Influence of Porcine Intervertebral Disc Matrix on Stem Cell Differentiation
Abstract
:1. Introduction
1.1. Differentiation of Progenitor Cells in Nucleus Pulposus Cells
1.2. Extracellular Matrix of Nucleus Pulposus
1.3. Biomaterials for NP Regeneration
1.4. Aim of the Study
2. Experimental Section
2.1. Isolation of Porcine Nucleus Pulposus Extract
2.2. Production and Purification of the Transglutaminase
2.3. Transglutaminase Mediated Glutamine and Lysine Biotinylation of Nucleus Pulposus Extract
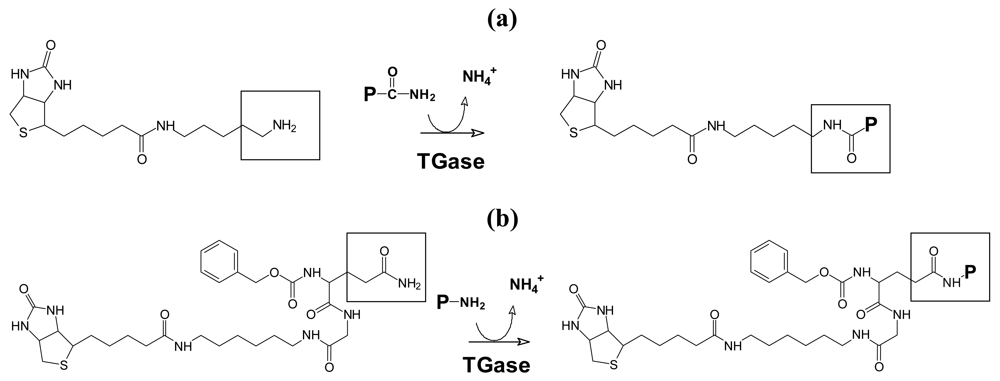
2.4. Preparation and Cultivation of the Cell-Matrix-Hydrogel
2.5. Live-Dead-Staining of Cells
2.6. Measurement of Cell Proliferation
2.7. Expression of Specific Marker Genes (RT-PCR)
| Gene Name | Gene Symbol | Reference Sequence (GI) | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|---|
| housekeeping gene | ||||
| glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 83641890 | AATCAAGTGGGG CGATGCTGGCGCT | AGTGTGGCAGGG ACTCCCCAGCAGT |
| genes coding for matrix proteins | ||||
| type I collagen | COL1A1 | 110349771 | GGGCCTCAGGGT GCTCGAGGATTC | AGGGCTGCCAGG GCTTCCAGTCAGA |
| type II collagen | COL2A1 | 111118973 | TGACGGTCCCTCT GGTGCCGAAGGT | CGGGGCCCTTCT CTCTCGGGCCTAA |
| type X collagen | COL10A1 | 98985802 | ACCCACAGGAGC CCCAGGAC | GCTATGCCAGCT GGGCCAGG |
| versican | VCAN | 255918075 | CCCACCGGTGAG GGGCTCCCT | GGTGCCTCCGTT AAGGCACGGGT |
| aggrecan | ACAN | 223462184 | CGCCGGTGTCGG GAGCAGCA | TGCGTTTGTAGGT GGTGGCTGTGC |
| genes coding for putative chondrocyte markers | ||||
| cartilage oligomeric protein | COMP | 40217842 | ACTGCAGGAAAC CAACGCGGCGC | GGTTCCGCACCA GCGGGCAGTT |
| integrin-binding sialoprotein | IBSP | 167466186 | AGGGCAAGGGCA CCTCGAAGA | TCATTGGCGCCC GTGTATTCGT |
| genes coding for putative NP cell markers | ||||
| neural cell adhesion molecule 1 | NCAM1 | 117320546 | GGCTTCGTGGAC TCGACCAGAG | TAGTGTCTGATG GGGGAGCCGC |
| annexin A3 | ANXA3 | 96304463 | GGCGCGGGAACA AACGAAGATGCC | GGCCGGCGTGTT CCTCACACAA |
| pleiotrophin | PTN | 42476152 | GGGCACACGGGA GGGCACTCG | TGGTCAGTTTGCC ACAGGGCTTGGA |
| glypican 3 | GPC3 | 257471005 | GCCGCCGGACGC CACCTGTC | GGCTGAATCAGG CAGGCCTGGGT |
| brachyury | T | 2558580 | AGCTCCCCTGGC ACCGAGAG | GCGTGGAGGGGA GGGAGAGG |
| paired box 1 | PAX1 | 153791841 | TGGCGCGCTACA ACGAGACC | GCGCCAGAGGAG GACCTTGC |
3. Results and Discussion
3.1. Cell Survival and Proliferation of in Gelatin Embedded and Differentiated hMSC-TERT
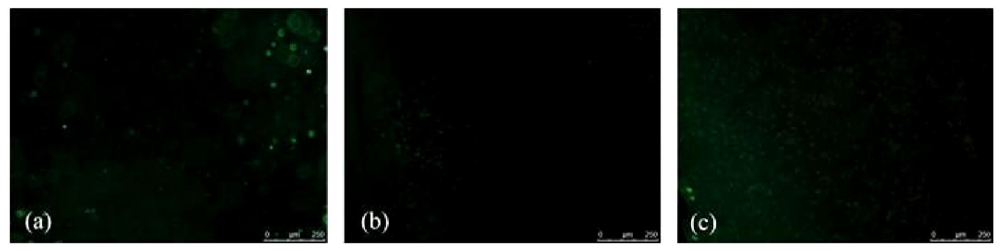
3.2. Morphological Changes after Differentiation of hMSC-TERT in Gelatin
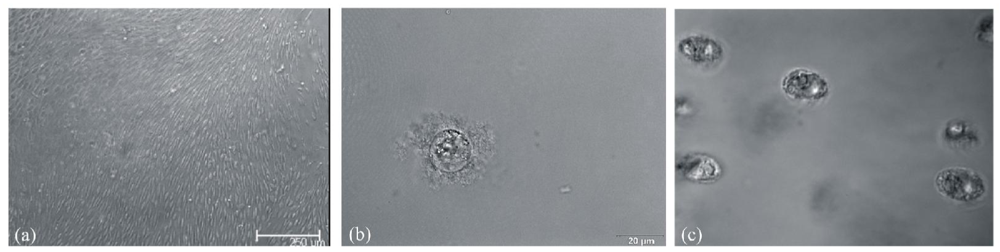
3.4. Gene Expression during hMSC-TERT Differentiation in Gelatin
