Efficacy of Chicken Litter and Wood Biochars and Their Activated Counterparts in Heavy Metal Clean up from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
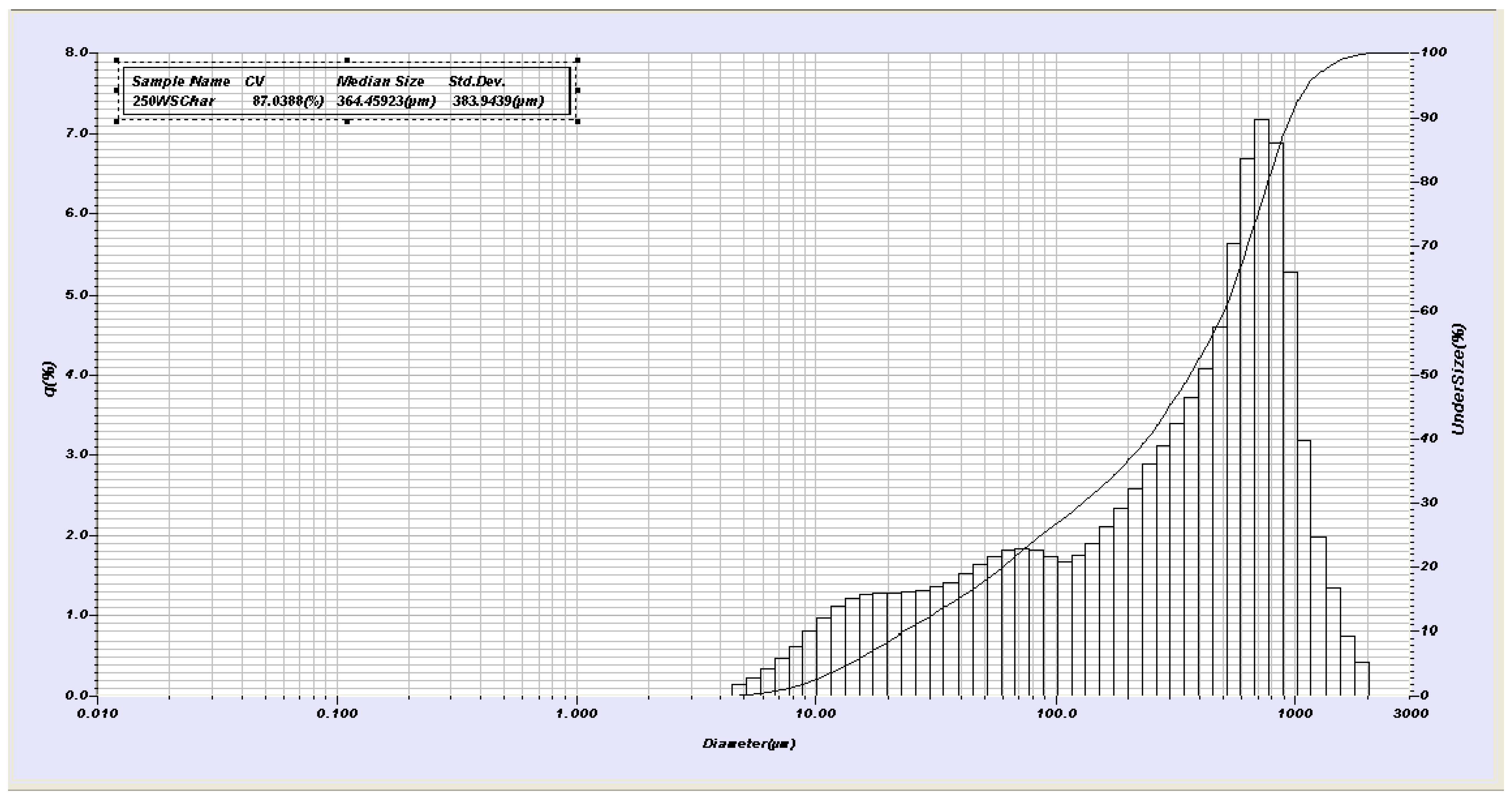
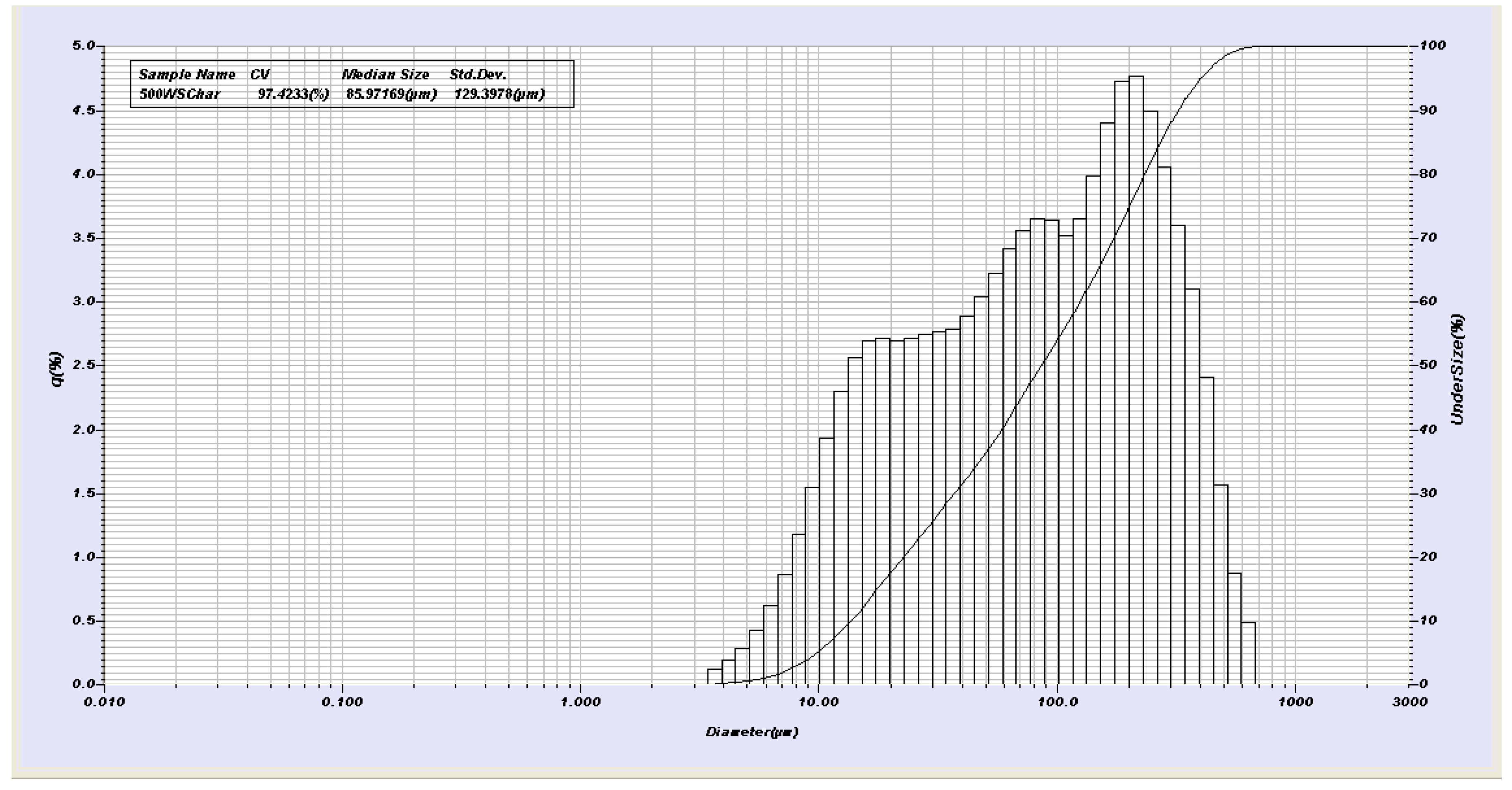
2.2. Physical and Chemical Measurements, Yield, Surface Area, pH
2.3. Ultimate, Elemental and Proximate Analysis
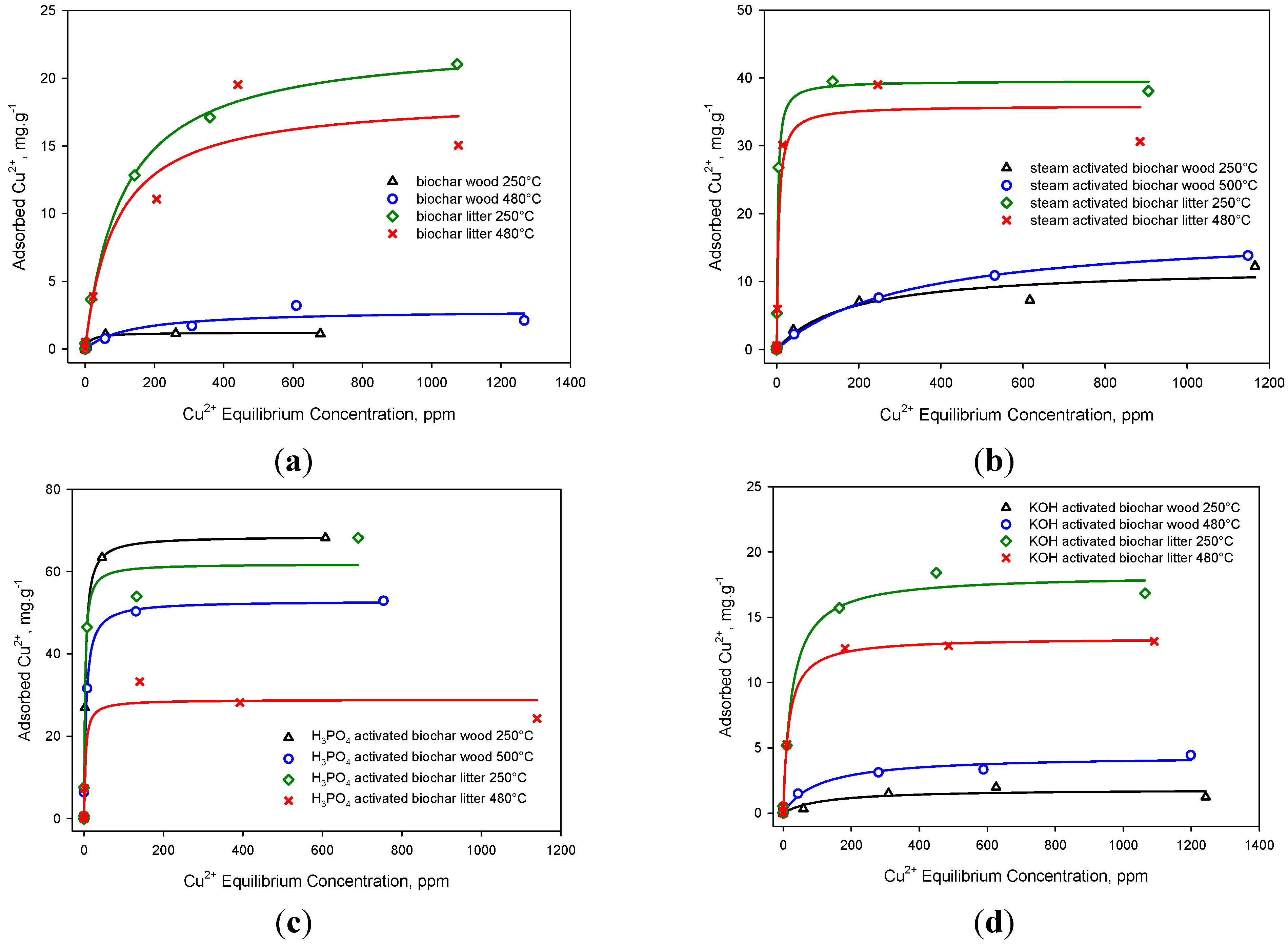
2.4. Copper Adsorption Isotherms
2.5. Arsenic Adsorption
2.6. Total Titratable Surface Charge
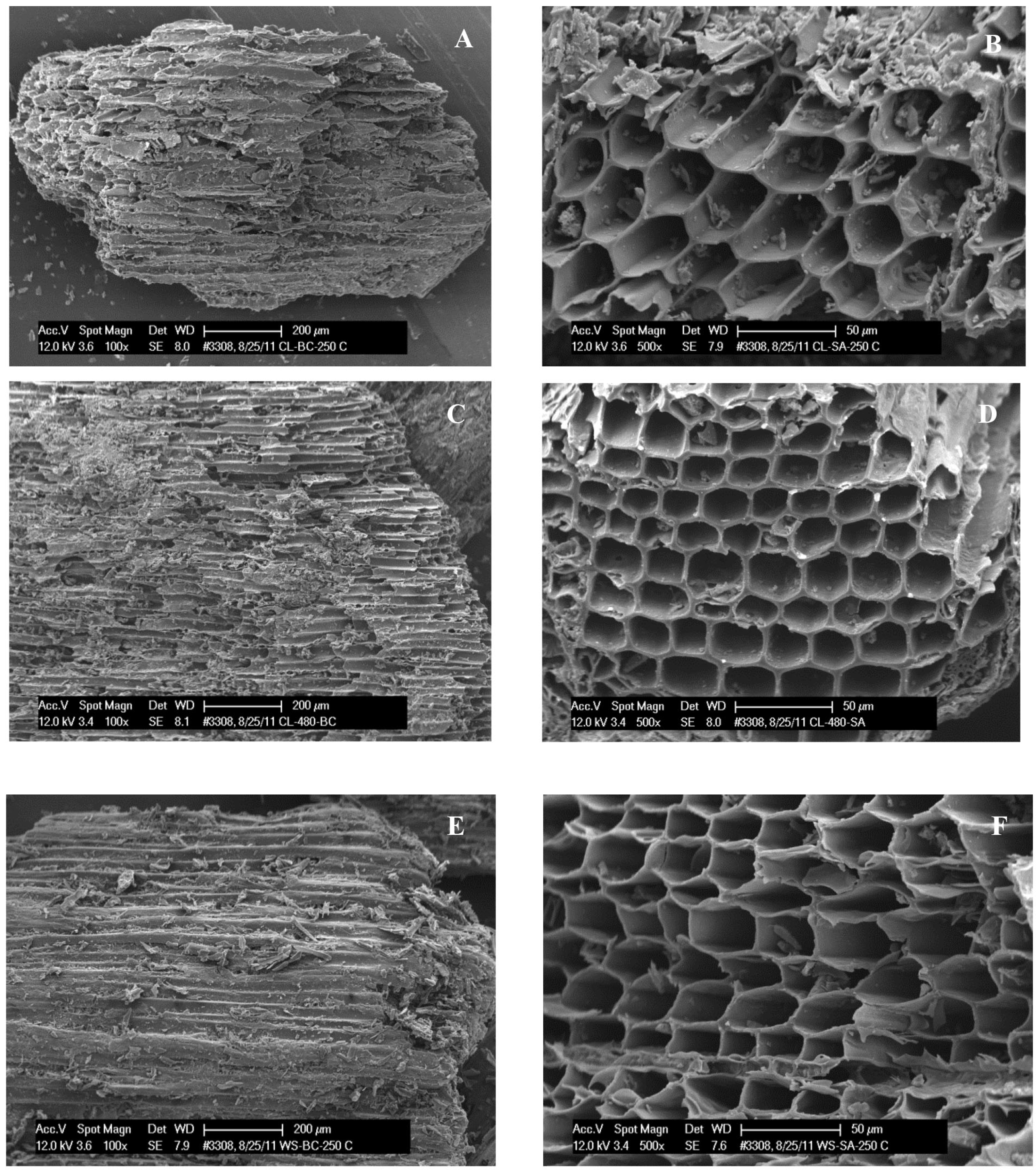
2.7. Scanning Electron Microscopy
2.8. Experimental Design and Statistical Analysis
3. Results and Discussion

| Effect | DF (2) | F-value (Pr) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B.E.T. | Ash | SC | VOC | Carbon | Oxygen | Hydrogen | Nitrogen | Ads Cap | ||
