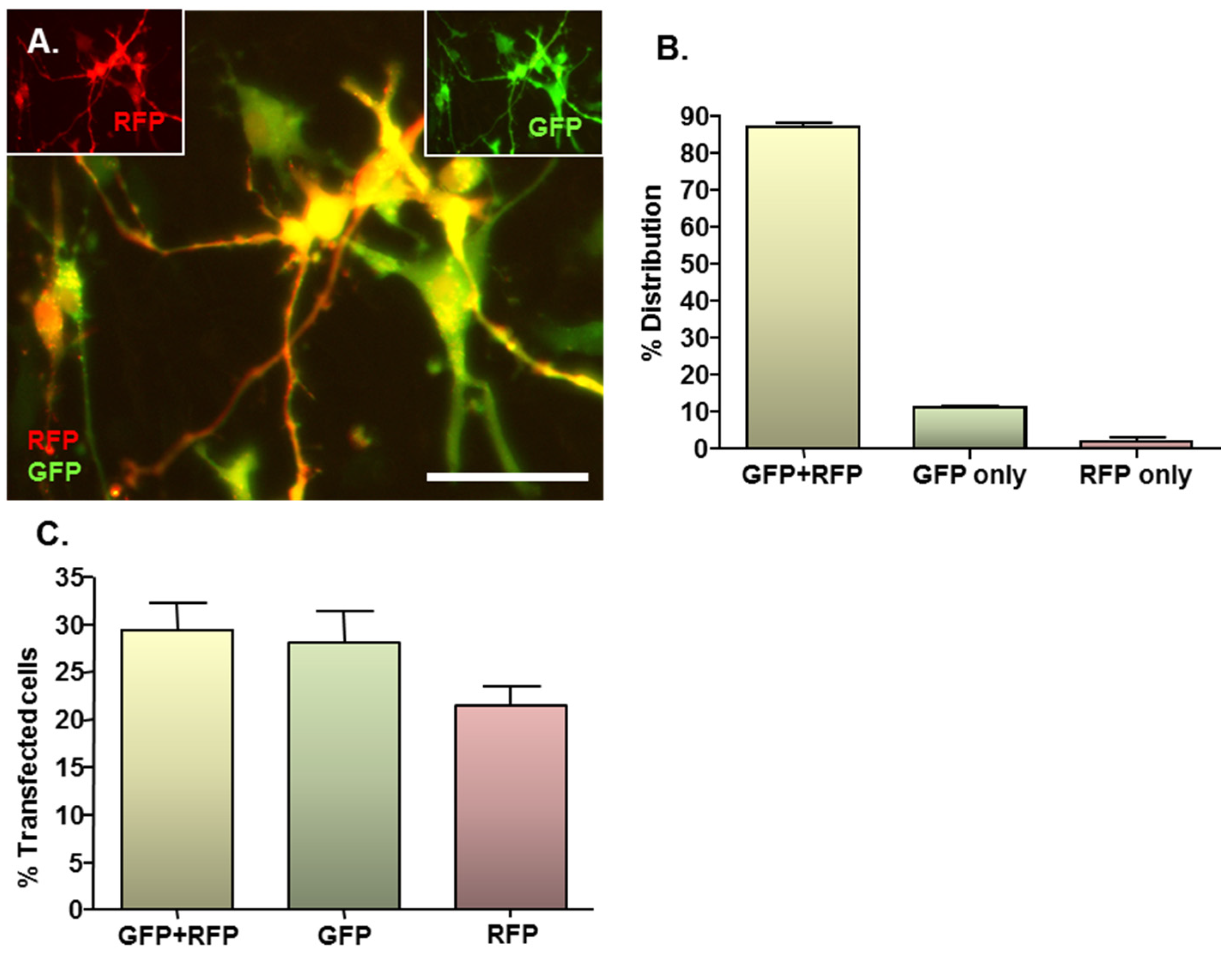
2.3. MNP-Mediated Combinatorial Gene Delivery
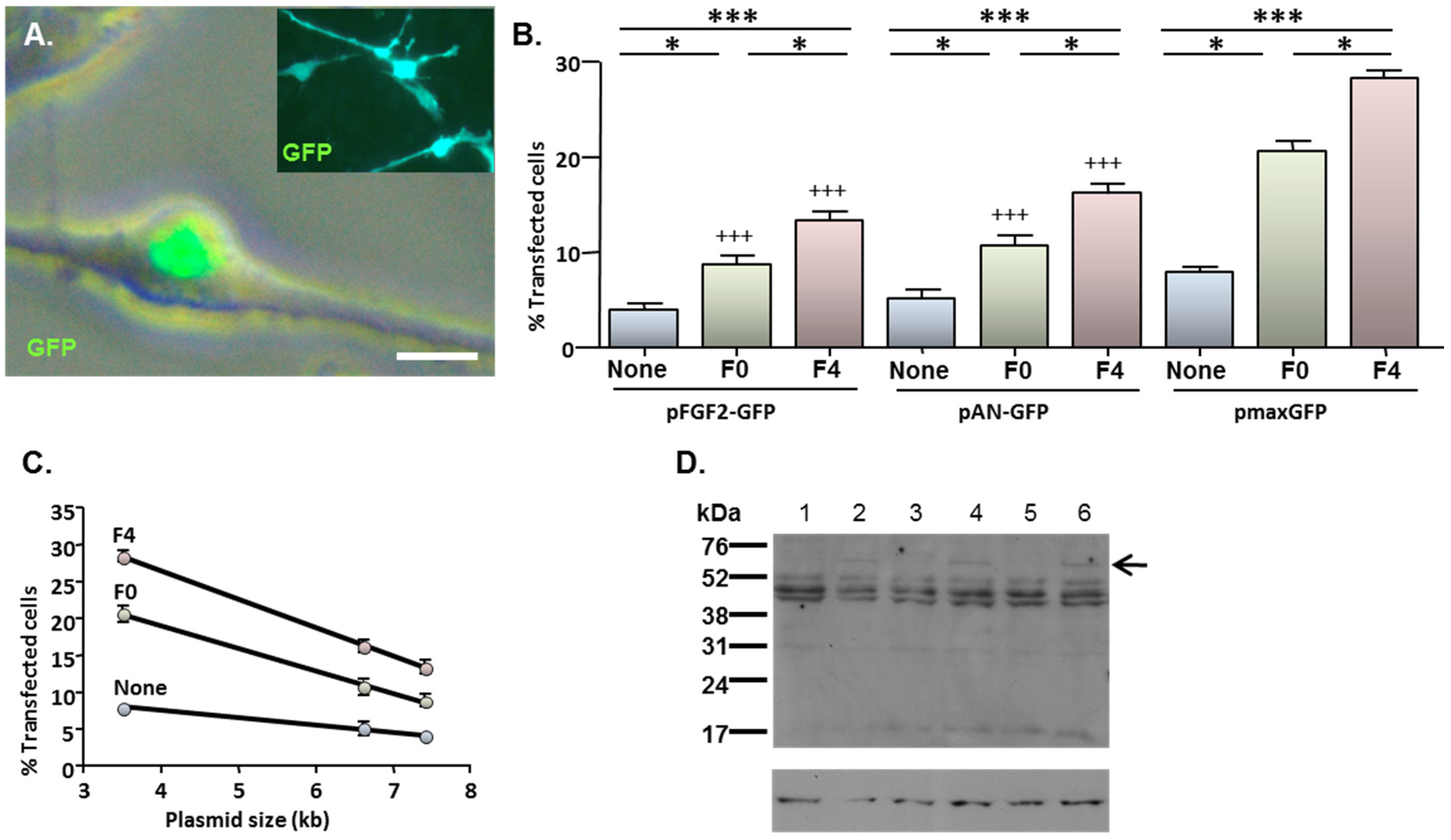
2.4. MNP-Mediated Functional Gene Delivery
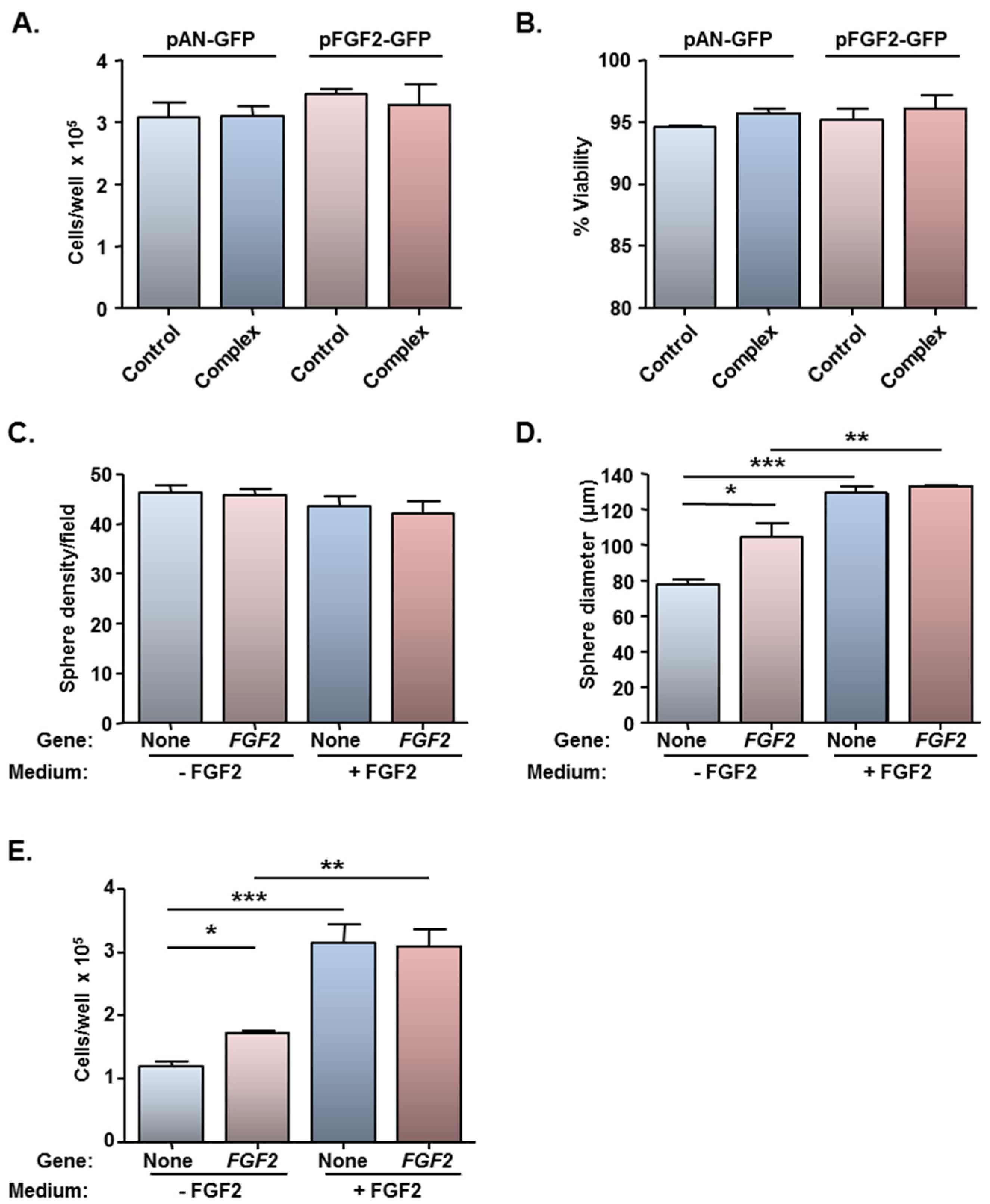
3. Experimental Section
3.1. Reagents
3.2. NSC Culture
3.3. MNP-Mediated Transfection of Monolayers
3.3.1. Single Gene Delivery (Reporter and Functional Genes)
3.3.2. Combinatorial Gene Delivery
3.4. Assessment of Transfection Efficiency, Toxicity and Proliferative Capacity
3.5. Immunoblotting
3.6. Microscopy and Image Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, S.U.; De Vellis, J. Stem cell-based cell therapy in neurological diseases: A review. J. Neurosci. Res. 2009, 87, 2183–2200. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, L. Neural stem cell-mediated therapy for rare brain diseases: Perspectives in the near future for LSDs and MNDs. Histol. Histopathol. 2011, 26, 1093–1109. [Google Scholar]
- Lindvall, O.; Kokaia, Z. Stem cells in human neurodegenerative disorders—Time for clinical translation? J. Clin. Invest. 2010, 120, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; Thakar, R.G.; Lomax, G.; Gibbons, D. Clinical trials for stem cell therapies. BMC Med. 2011, 9. [Google Scholar] [CrossRef]
- Lévesque, M.F.; Neuman, T.; Rezak, M. Therapeutic microinjection of autologous adult human neural stem cells and differentiated neurons for Parkinson’s disease: Five-year post-operative outcome. Open Stem Cell J. 2009, 1, 10–19. [Google Scholar] [CrossRef]
- Bjorklund, L.M.; Sánchez-Pernaute, R.; Chung, S.; Andersson, T.; Chen, I.Y.; McNaught, K.S.; Brownell, A.L.; Jenkins, B.G.; Wahlestedt, C.; Kim, K.S.; et al. Embryonic stem cells develop into functional dopaminergic neurons after transplantation in a Parkinson rat model. Proc. Natl. Acad. Sci. USA 2002, 99, 2344–2349. [Google Scholar] [CrossRef]