| NC | 7% (w/v) gelatin -NP extract +TGF-b3 | 12.5% (w/v) gelatin -NP extract +TGF-b3 | 12.5% (w/v) gelatin +NP extract +TGF-b3 | 12.5% (w/v) gelatin +NP extract -TGF-b | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| differentiation time [d] | - | 4 | 7 | 11 | 14 | 17 | 21 | 4 | 7 | 11 | 14 | 17 | 21 | 4 | 7 | 11 | 14 | 17 | 21 | 4 | 7 | 11 | 14 | 17 | 21 | |
| gene symbol | ||||||||||||||||||||||||||
| putative NP cell marker | ANAX3 | |||||||||||||||||||||||||
| GPC3 | ||||||||||||||||||||||||||
| NCAM1 | ||||||||||||||||||||||||||
| PAX1 | ||||||||||||||||||||||||||
| PTN | ||||||||||||||||||||||||||
| T | ||||||||||||||||||||||||||
| putative chrondrocyte marker | COMP | |||||||||||||||||||||||||
| IBSP | ||||||||||||||||||||||||||
| matrix proteins | COL1A1 | |||||||||||||||||||||||||
| COL2A1 | ||||||||||||||||||||||||||
| COL10A1 | ||||||||||||||||||||||||||
| ACAN | ||||||||||||||||||||||||||
| VCAN | ||||||||||||||||||||||||||
| housekeeping gene | GAPDH | |||||||||||||||||||||||||
3.4.1. Influence of Gelatin Concentration
3.4.2. Influence of Nucleus Pulposus Extract
3.4.3. Influence of TGF-β3
3.5. Determination of Transglutaminase Substrates in Nucleus Pulposus

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Freimark, D.; Czermak, P. Cell-based regeneration of intervertebral disc defects: Review and concepts. Int. J. Artif. Organs 2009, 32, 197–203. [Google Scholar]
- Horner, H.A.; Roberts, S.; Bielby, R.C.; Menage, J.; Evans, H.; Urban, J.P. Cells from different regions of the intervertebral disc: Effect of culture system on matrix expression and cell phenotype. Spine 2002, 27, 1018–1028. [Google Scholar]
- Risbud, M.V.; Schaer, T.P.; Shapiro, I.M. Toward an understanding of the role of notochordal cells in the adult intervertebral disc: From discord to accord. Dev. Dyn. 2010, 239, 2141–2148. [Google Scholar]
- Sakai, D.; Mochida, J.; Iwashina, T.; Watanabe, T.; Nakai, T.; Ando, K.; Hotta, T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: Potential and limitations for stem cell therapy in disc regeneration. Spine 2005, 30, 2379–2387. [Google Scholar]
- Richardson, S.M.; Walker, R.V.; Parker, S.; Rhodes, N.P.; Hunt, J.A.; Freemont, A.J.; Hoyland, J.A. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells 2006, 24, 707–716. [Google Scholar]
- Ehlicke, F.; Freimark, D.; Heil, B.; Dorresteijn, A.; Czermak, P. Intervertebral disc regeneration: Influence of growth factors on differentiation of human mesenchymal stem cells (hMSC). Int. J. Artif. Organs 2010, 33, 244–252. [Google Scholar]
- Acosta, F.L., Jr.; Lotz, J.; Ames, C.P. The potential role of mesenchymal stem cell therapy for intervertebral disc degeneration: A critical overview. Neurosurg Focus 2005, 19, E4. [Google Scholar]