| Feedstock | 1 | 3.1 (0.177) | 239 (0.001) | 3.9 (0.188) | 11.7 (0.042) | 201 (0.001) | 0.7 (0.465) | 16.6 (0.027) | 54.9 (0.005) | 104.3 (<0.0001) |
| Temperature | 1 | 5.9 (0.098) | 27.5 (0.013) | 39.3 (0.025) | 77.2 (0.003) | 4.8 (0.117) | 35.1 (0.010) | 19.6 (0.021) | 0.08 (0.801) | 44.8 (<0.0001) |
| Feedstock × Temperature | 1 | 4.5 (0.123) | 17.4 (0.025) | 1.8 (0.309) | 0.3 (0.612) | 3.9 (0.144) | 0.1 (0.766) | 1.2 (0.352) | 0.7 (0.472) | 28.3 (<0.0001) |
| Activation | 3 | 70.2 (0.003) | 11.0 (0.040) | 118 (0.008) | 103 (0.002) | 7.4 (0.067) | 40.3 (0.006) | 57.8 (0.004) | 15.9 (0.024) | 504.1 (<0.0001) |
| Feedstock × Activation | 3 | 0.72 (0.603) | 9.4 (0.049) | 7.7 (0.115) | 1.8 (0.323) | 11.6 (0.037) | 1.2 (0.430) | 0.6 (0.654) | 15.9 (0.024) | 83.3 (<0.0001) |
| Temperature × Activation | 3 | 11.0 (0.040) | 1.4 (0.401) | 8.2 (0.111) | 36.7 (0.007) | 2.3 (0.251) | 9.7 (0.047) | 8.4 (0.057) | 0.6 (0.676) | 40.8 (<0.0001) |
