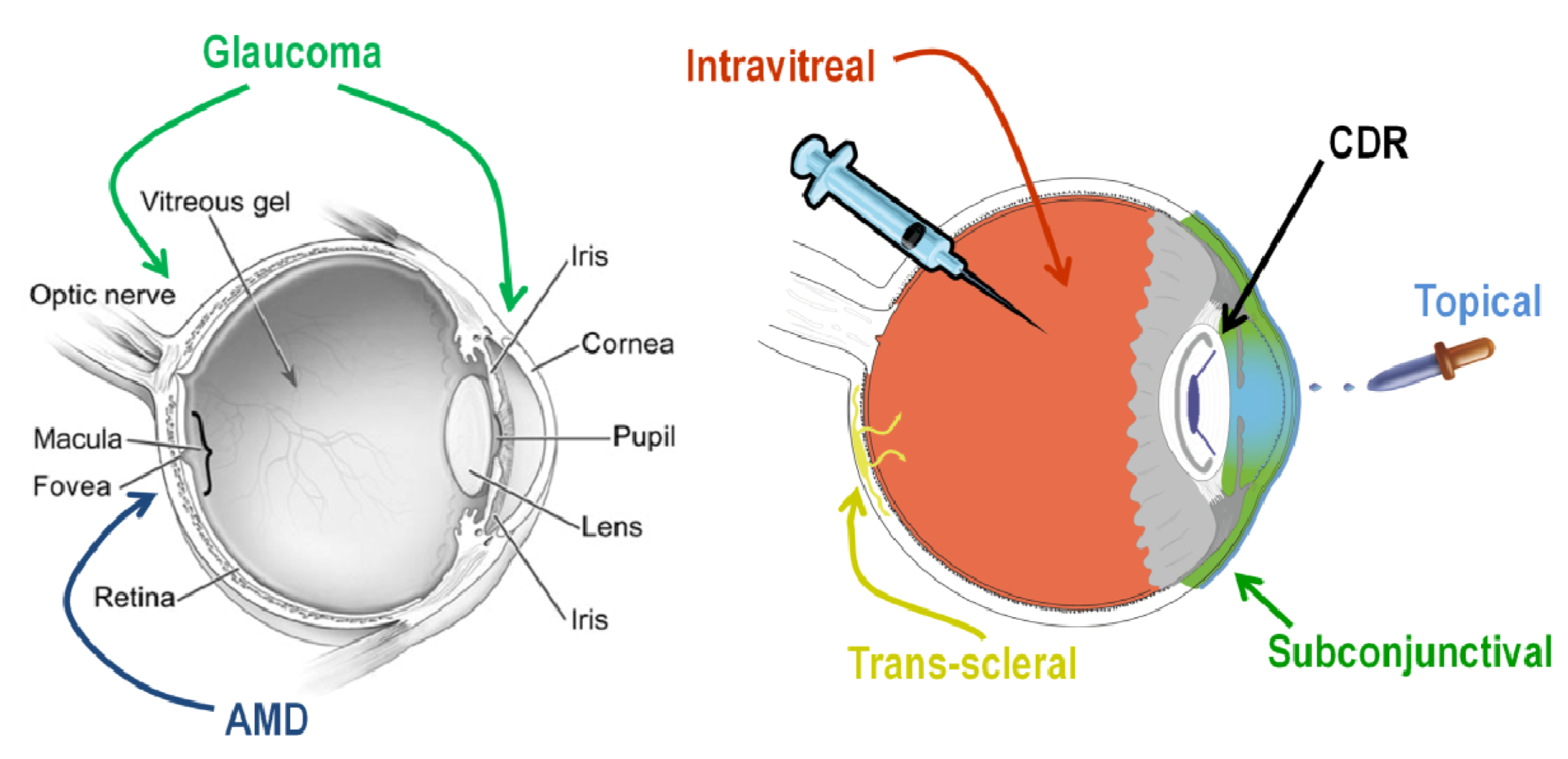
Despite not being currently FDA approved for ocular applications, Avastin (bevacizumab; Genentech, San Francisco, CA, USA) was used as the pharmacological agent in this study. Lucentis® (ranibizumab; Genentech, San Francisco, CA, USA), the Fab fragment of Avastin, is an FDA-approved treatment for AMD. While Lucentis is a prescription medicine for the treatment of AMD, Avastin is being investigated as a lower-cost potential alternative therapy and is often used off-label for the clinical treatment of AMD [
3]. Recent studies show similar outcomes in the ocular anti-angiogenic efficacies and safety of the two drugs [
3].