- Le Maitre, C.L.; Pockert, A.; Buttle, D.J.; Freemont, A.J.; Hoyland, J.A. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem. Soc. Trans. 2007, 35, 652–655. [Google Scholar]
- Hunt, N.C.; Grover, L.M. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol. Lett. 2010, 32, 733–742. [Google Scholar]
- Gokorsch, S.; Nehring, D.; Grottke, C.; Czermak, P. Hydrodynamic stimulation and long term cultivation of nucleus pulposus cells: A new bioreactor system to induce extracellular matrix synthesis by nucleus pulposus cells dependent on intermittent hydrostatic pressure. Int. J. Artif. Organs 2004, 27, 962–970. [Google Scholar]
- Gokorsch, S.; Weber, C.; Wedler, T.; Czermak, P. A stimulation unit for the application of mechanical strain on tissue engineered anulus fibrosus cells: A new system to induce extracellular matrix synthesis by anulus fibrosus cells dependent on cyclic mechanical strain. Int. J. Artif. Organs 2005, 28, 1242–1250. [Google Scholar]
- Sebastine, I.M.; Williams, D.J. Current developments in tissue engineering of nucleus pulposus for the treatment of intervertebral disc degeneration. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2007, 2007, 6401–6406. [Google Scholar]
- Calderon, L.; Collin, E.; Velasco-Bayon, D.; Murphy, M.; O'Halloran, D.; Pandit, A. Type II collagen-hyaluronan hydrogel—A step towards a scaffold for intervertebral disc tissue engineering. Eur. Cell Mater. 2010, 20, 134–148. [Google Scholar]
- Gerber, U.; Jucknischke, U.; Putzien, S.; Fuchsbauer, H.L. A rapid and simple method for the purification of transglutaminase from Streptoverticillium mobaraense. Biochem. J. 1994, 299, 825–829. [Google Scholar]
- Sarafeddinov, A.; Schmidt, S.; Adolf, F.; Mainusch, M.; Bender, A.; Fuchsbauer, H.L. A novel transglutaminase substrate from Streptomyces mobaraensis triggers autolysis of neutral metalloproteases. Biosci. Biotechnol. Biochem. 2009, 73, 993–999. [Google Scholar]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.S.; Jensen, T.G.; Kassem, M. Telomerase expression extends the proliferative life-span and maintains the osteogenic potential of human bone marrow stromal cells. Nat. Biotech. 2002, 20, 592–596. [Google Scholar]
- Weber, C.; Pohl, S.; Portner, R.; Wallrapp, C.; Kassem, M.; Geigle, P.; Czermak, P. Expansion and Harvesting of hMSC-TERT. Open Biomed. Eng. J. 2007, 1, 38–46. [Google Scholar]
- Weber, C; Pohl, S; Poertner, R; Pino-Grace, P; Freimark, D; Wallrapp, C; Geigle, P; Czermak, P. Production process for stem cell-based therapeutic implants—Expansion of the production cell line and cultivation of encapsulated cells. Adv. Biochem. Eng. Biotechnol. 2010, 123, 143–162. [Google Scholar]
