Assessment of Photosynthetic Pigment and Water Contents in Intact Sunflower Plants from Spectral Indices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Greenhouse and Growth Chamber for Sunflower Cultivation
2.2. Sunflower Measurements under Drought Conditions
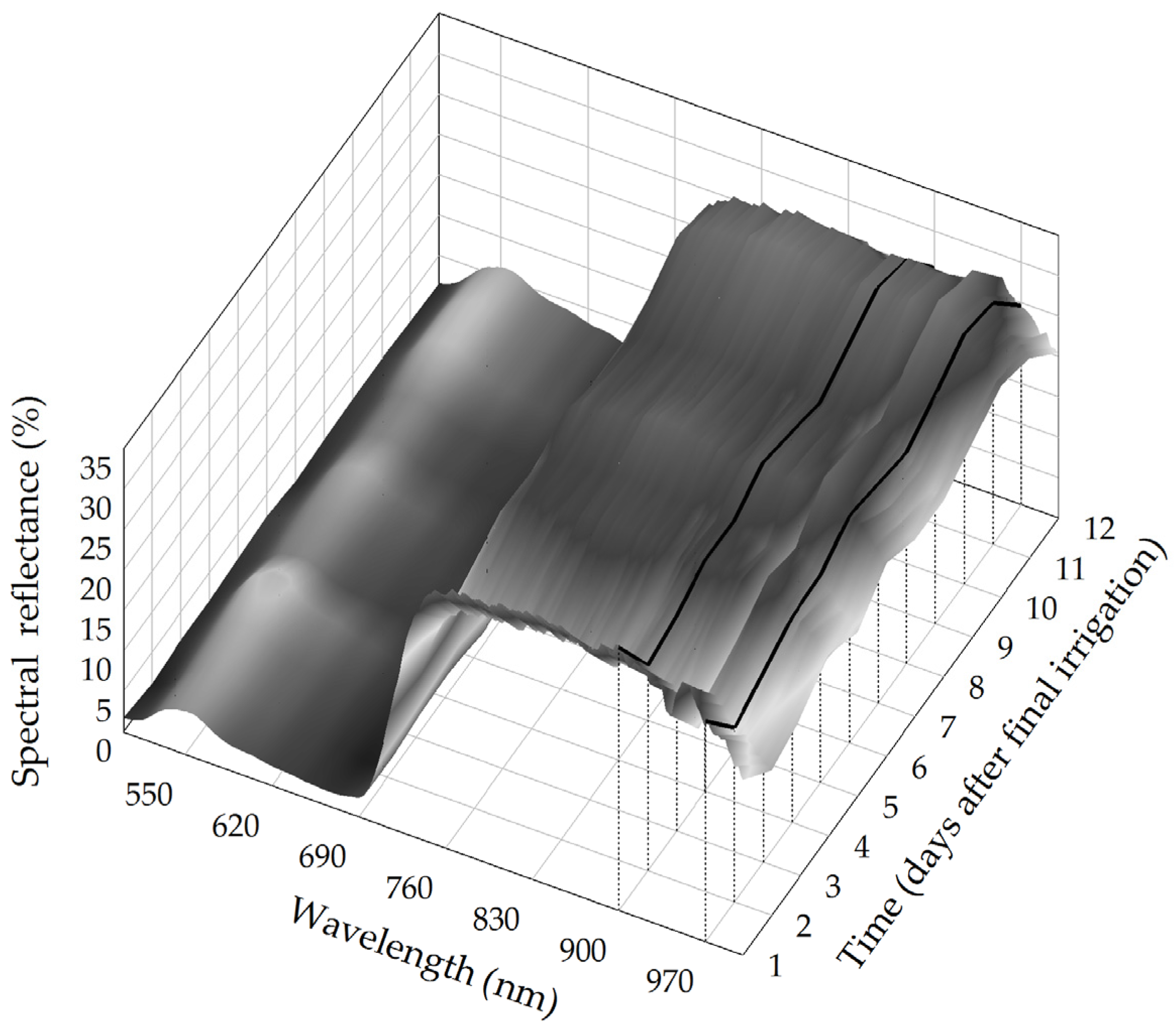
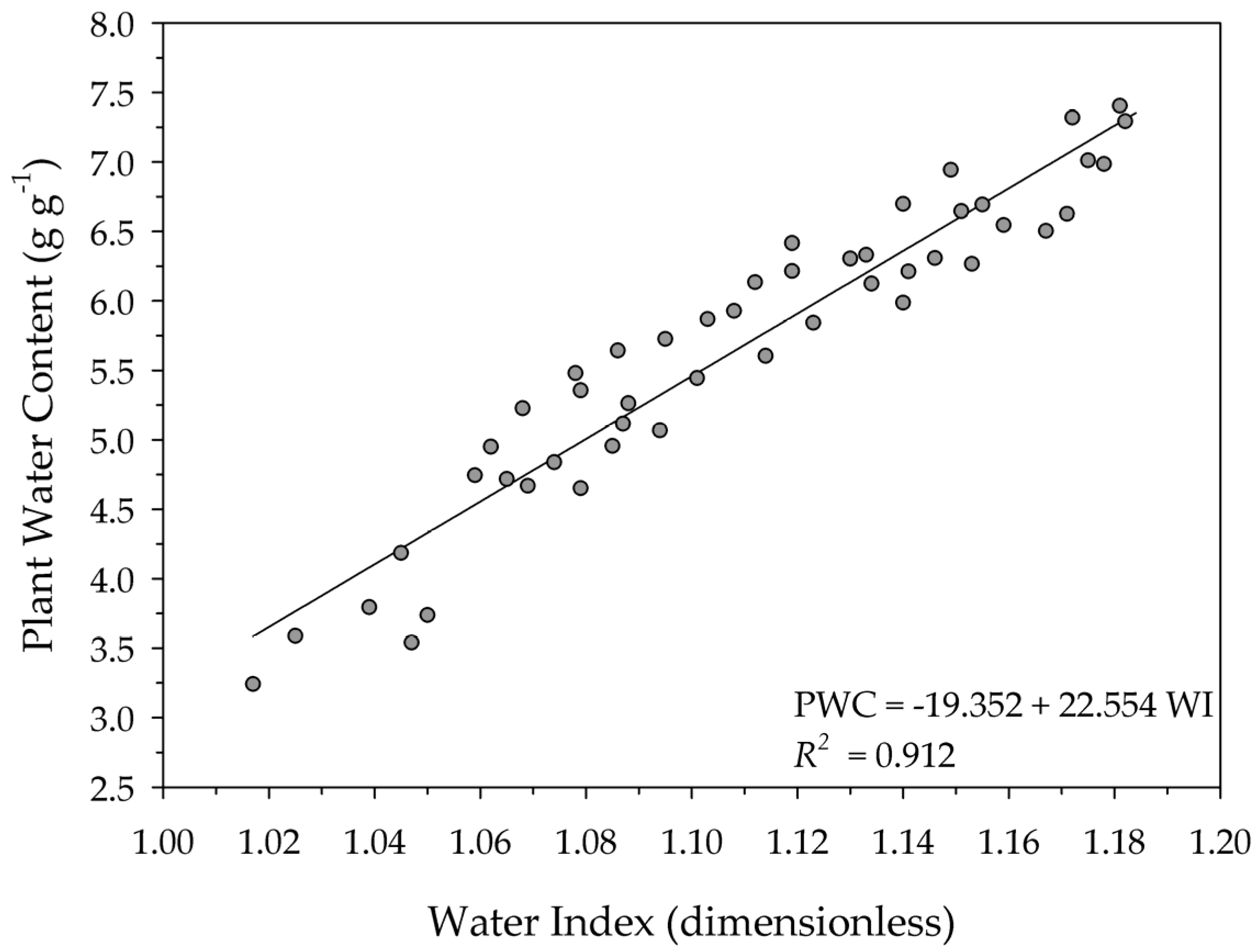
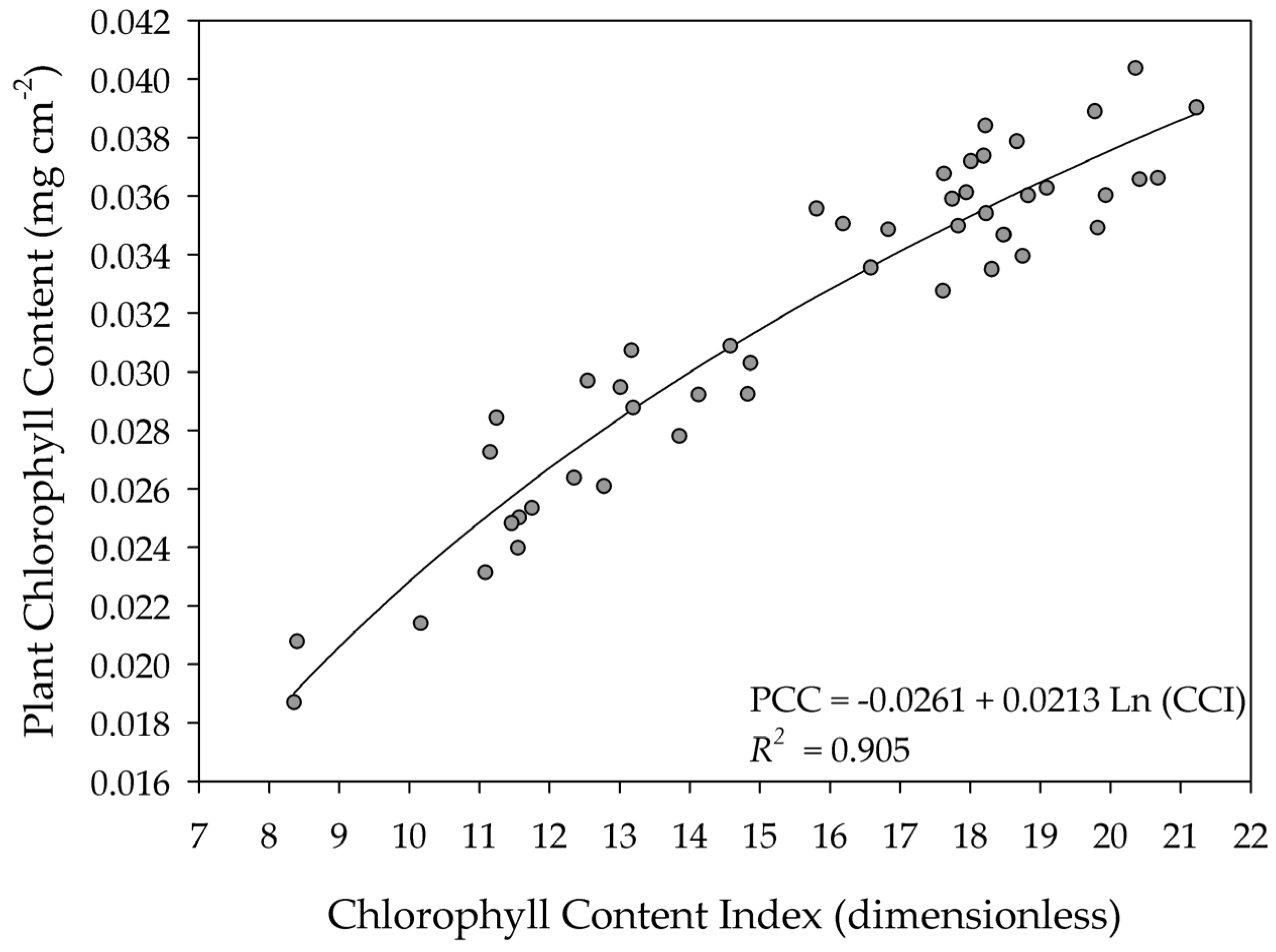
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ullah, A.; Skidmore, A.K.; Groen, T.A.; Schlerf, M. Evaluation of three proposed indices for the retrieval of leaf water content from the mid-wave infrared (2–6 μm) spectra. Agric. For. Meteorol. 2013, 171–172, 65–71. [Google Scholar] [CrossRef]
- Silla, F.; González-Gil, A.; González-Molina, M.E.; Mediavilla, S.; Escudero, A. Estimation of chlorophyll in Quercus leaves using a portable chlorophyll meter: Effects of species and leaf age. Ann. For. Sci. 2010, 67, 1–7. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Estimating chlorophyll content from hyperspectral vegetation indices: Modeling and validation. Agric. For. Meteorol. 2008, 148, 1230–1241. [Google Scholar] [CrossRef]
- Hawkins, T.S.; Gardiner, E.S.; Comer, G.S. Modeling the relationship between extractable chlorophyll and SPAD-502 readings for endangered plant species research. J. Nat. Conserv. 2009, 17, 123–127. [Google Scholar] [CrossRef]
- Ghobadi, M.; Taherabadi, S.; Ghobadi, M.; Mohammadi, G.; Jalali-Honarmand, S. Antioxidant capacity, photosynthetic characteristics and water relations of sunflower (Helianthus annuus L.) cultivars in response to drought stress. Ind. Crops Prod. 2013, 50, 29–38. [Google Scholar] [CrossRef]
