Phytochemical Analysis and Antioxidant Properties in Colored Tiggiano Carrots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Detection of Phenolic Acids
2.3. Total Content of Anthocyanins
2.4. Sugar and Organic Acid Extraction and Quantification
2.5. Carotenoid Content
2.6. Determination of Antioxidant Activity
2.7. Statistical Analysis
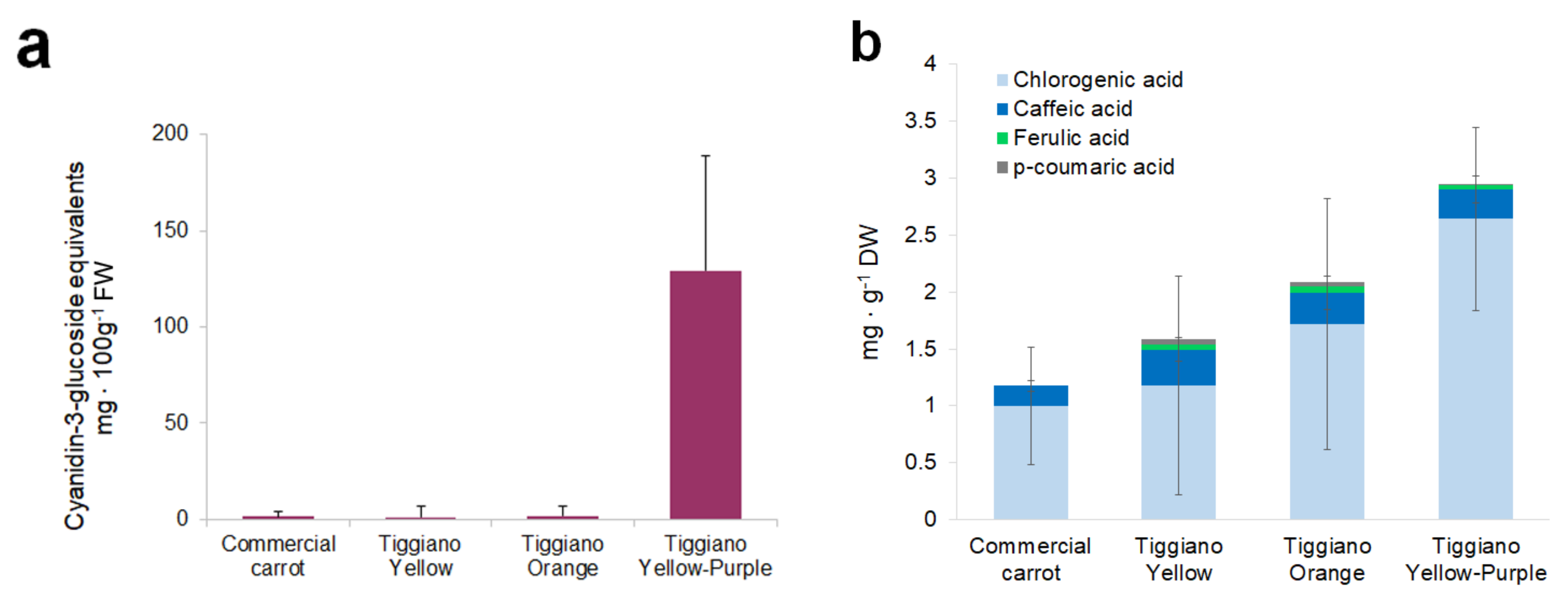
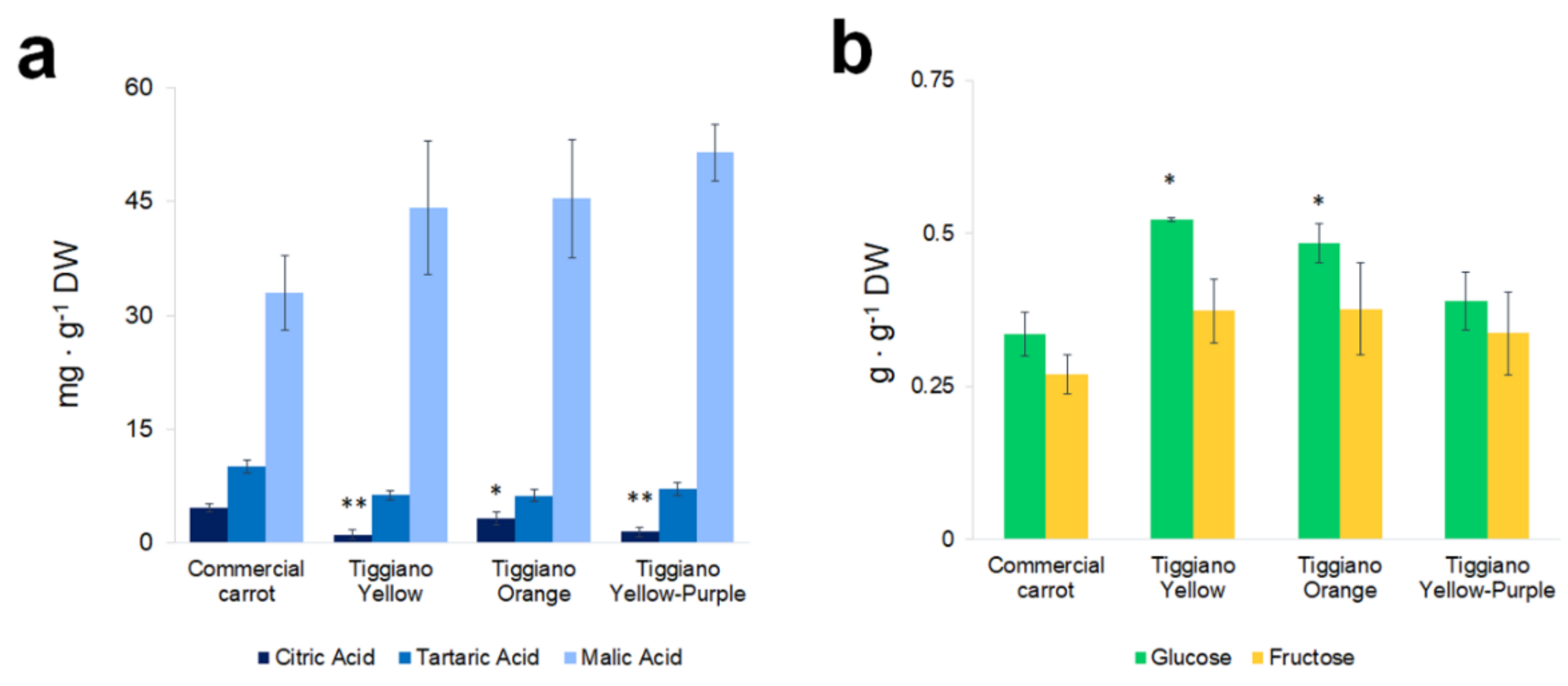
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Silva Dias, J.C. Nutritional and health benefits of carrots and their seed extracts. Food Nutr. Sci. 2014, 5, 2147–2156. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistic Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- ISTAT. Italian National Institute of Statistics, Rome. 2017. Available online: http://agri.istat.it/ (accessed on 1 February 2018).
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Simon, P.W.; Tanumihardjo, S.A. Antioxidant phytochemicals and antioxidant capacity of biofortified carrots (Daucus carota L.) of various colors. J. Agric. Food Chem. 2009, 57, 4142. [Google Scholar] [CrossRef] [PubMed]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Comp. Rev. Food Sci. Food Saf. 2010, 9, 223. [Google Scholar] [CrossRef]
- Simon, P.W. Domestication, historical development, and modern breeding of carrot. Plant Breed. Rev. 2000, 19, 157–190. [Google Scholar]
- Cefola, M.; Pace, B.; Renna, M.; Santamaria, P.; Signore, A.; Serio, F. Compositional analysis and antioxidant profile of yellow, orange and purple Polignano carrots. Ital. J. Food Sci. 2012, 24, 284–291. [Google Scholar]
- Renna, M.; Serio, F.; Signore, A.; Santamaria, P. The yellow-purple Polignano carrot (Daucus carota L.): A multicolored landrace from the Puglia region (Southern Italy) at risk of genetic erosion. Gen. Res. Crop Evol. 2014, 61, 1611–1619. [Google Scholar] [CrossRef]
- Elia, A.; Santamaria, P. Biodiversity in vegetable crops, a heritage to save: The case of Puglia region. Ital. J. Agron. 2013, 8, 4. [Google Scholar] [CrossRef]
- Signore, A.; Renna, M.; D’Imperio, M.; Serio, F.; Santamaria, P. Preliminary evidences of biofortification with iodine of “Carota di Polignano”, an Italian carrot landrace. Front. Plant Sci. 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Accogli, R.; Marchiori, S. Verifica agronomica di Daucus carota L. varietà locale carota de Santu Pati. In Progetto Co.Al.Ta. 1 “Analisi e Valutazione di Ordinamenti Produttivi Alternativi Nelle Aree a Riconversione del Tabacco” Risultati del 1° Anno di Attività; A Cura dell’Istituto INEA: Rome, Italy, 2006. [Google Scholar]