| Feedstock Source | Sample | Y % db | B.E.T. m2/g | pH | SC meq H+/g | Q0 Cu2+ (mg/g) | b (L/mg) | r2 | P |
|---|---|---|---|---|---|---|---|---|---|
| WS 250 °C | BC | - | 0.03 | 5.3 | 1.57 | 1.22 | 0.0656 | 0.98 | <0.0001 |
| SA | 43.0 | 573 | 8.8 | 0.00 | 12.2 | 0.0058 | 0.94 | 0.0004 | |
| AA | 17.9 | 851 | 2.5 | 3.00 | 68.6 | 0.2689 | 0.99 | <0.0001 | |
| BA | 40.2 | 27 | 6.7 | 0.36 | 1.84 | 0.0085 | 0.84 | 0.0039 | |
| WS 500 °C | BC | - | 0.00 | 5.6 | 0.37 | 2.90 | 0.0074 | 0.89 | 0.0016 |
| SA | 76.7 | 511 | 8.1 | 0.00 | 17.7 | 0.0031 | 1.00 | <0.0001 | |
| AA | 24.8 | 538 | 2.2 | 2.11 | 52.8 | 0.1855 | 0.99 | <0.0001 | |
| BA | 47.8 | 360 | 6.7 | 0.04 | 4.41 | 0.010 | 0.98 | <0.0001 | |
| CL 250 °C | BC | - | 0.45 | 6.2 | 1.28 | 22.8 | 0.0092 | 1.00 | <0.0001 |