- Levizou, E.; Manetas, Y. Photosynthetic pigment contents in twigs of 24 woody species assessed by in vivo reflectance spectroscopy indicate low chlorophyll levels but high carotenoid/chlorophyll ratios. Environ. Exp. Bot. 2007, 59, 293–298. [Google Scholar] [CrossRef]
- Rodríguez-Pérez, J.R.; Riaño, D.; Carlisle, E.; Ustin, S.; Smart, D.R. Evaluation of hyperspectral reflectance indexes to detect grapevine water status in vineyards. Am. J. Enol. Vitic. 2007, 58, 302–317. [Google Scholar]
- Xiaobo, Z.; Jiyong, S.; Limin, H.; Jiewen, Z.; Hanpin, M.; Zhenwei, C.; Yanxiao, L.; Holmes, M. In vivo noninvasive detection of chlorophyll distribution in cucumber (Cucumis sativus) leaves by indices based on hyperspectral imaging. Anal. Chim. Acta 2011, 706, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yi, Q.; Bao, A.; Wang, Q.; Zhao, J. Estimation of leaf water content in cotton by means of hyperspectral indices. Comput. Electron. Agric. 2013, 90, 144–151. [Google Scholar] [CrossRef]
- Sato, T.; Maw, A.A.; Katsuta, M. Nondestructive near-infrared reflectance spectroscopy of sesame (Sesamum indicum L.) components by single seed analysis. Plant Prod. Sci. 2006, 9, 161–164. [Google Scholar] [CrossRef]
- Vaiphasa, C.; Skidmore, A.K.; de Boer, W.F.; Vaiphasa, T. A hyperspectral band selector for plant species discrimination. ISPRS J. Photogramm. Remote Sens. 2007, 62, 225–235. [Google Scholar] [CrossRef]
- Min, M.; Lee, W.S.; Burks, T.F.; Jordan, J.D.; Schumann, A.W.; Schueller, J.K.; Xie, H. Design of a hyperspectral nitrogen sensing system for orange leaves. Comput. Electron. Agric. 2008, 63, 215–226. [Google Scholar] [CrossRef]
- Steidle Neto, A.J.; Grossi, J.A.S.; Lopes, D.C.; Anastácio, E.A. Potential of spectral reflectance as postharvest classification tool for flower development of calla lily (Zantedeschia aethiopica (L.) Spreng.). Chil. J. Agric. Res. 2009, 69, 588–592. [Google Scholar] [CrossRef]