- Cuevas Montilla, E.; Rodriguez Arzaba, M.; Hillebrand, S.; Winterhalter, P. Anthocyanin composition of black carrot (Daucus carota ssp. sativus var. atrorubens Alef.) cultivars Antonina, Beta Sweet, Deep Purple, and Purple Haze. J. Agric. Food Chem. 2011, 59, 3385–3390. [Google Scholar]
- Yildiz, M.; Willis, D.K; Cavagnaro, P.F.; Iorizzo, M.; Abak, K.; Simon, P.W. Expression and mapping of anthocyanin biosynthesis genes in carrot. Theor. Appl. Genet. 2013, 126, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-S.; Huang, Y.; Wang, F.; Song, X.; Wang, G.-L.; Xiong, A.-S. Transcript profiling of structural genes involved in cyanidin-based anthocyanin biosynthesis between purple and non-purple carrot (Daucus carota L.) cultivars reveals distinct patterns. BMC Plant Biol. 2014, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-S.; Feng, K.; Que, F.; Wang, F.; Xiong, A.-S. A MYB transcription factor, DcMYB6, is involved in regulating anthocyanin biosynthesis in purple carrot taproots. Sci. Rep. 2017, 7, 45324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Peterek, S.; Schijlen, E.G.; Hall, R.D.; Bovy, A.G.; Luo, J.; Martin, C. Enrichment of tomato fruit with health-promoting anthocyanins by expression of selected transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, M.L.; Butelli, E.; Martin, C.; Carding, S.R. Flavonoids from engineered tomatoes inhibit gut barrier pro-inflammatory cytokines and chemokines, via SAPK/JNK and p38 MAPK pathways. Front. Nutr. 2017, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Galleggiante, V.; De Santis, S.; Cavalcanti, E.; Scarano, A.; De Benedictis, M.; Serino, G.; Caruso, M.L.; Mastronardi, M.; Pinto, A.; Campiglia, P.; et al. Dendritic cells modulate iron homeostasis and inflammatory abilities following quercetin exposure. Curr. Pharm. Des. 2017, 23, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Santino, A.; Scarano, A.; De Santis, S.; De Benedictis, M.; Giovinazzo, G.; Chieppa, M. Gut microbiota modulation and anti-inflammatory properties of dietary polyphenols in IBD: New and consolidated perspectives. Curr. Pharm. Des. 