- Ahmed, R.P.H.; Ashraf, M.; Buccini, S.; Shujia, J.; Haider, K.H. Cardiac tumorigenic potential of induced pluripotent stem cells in an immunocompetent host with myocardial infarction. Regen. Med. 2011, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.L.; Shen, J.S.; Ohashi, T.; Maeda, H.; Kim, S.U.; Eto, Y. Brain transplantation of genetically engineered human neural stem cells globally corrects brain lesions in the mucopolysaccharidosis type VII mouse. J. Neurosci. Res. 2003, 74, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.; Tang, Y. Genetic modification of stem cells for transplantation. Adv. Drug Deliv. Rev. 2008, 60, 160–172. [Google Scholar]
- Kageyama, R.; Hirata, H.; Hatakeyama, J. Retroviral vectors for gene delivery to neural precursor cells. Int. Rev. Neurobiol. 2003, 55, 123–147. [Google Scholar] [PubMed]
- Jandial, R.; Singec, I.; Ames, C.P.; Snyder, E.Y. Genetic modification of neural stem cells. Mol. Ther. 2008, 16, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Falk, A.; Holmström, N.; Carlén, M.; Cassidy, R.; Lundberg, C.; Frisén, J. Gene delivery to adult neural stem cells. Exp. Cell. Res. 2002, 279, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Ye, Y.; Svendsen, C.N. Transduction of human neural progenitor cells using recombinant adeno-associated viral vectors. Gene Ther. 2002, 9, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kameda, M.; Shingo, T.; Takahash, K.; Muraoka, K.; Kurozumi, K.; Yasuhara, T.; Maruo, T.; Tsuboi, T.; Uozumi, T.; Matsui, T.; et al. Adult neural stem and progenitor cells modified to secrete GDNF can protect, migrate and integrate after intracerebral transplantation in rats with transient forebrain ischemia. Eur. J. Neurosci. 2007, 26, 1462–1478. [Google Scholar] [CrossRef]
- Dieterlen, M.T.; Wegner, F.; Schwarz, S.C.; Milosevic, J.; Schneider, B.; Busch, M.; Römuss, U.; Brandt, A.; Storch, A.; Schwarz, J. Non-viral gene transfer by nucleofection allows stable gene expression in human neural progenitor cells. J. Neurosci. Methods 2009, 178, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, M.A.; Simanek, E.E. Nonviral vectors for gene delivery. Chem. Rev. 2009, 109, 259–302. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Nazarali, A.; Foldvari, M. Non-viral nucleic acid delivery: Key challenges and future directions. Curr. Drug Deliv. 2011, 8, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.I.; Pickard, M.R.; Granger, N.; Chari, D.M. Magnetic nanoparticle-mediated gene transfer to oligodendrocyte precursor cell transplant populations is enhanced by magnetofection strategies. ACS Nano 2011, 5, 6527–6538. [Google Scholar] [CrossRef] [PubMed]
- Pickard, M.; Chari, D. Enhancement of magnetic nanoparticle-mediated gene transfer to astrocytes by “magnetofection”: Effects of static and oscillating fields. Nanomedicine (Lond) 2010, 5, 217–232. [Google Scholar] [CrossRef]
- Pickard, M.R.; Barraud, P.; Chari, D.M. The transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticles. Biomaterials 2011, 32, 2274–2284. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zelphati, O.; Mykhaylyk, O. Magnetically enhanced nucleic acid delivery. Adv. Drug Deliv. Rev. 2011, 63, 1300–1331. [Google Scholar] [CrossRef] [PubMed]
- Sapet, C.; Laurent, N.; de Chevigny, A.; Le Gourrierec, L.; Bertosio, E.; Zelphati, O.; Béclin, C. High transfection efficiency of neural stem cells with magnetofection. Biotechniques 2011, 50, 187–189. [Google Scholar] [PubMed]
- Adams, C.; Pickard, M.; Chari, D. Magnetic nanoparticle mediated transfection of neural stem cell suspension cultures is enhanced by applied oscillating magnetic fields. Nanomedicine 2013, 9, 737–741. [Google Scholar] [CrossRef] [PubMed]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar]
- Cesnulevicius, K.; Timmer, M.; Wesemann, M.; Thomas, T.; Barkhausen, T.; Grothe, C. Nucleofection is the most efficient nonviral transfection method for neuronal stem cells derived from ventral mesencephali with no changes in cell composition or dopaminergic fate. Stem Cells 2006, 24, 2776–2791. [Google Scholar] [CrossRef] [PubMed]
- Dhara, S.K.; Majumder, A.; Dodla, M.C.; Stice, S.L. Nonviral gene delivery in neural progenitors derived from human pluripotent stem cells. Methods Mol. Biol. 2011, 767, 343–354. [Google Scholar] [PubMed]