3.3. In vitro Cytotoxicity
The interface between an implanted material and the surrounding biological tissue is the site of action wherein the host’s response to an implant is most markedly manifested. Materials implanted into both hard and soft tissues generate some degree of cellular response. The severity of the host’s response to an implant can be attributed to a number of factors, including the surgical technique, the size, shape and surface properties of the implant and the nature of the tissues at the implant location. The interfacial interactions are a major factor in determining the success or failure of an implant. Therefore, the determination of an implant’s impact on the host tissues is important to evaluate. This impact can only fully be anticipated through statistically powered in vivo usage of the implant; however, first step biocompatibility testing of biomedical devices can be shown through the use of in vitro cytotoxicity testing, as discussed in ISO 10993. Implantation into a host tissue generates a host cellular response. Activated cells produce cytokines, including MCP-1, TGF-β1, IL-1β, TNF, MIP-1α and MIP-1β. These cytokines are influential in regulating the host’s wound healing response. Wound healing involves a number of cells, including fibroblasts, monocytes, macrophages and endothelial cells. For this study, we performed cell culture with L-929 fibroblasts and J774A.1 macrophages. Both cell lines were individually cultured on the primary component of the CDR, Bionate II (DSM). Cell culture media was harvested after each day of the experiment, and assay cytokines were quantified through the use of CBA on media harvested from days 1, 3 and 5. Fibroblast cell culture media was quantified for the presence of MCP-1 and TGF-β1; macrophage cell culture media was quantified for the presence of MCP-1, TNF, MIP-1α and MIP-1β cytokines. These cytokine concentrations were compared to concentrations produced by cells cultured on incubation gold standard tissue culture polystyrene (TCPS).
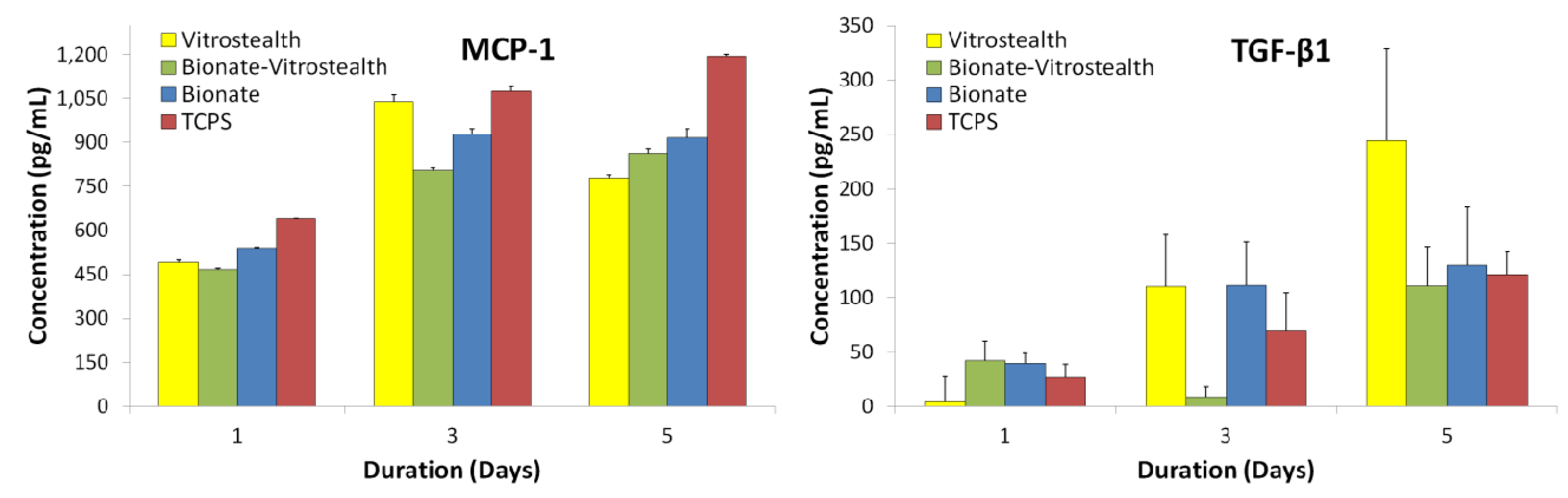
L-929 fibroblasts were incubated on each surface and growth media was quantified 1, 3 and 5 days post-incubation for inflammatory cytokines (
Figure 5). The production of MCP-1 and TGF-β1 proinflammatory cytokines had a general increasing trend over incubation time, which was to be expected as the cell populations increased over time. It was expected that a PEG coating (Vitrostealth) of the biomaterial would decrease the production of proinflammatory cytokines, but this correlation was not seen with our data. The production of TGF-β1 appeared to increase most dramatically for the fibroblasts cultured with the Vitrostealth coating. For both fibroblast produced quantified cytokines, the cellular populations appear to show little difference from the negative control.
Figure 5.