- Rio, D.C.; Ares, M., Jr.; Hannon, G.J.; Nilsen, T.W. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb Protoc. 2010, 2010. pdb prot5439. [Google Scholar]
- Rozen, S.; Skaletsky, H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol. Biol. 2000, 132, 365–386. [Google Scholar]
- Mattii, L.; Battolla, B.; D'Alessandro, D.; Trombi, L.; Pacini, S.; Cascone, M.G.; Lazzeri, L.; Bernardini, N.; Dolfi, A.; Galimberti, S.; Petrini, M. Gelatin/PLLA sponge-like scaffolds allow proliferation and osteogenic differentiation of human mesenchymal stromal cells. Macromol. Biosci. 2008, 8, 819–826. [Google Scholar]
- Shin, Y.M.; Kim, K.S.; Lim, Y.M.; Nho, Y.C.; Shin, H. Modulation of spreading, proliferation, and differentiation of human mesenchymal stem cells on gelatin-immobilized poly(l-lactide-co-Ïμ-caprolactone) substrates. Biomacromolecules 2008, 9, 1772–1781. [Google Scholar]
- Yang, S.H.; Chen, P.Q.; Chen, Y.F.; Lin, F.H. An in-vitro study on regeneration of human nucleus pulposus by using gelatin/chondroitin-6-sulfate/hyaluronan tri-copolymer scaffold. Artif. Organ. 2005, 29, 806–814. [Google Scholar]
- Fujita, N.; Miyamoto, T.; Imai, J.I.; Hosogane, N.; Suzuki, T.; Yagi, M.; Morita, K.; Ninomiya, K.; Miyamoto, K.; Takaishi, H.; Matsumoto, M.; Morioka, H.; Yabe, H.; Chiba, K.; Watanabe, S.; Toyama, Y.; Suda, T. CD24 is expressed specifically in the nucleus pulposus of intervertebral discs. Biochem. Biophys. Res. Commun. 2005, 338, 1890–1896. [Google Scholar]
- Lee, C.; Sakai, D.; Nakai, T.; Toyama, K.; Mochida, J.; Alini, M.; Grad, S. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur. Spine J. 2007, 16, 2174–2185. [Google Scholar]
- Sakai, D.; Nakai, T.; Mochida, J.; Alini, M.; Grad, S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine (Phila Pa 1976) 2009, 34, 1448–1456. [Google Scholar]
- Rutges, J.; Creemers, L.B.; Dhert, W.; Milz, S.; Sakai, D.; Mochida, J.; Alini, M.; Grad, S. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: Potential associations with aging and degeneration. Osteoarthritis Cartilage 2009, 18, 416–423. [Google Scholar]
- Minogue, B.; Richardson, S.; Zeef, L.; Freemont, A.; Hoyland, J. Transcriptional profiling of bovine intervertebral disc cells: Implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthristis Res. Ther. 2010, 12, R22–R29. [Google Scholar]
- Minogue, B.M.; Richardson, S.M.; Zeef, L.A.H.; Freemont, A.J.; Hoyland, J.A. Characterization of the human nucleus pulposus cell phenotype and evaluation of novel marker gene expression to define adult stem cell differentiation. Arthritis Rheum. 2010, 62, 3695–3705. [Google Scholar]