- Cañasveras, J.C.; Barrón, V.; Del Campillo, M.C.; Rossel, R.A.V. Reflectance spectroscopy: A tool for predicting soil properties related to the incidence of Fe chlorosis. Span. J. Agric. Res. 2012, 10, 1133–1142. [Google Scholar] [CrossRef]
- Huang, W.; Li, J.; Wang, Q.; Chen, L. Development of a multispectral imaging system for online detection of bruises on apples. J. Food Eng. 2015, 146, 62–71. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Kӧppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar]
- Steidle Neto, A.J.; Zolnier, S.; Marouelli, W.A. Transpiration of tomato crop cultivated in substrate and its influence on the leaching fraction and electrical conductivity of the drained nutrient solution. Acta Sci. Agron. 2010, 32, 721–727. [Google Scholar]
- Xing, J.; de Baerdemaeker, J. Bruise detection on “Jonagold” apples using hyperspectral imaging. Postharvest Biol. Technol. 2005, 37, 152–162. [Google Scholar] [CrossRef]
- Claudio, H.C.; Cheng, Y.; Fuentes, D.A.; Gamon, J.A.; Luo, H.; Oechel, W.; Qiu, H.; Rahman, A.F.; Sims, D.A. Monitoring drought effects on vegetation water content and fluxes in chaparral with the 970 nm water band index. Remote Sen. Environ. 2006, 103, 304–311. [Google Scholar] [CrossRef]
- Peñuelas, J.; Piñol, J.; Ogaya, R.; Filella, I. Estimation of plant water content by the reflectance Water Index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Estimation of vegetation water content and photosynthetic tissue area from spectral reflectance: A comparison of indices based on liquid water and chlorophyll absorption features. Remote Sens. Environ. 2003, 84, 526–537. [Google Scholar] [CrossRef]