MCP-1 and TGF-β1 proinflammatory cytokines produced by L-929 fibroblasts were quantified by cytometric bead array (CBA) and are compared to cells cultured on tissue culture polystyrene.
Figure 5.
MCP-1 and TGF-β1 proinflammatory cytokines produced by L-929 fibroblasts were quantified by cytometric bead array (CBA) and are compared to cells cultured on tissue culture polystyrene.
J774A.1 macrophages were cultured experimentally similar to the L-929 fibroblasts, and media was harvested and quantified for days 1, 3 and 5. MCP-1, TNF, MIP-1α and MIP-1β proinflammatory cytokines were measured using CBA (
Figure 6). Cells cultured on the Bionate II material tended to show similar cytokine concentrations to the negative control throughout the duration of the experiment. In addition, the macrophage cells cultured on each surface did not show signs of proliferative or morphological toxicity. This would indicate that the polycarbonate urethane (Bionate II) was not aggravating the cells. However, cells that were cultured on Vitrostealth generally tended to express higher concentrations of TNF and MCP-1. However, this increase in TNF (~100 pg/mL) and MCP-1 (~60 pg/mL) is not significant on these volume scales. While no material is perfectly biocompatible, these
in vitro data indicate that each of the tested biomaterials has a very similar impact on cultured fibroblasts and macrophages as the gold standard in cell culture, TCPS. This is seen in the similar levels of cytokine production in each of the samples.
It should be noted that cellular adhesion is also an important indicator of material biocompatibility. During this study, this property was not implicitly measured for quantitative analysis, but a qualitative analysis shows cellular adhesion on each material surface to be comparable. Macrophage cell adhesion was unaffected by the material type, showing similar growth and adhesion on each of the materials. Fibroblast cellular adhesion appears to be impacted by each of the materials, resulting in a slight morphological change despite remaining viable.
Figure 6.
MCP-1, TNF, MIP-1α and MIP-1β proinflammatory cytokines produced by J774A.1 macrophages were quantified by CBA and are compared to cells cultured on tissue culture polystyrene.
Figure 6.
MCP-1, TNF, MIP-1α and MIP-1β proinflammatory cytokines produced by J774A.1 macrophages were quantified by CBA and are compared to cells cultured on tissue culture polystyrene.
Surface roughness was measured for samples of TCPS, Bionate II and Vitrostealth-coated Bionate II. Contact angle measurements were also taken for TCPS and Bionate II samples. These data are shown in
Table 1. The data indicate that Bionate II was relatively rough in comparison to the other two surfaces. This may have induced some degree of increased cellular toxicity in comparison to the other samples; however, surface contact angles for both Bionate II and TCPS were very similar, and the cellular release of proinflammatory cytokines also indicates that the increased roughness of the Bionate II samples had little effect on the cytotoxicity of the samples.
Table 1.
Surface measurements of roughness and hydrophilicity.
Table 1.
Surface measurements of roughness and hydrophilicity.
| Material | Surface roughness (Ra, Å) | RMS roughness (Rq, Å) | Contact angle (degrees) |
|---|
| TCPS | 177.7 ± 46.6 | 227.1 ± 62.6 | 76.5 ± 2.3 |
| Bionate II | 754.3 ± 372.2 | 953.5 ± 452.3 | 77.0 ± 2.5 |
| Vitrostealth | 98.8 ± 36.4 | 123.1 ± 47.0 | N/A |
3.4. Drug Release Kinetics—In vitro/in vivo
The current standard of care for the treatment of AMD is monthly intravitreal injections of Lucentis (ranibizumab; Genentech). However, Avastin (bevacizumab; Genentech) is a drug that is increasingly being used off label as a replacement for Lucentis for AMD treatment. For our drug release kinetics assessments, we have used Avastin. For clinical treatment, each monthly bolus injection contains 1.25 mg, and thus, the daily rate delivered is 41.7 μg/day. We used this as an initial target rate to be further refined with future in vivo experimentation. It is unclear if more or less drug than this is needed, as the implant location is intended for the capsular bag as compared to intravitreally placed. Furthermore, sustained release drug delivery generally requires less drug, and therefore, a clinically relevant delivery rate will likely differ. Delineating macromolecular drug delivery from CDRs placed in the capsular bag at the time of cataract surgery is ongoing.