- Schulze-Tanzil, G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anatomy 2009, 191, 325–338. [Google Scholar]
- Mwale, F.; Roughley, P.; Antoniou, J. Distinction between the extracellular matrix of the nucleus pulposus and hyaline cartilage: A requisite for tissue engineering of intervertebral disc. Eur. Cell Mater. 2004, 8, 58–63. [Google Scholar]
- Kwan, K.M.; Pang, M.K.M.; Zhou, S.; Cowan, S.K.; Kong, R.Y.C.; Pfordte, T.; Olsen, B.R.; Sillence, D.O.; Tam, P.P.L.; Cheah, K.S.E. Abnormal compartmentalization of cartilage matrix components in mice lacking Collagen X: Implications for function. J. Cell Biol. 1997, 136, 459–471. [Google Scholar]
- Ogata, Y. Bone sialoprotein and its transcriptional regulatory mechanism. J. Period. Res. 2008, 43, 127–135. [Google Scholar]
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol. 2008, 9. [Google Scholar] [CrossRef]
- McGaughran, J.M.; Oates, A.; Donnai, D.; Read, A.P.; Tassabehji, M. Mutations in PAX1 may be associated with Klippel-Feil syndrome. Eur. J. Human Genet. 2003, 11, 468–474. [Google Scholar]
- Woods, A.; Wang, G.; Beier, F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J. Cell Physiol. 2007, 213, 1–8. [Google Scholar]
- Technau, U.; Scholz, C.B. Origin and evolution of endoderm and mesoderm. Int. J. Dev. Biol. 2003, 47, 531–539. [Google Scholar]
- Aladin, D.M.; Cheung, K.M.; Ngan, A.H.; Chan, D.; Leung, V.Y.; Lim, C.T.; Luk, K.D.; Lu, W.W. Nanostructure of collagen fibrils in human nucleus pulposus and its correlation with macroscale tissue mechanics. J. Orthop. Res. 2010, 28, 497–502. [Google Scholar]
- Gorensek, M.; Jaksimovic, C.; Kregar-Velikonja, N.; Gorensek, M.; Knezevic, M.; Jeras, M.; Pavlovcic, V.; Cor, A. Nucleus pulposus repair with cultured autologous elastic cartilage derived chondrocytes. Cell Mol. Biol. Lett. 2004, 9, 363–373. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salzig, D.; Schmiermund, A.; Gebauer, E.; Fuchsbauer, H.-L.; Czermak, P. Influence of Porcine Intervertebral Disc Matrix on Stem Cell Differentiation. J. Funct. Biomater. 2011, 2, 155-172. https://doi.org/10.3390/jfb2030155
Salzig D, Schmiermund A, Gebauer E, Fuchsbauer H-L, Czermak P. Influence of Porcine Intervertebral Disc Matrix on Stem Cell Differentiation. Journal of Functional Biomaterials. 2011; 2(3):155-172. https://doi.org/10.3390/jfb2030155
Chicago/Turabian StyleSalzig, Denise, Alexandra Schmiermund, Elke Gebauer, Hans-Lothar Fuchsbauer, and Peter Czermak. 2011. "Influence of Porcine Intervertebral Disc Matrix on Stem Cell Differentiation" Journal of Functional Biomaterials 2, no. 3: 155-172. https://doi.org/10.3390/jfb2030155