- Jenkins, S.I.; Pickard, M.R.; Furness, D.N.; Yiu, H.H.; Chari, D.M. Differences in magnetic particle uptake by CNS neuroglial subclasses: Implications for neural tissue engineering. Nanomedicine (Lond) 2013, 8, 951–968. [Google Scholar] [CrossRef]
- Campos, L.S. Neurospheres: Insights into neural stem cell biology. J. Neurosci. Res. 2004, 78, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Akesson, E.; Piao, J.H.; Samuelsson, E.B.; Holmberg, L.; Kjaeldgaard, A.; Falci, S.; Sundström, E.; Seiger, A. Long-term culture and neuronal survival after intraspinal transplantation of human spinal cord-derived neurospheres. Physiol. Behav. 2007, 92, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Kulbatski, I.; Parr, A.; Mohareb, M.; Tator, C.H. Adult spinal cord stem/progenitor cells transplanted as neurospheres preferentially differentiate into oligodendrocytes in the adult rat spinal cord. Cell. Transpl. 2008, 17, 735–751. [Google Scholar] [CrossRef]
- Neri, M.; Ricca, A.; di Girolamo, I.; Alcalá-Franco, B.; Cavazzin, C.; Orlacchio, A.; Martino, S.; Naldini, L.; Gritti, A. Neural stem cell gene therapy ameliorates pathology and function in a mouse model of globoid cell leukodystrophy. Stem Cells 2011, 29, 1559–1571. [Google Scholar] [CrossRef] [PubMed]
- Kouroupi, G.; Lavdas, A.A.; Gaitanou, M.; Thomaidou, D.; Stylianopoulou, F.; Matsas, R. Lentivirus-mediated expression of insulin-like growth factor-I promotes neural stem/precursor cell proliferation and enhances their potential to generate neurons. J. Neurochem. 2010, 115, 460–474. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Li, M.; Song, T.; Qian, Y.; Xiao, X.; Chen, X.; Zhang, P.; Feng, X.; Parker, T.; Liu, Y. Retrovirus delivered neurotrophin-3 promotes survival, proliferation and neuronal differentiation of human fetal neural stem cells in vitro. Brain Res. Bull. 2008, 77, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Park, K.I.; Himes, B.T.; Stieg, P.E.; Tessler, A.; Fischer, I.; Snyder, E.Y. Neural stem cells may be uniquely suited for combined gene therapy and cell replacement: Evidence from engraftment of Neurotrophin-3-expressing stem cells in hypoxic-ischemic brain injury. Exp. Neurol. 