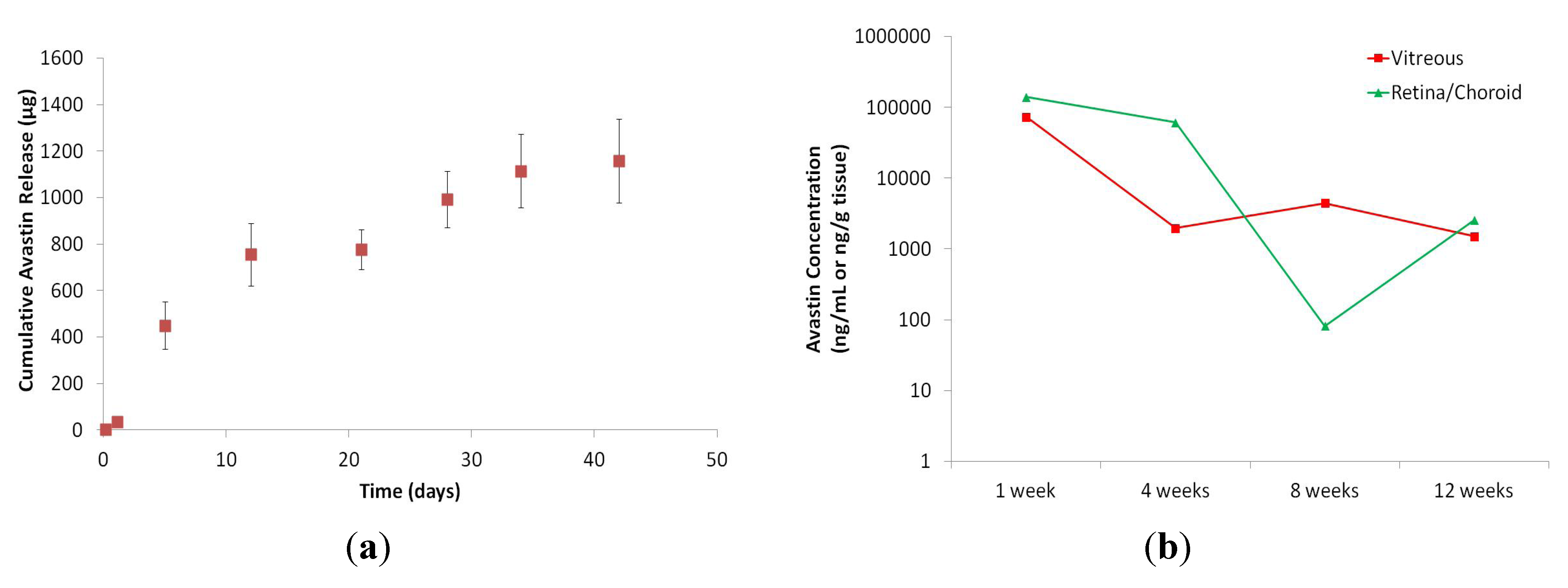
Drug release for the CDR was assessed
in vitro and for a preliminary
in vivo study. These experiments were performed as a first assessment of CDR device efficacy.
In vitro media was harvested over time, as previously described, and assessed for Avastin concentrations at predetermined time points. The cumulative
in vitro drug release accounts for ~30% of the total loaded drug, where release plateaued at day 42 (
Figure 7). Explanations for the unaccounted drug include non-specific binding with the CDR, protein degradation from elevated temperatures and aggregation from the elevated concentration of 100 mg/mL. The presence of these phenomena were confirmed, but not quantified explicitly. This study showed a two phase drug release profile. The first 10 days show a drug release of about 80 μg/day. After the first 10 days, the rate of drug release slows down to about 16.5 μg/day. Future work with the CDR will include the tuning of the rate of drug release to incorporate near-zero order release kinetics and stability optimization, as measured by charge variance analysis, to maintain a therapeutic effect, as determined in an appropriate disease model.
In vivo drug concentrations were quantified after implantation into 4 rabbits, concurrent to our
in vitro assessment. The rabbits were sacrificed at 1, 4, 8 and 12 weeks. Tissues were harvested and ELISA was performed. Ocular drug distribution was assessed upon sacrifice of implanted rabbits at predetermined time points, as shown in
Figure 7. To the best of our knowledge, there is no published work showing the penetration of a large molecule from the anterior chamber to the posterior chamber. This experiment clearly shows the ability of a large molecule, such as Avastin (149 kDa), to penetrate into retinal tissues. During week 1, the drug concentration is found to be about 100 μg/mL and, over the course of 12 weeks, decreased to a concentration of about 1 μg/mL. However, for this experiment, only a single rabbit (n = 2 eyes) was assessed for each timepoint, and a statistically powered experiment will need to be performed in order to determine a correlation between the
in vitro and
in vivo release profiles.
Figure 7.
CDR sustained drug release was quantified by measuring Avastin release over time. (a) This figure shows in vitro release of Avastin over 40+ days; (b) In vivo release of Avastin was detected in rabbit tissues out to at least 12 weeks.
Figure 7.
CDR sustained drug release was quantified by measuring Avastin release over time. (a) This figure shows in vitro release of Avastin over 40+ days; (b) In vivo release of Avastin was detected in rabbit tissues out to at least 12 weeks.