| SA | 31.8 | 592 | 10.5 | 0.06 | 39.5 | 0.3711 | 0.99 | <0.0001 | |
| AA | 32.0 | 789 | 3.9 | 2.15 | 61.9 | 0.3861 | 0.98 | <0.0001 | |
| BA | 40.2 | 122 | 8.0 | 0.70 | 18.3 | 0.0391 | 0.99 | <0.0001 | |
| CL 480 °C | BC | - | 1.56 | 8.7 | 0.22 | 18.6 | 0.0113 | 0.94 | 0.0004 |
| SA | 68.5 | 420 | 10.9 | 0.00 | 35.9 | 0.2182 | 0.97 | <0.0001 | |
| AA | 42.9 | 320 | 5.6 | 0.77 | 28.8 | 0.2739 | 0.96 | <0.0001 | |
| BA | 53.0 | 118 | 7.7 | 0.00 | 13.4 | 0.0612 | 1.00 | <0.0001 |
| Feedstock | Sample (2) | Moisture, % db | VM % | Fixed C % | Ash % | C % | H % | O % |
|---|---|---|---|---|---|---|---|---|
| Wood chip | 4.60 ± 0.08 | 56.9 ± 0.5 | 40.0 ± 0.5 | 3.08 ± 0.03 | 61.1 ± 1.1 | 5.42 ± 0.32 | 24.3 ± 1.7 | |
| WS 250 °C | BC | 3.96 ± 0.11 | 59.1 ± 0.7 | 38.3 ± 0.4 | 2.6 ± 0.8 | 62.8 ± 0.1 | 7.41 ± 0.62 | 25.8 ± 2.4 |