2006, 199, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Bowers, W.J.; Breakefield, X.O.; Sena-Esteves, M. Genetic therapy for the nervous system. Hum. Mol. Genet. 2011, 20, R1–R14. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Shim, J.W.; Oh, Y.J.; Son, H.; Lee, Y.S.; Lee, S.H. Co-transfection with cDNA encoding the Bcl family of anti-apoptotic proteins improves the efficiency of transfection in primary fetal neural stem cells. J. Neurosci. Methods 2002, 117, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, R.B.; Faijerson, J.; Eriksson, P.S. Efficient non-viral transfection of adult neural stem/progenitor cells, without affecting viability, proliferation or differentiation. J. Gene Med. 2006, 8, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.D.; Kingston, P.A. Plasmid-mediated gene therapy for cardiovascular disease. Cardiovasc. Res. 2011, 91, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pickard, M.R.; Adams, C.F.; Barraud, P.; Chari, D.M. Using Magnetic Nanoparticles for Gene Transfer to Neural Stem Cells: Stem Cell Propagation Method Influences Outcomes. J. Funct. Biomater. 2015, 6, 259-276. https://doi.org/10.3390/jfb6020259
Pickard MR, Adams CF, Barraud P, Chari DM. Using Magnetic Nanoparticles for Gene Transfer to Neural Stem Cells: Stem Cell Propagation Method Influences Outcomes. Journal of Functional Biomaterials. 2015; 6(2):259-276. https://doi.org/10.3390/jfb6020259
Chicago/Turabian StylePickard, Mark R., Christopher F. Adams, Perrine Barraud, and Divya M. Chari. 2015. "Using Magnetic Nanoparticles for Gene Transfer to Neural Stem Cells: Stem Cell Propagation Method Influences Outcomes" Journal of Functional Biomaterials 6, no. 2: 259-276. https://doi.org/10.3390/jfb6020259