2017, 23, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Butelli, E.; De Santis, S.; Cavalcanti, E.; Hill, L.; De Angelis, M.; Giovinazzo, G.; Chieppa, M.; Martin, C.; Santino, A. Combined dietary anthocyanins, flavonols, and stilbenoids alleviate inflammatory bowel disease symptoms in mice. Front. Nutr. 2018, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOC Int. 2005, 88, 1269–1278. [Google Scholar]
- Gerardi, C.; Tommasi, N.; Albano, C.; Pinthus, E.; Rescio, L.; Blando, F.; Mita, G. Prunus mahaleb L. fruit extracts: A novel source for natural pigments. Eur. Food Res. Technol. 2015, 241, 683–695. [Google Scholar] [CrossRef]
- Koch, T.; Goldman, I. A one-pass semi-quantitative method for extraction and analysis of carotenoids and tocopherols in carrot. HortScience 2004, 39, 1260–1261. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2012, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of sugars, organic acids, and total phenolics in 25 wild or cultivated berry species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Yun, S.K.; Jun, J.H.; Yoon, I.K.; Nam, E.Y.; Kwon, J.H. Assessment of organic acid and sugar composition in apricot, plumcot, plum, and peach during fruit development. J. Appl. Bot. Food. Qual. 2014, 87, 24–29. [Google Scholar]
- Bajec, M.R.; Pickering, G.J. Astringency: Mechanisms and Perception. Crit. Rev. Food Sci. Nut. 2008, 48, 858–875. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, A.; Gerardi, C.; D’Amico, L.; Accogli, R.; Santino, A. Phytochemical Analysis and Antioxidant Properties in Colored Tiggiano Carrots. Agriculture 2018, 8, 102. https://doi.org/10.3390/agriculture8070102
Scarano A, Gerardi C, D’Amico L, Accogli R, Santino A. Phytochemical Analysis and Antioxidant Properties in Colored Tiggiano Carrots. Agriculture. 2018; 8(7):102. https://doi.org/10.3390/agriculture8070102
Chicago/Turabian StyleScarano, Aurelia, Carmela Gerardi, Leone D’Amico, Rita Accogli, and Angelo Santino. 2018. "Phytochemical Analysis and Antioxidant Properties in Colored Tiggiano Carrots" Agriculture 8, no. 7: 102. https://doi.org/10.3390/agriculture8070102
APA StyleScarano, A., Gerardi, C., D’Amico, L., Accogli, R., & Santino, A. (2018). Phytochemical Analysis and Antioxidant Properties in Colored Tiggiano Carrots. Agriculture, 8(7), 102. https://doi.org/10.3390/agriculture8070102