- Serrano, L.; González-Flor, C.; Gorchs, G. Assessing vineyard water status using the reflectance based water index. Agric. Ecosyst. Environ. 2010, 139, 490–499. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ponzoni, F.J.; Shimabukuro, Y.E.; Kuplich, T.M. Sensoriamento Remoto da Vegetação, 2nd ed.; Oficina de Textos: São Paulo, Brazil, 2012; pp. 7–40. [Google Scholar]
- Peñuelas, J.; Inoue, Y. Reflectance indices indicative of changes in water and pigment contents of peanut and wheat leaves. Photosynthetica 1999, 36, 355–360. [Google Scholar] [CrossRef]
- Cheng, T.; Rivard, B.; Sánchez-Azofeifa, A.G. Spectroscopic determination of leaf water content using continuous wavelet analysis. Remote Sens. Environ. 2011, 115, 659–670. [Google Scholar] [CrossRef]
- Richardson, A.D.; Duigan, S.P.; Berlyn, G.P. An evaluation of noninvasive methods to estimate foliar chlorophyll content. New Phytol. 2002, 153, 185–194. [Google Scholar] [CrossRef]
- Jifon, J.L.; Syvertsen, J.P.; Whaley, E. Growth environment and leaf anatomy affect nondestructive estimates of chlorophyll and nitrogen in Citrus sp. leaves. J. Am. Soc. Hortic. Sci. 2005, 130, 152–158. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Parry, C.; Blonquist, J.M., Jr.; Bugbee, B. In situ measurement of leaf chlorophyll concentration: Analysis of the optical/absolute relationship. Plant Cell Environ. 2014, 37, 2508–2520. [Google Scholar] [CrossRef] [PubMed]
- De Maria, S.; Puschenreiter, M.; Rivelli, A.R. Cadmium accumulation and physiological response of sunflower plants to Cd during the vegetative growing cycle. Plant Soil Environ. 2013, 59, 254–261. [Google Scholar]