| BC SA | 1.89 ± 0.07 | 6.0 ± 0.3 | 88.4 ± 1.2 | 5.6 ± 1.2 | 89.6 ± 0.5 | 2.07 ± 0.26 | 3.7 ± 0.4 | |
| BC AA | 3.05 ± 0.10 | 35.8 ± 0.1 | 57.8 ± 2.2 | 6.4 ± 2.2 | 67.1 ± 0.2 | 2.22 ± 0.04 | 27.7 ± 0.6 | |
| BC BA | 3.43 ± 0.19 | 19.9 ± 0.2 | 77.6 ± 0.6 | 2.6 ± 0.7 | 73.5 ± 3.5 | 4.23 ± 0.71 | 8.2 ± 0.8 | |
| WS 500 °C | BC | 7.74 ± 0.03 | 14.2 ± 0.6 | 80.3 ± 0.8 | 5.6 ± 0.3 | 81.9 ± 0.5 | 4.03 ± 0.38 | 6.0 ± 0.8 |
| BC SA | 1.56 ± 0.06 | 6.3 ± 0.6 | 87.1 ± 0.4 | 6.6 ± 0.9 | 90.0 ± 0.1 | 2.39 ± 0.42 | 4.1 ± 0.5 | |
| BC AA | 2.41 ± 0.10 | 30.2 ± 0.4 | 62.8 ± 7.1 | 7.1 ± 1.9 | 70.1 ± 0.3 | 2.32 ± 0.07 | 21.5 ± 1.4 | |
| BC BA | 3.27 ± 0.30 | 12.1 ± 0.2 | 82.0 ± 0.9 | 5.9 ± 0.8 | 80.4 ± 0.4 | 2.67 ± 0.33 | 5.6 ± 0.3 | |
| chicken litter | 41.4 ± 0.9 | 70.6 ± 0.5 | 17.8 ± 0.8 | 11.6 ± 1.0 | 38.3 ± 2.4 | 4.36 ± 0.55 | 32.7 ± 1.2 | |