- Moschen, S.; Luoni, S.B.; Di Rienzo, J.A.; Del Pilar Caro, M.; Tohge, T.; Watanabe, M.; Hollmann, J.; González, S.; Rivarola, M.; García-García, F.; et al. Integrating transcriptomic and metabolomic analysis to understand natural leaf senescence in sunflower. Plant Biotechnol. J. 2016, 14, 719–734. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, A.K.; Perkins, T.D. Evaluation of a portable chlorophyll meter to estimate chlorophyll and nitrogen contents in sugar maple (Acer saccharum Marsh.) leaves. For. Ecol. Manag. 2004, 200, 113–117. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Ng, Y.F.; Tan, S.N.; Chew, A.Y.L. Effect of fertilizer application on photosynthesis and oil yield of Jatropha curcas L. Photosynthetica 2010, 48, 208–218. [Google Scholar] [CrossRef]
- Dalil, B.; Ghassemi-Golezani, K.; Moghaddam, M.; Raey, Y. Effects of seed viability and water supply on leaf chlorophyll content and grain yield of maize (Zea mays). J. Food Agric. Environ. 2010, 8, 399–402. [Google Scholar]
- Kiani, S.P.; Maury, P.; Sarrafi, A.; Grieu, P. QTL analysis of chlorophyll fluorescence parameters in sunflower (Helianthus annuus L.) under well-watered and water-stressed conditions. Plant Sci. 2008, 175, 565–573. [Google Scholar] [CrossRef]
- Bannari, A.; Khurshid, K.S.; Staenz, K.; Schwarz, J.W. A comparison of hyperspectral chlorophyll indices for wheat crop chlorophyll content estimation using laboratory reflectance measurements. IEEE Trans. Geosci. Remote 2007, 45, 3063–3074. [Google Scholar] [CrossRef]
| Water Index Range | Vegetation | Reference |
|---|---|---|
| 0.88–1.15 | Trees, shrubs, and grasses | Peñuelas et al. [20] |
| 0.99–1.03 | Wheat and peanut | Peñuelas and Inoue [25] |
| 0.96–1.23 | Annual crops, vines, trees, and shrubs | Sims and Gamon [21] |
| 0.93–1.10 | Semiarid shrubland ecosystem (chaparral) | Claudio et al. [19] |
| 0.97–1.03 | Grapevine | Rodríguez-Pérez et al. [7] |
| 0.96–1.04 | Species of tropical forests | Cheng et al. [26] |
| 1.01–1.18 | Sunflower | In this study |
| Chlorophyll Content Index Range | Vegetation | Reference |
|---|---|---|
| 1.0–23.0 | Paper birch | Richardson et al. [27] |
| 2.4–23.7 | Sugar maple | Van den Berg and Perkins [33] |
| 3.0–34.0 | Lemon | Jifon et al. [28] |
| 7.3–38.0 | Physic nut | Yong et al. [34] |
| 5.0–27.5 | Maize | Dalil et al. [35] |
| 1.0–36.0 | Kiwi | Cerovic et al. [29] |
| 4.4–25.5 | Sunflower | In this study |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steidle Neto, A.J.; Lopes, D.D.C.; Borges Júnior, J.C.F. Assessment of Photosynthetic Pigment and Water Contents in Intact Sunflower Plants from Spectral Indices. Agriculture 2017, 7, 8. https://doi.org/10.3390/agriculture7020008
Steidle Neto AJ, Lopes DDC, Borges Júnior JCF. Assessment of Photosynthetic Pigment and Water Contents in Intact Sunflower Plants from Spectral Indices. Agriculture. 2017; 7(2):8. https://doi.org/10.3390/agriculture7020008
Chicago/Turabian StyleSteidle Neto, Antonio José, Daniela De Carvalho Lopes, and João Carlos Ferreira Borges Júnior. 2017. "Assessment of Photosynthetic Pigment and Water Contents in Intact Sunflower Plants from Spectral Indices" Agriculture 7, no. 2: 8. https://doi.org/10.3390/agriculture7020008
APA StyleSteidle Neto, A. J., Lopes, D. D. C., & Borges Júnior, J. C. F. (2017). Assessment of Photosynthetic Pigment and Water Contents in Intact Sunflower Plants from Spectral Indices. Agriculture, 7(2), 8. https://doi.org/10.3390/agriculture7020008