| CL 250 °C | BC | 3.64 ± 0.04 | 59.5 ± 0.2 | 27.0 ± 0.4 | 13.5 ± 0.2 | 46.3 ± 0.2 | 5.74 ± 0.37 | 24.2 ± 0.9 |
| BC SA | 1.50 ± 0.05 | 7.31 ± 0.49 | 44.7 ± 1.0 | 48.0 ± 1.4 | 46.0 ± 3.7 | 0.83 ± 0.16 | 8.4 ± 0.7 | |
| BC AA | 22.2 ± 0.6 | 32.8 ± 1.3 | 49.6 ± 1.9 | 17.5 ± 3.2 | 52.2 ± 1.1 | 1.75 ± 0.14 | 28.4 ± 2.3 | |
| BC BA | 2.24 ± 0.13 | 22.1 ± 0.3 | 55.7 ± 2.1 | 22.1 ± 2.3 | 62.1 ± 0.3 | 3.04 ± 0.53 | 10.2 ± 0.3 | |
| CL 480 °C | BC | 4.36 ± 0.33 | 21.8 ± 0.5 | 38.1 ± 3.8 | 40.1 ± 3.5 | 51.2 ± 0.6 | 3.51 ± 0.50 | 8.9 ± 0.2 |
| BC SA | 1.07 ± 0.20 | 6.97 ± 0.58 | 37.8 ± 5.3 | 55.2 ± 5.9 | 48.4 ± 0.6 | 0.71 ± 0.13 | 6.2 ± 0.5 | |
| BC AA | 10.1 ± 0.3 | 25.0 ± 0.4 | 38.0 ± 1.8 | 37.0 ± 2.1 | 50.3 ± 4.4 | 1.40 ± 0.20 | 15.3 ± 0.7 | |
| BC BA | 4.80 ± 0.66 | 20.8 ± 0.7 | 40.1 ± 9.9 | 39.2 ± 9.3 | 58.4 ± 0.5 | 2.80 ± 0.09 | 9.4 ± 0.4 |
| Sample | T, °C | P | K | Ca | Mg | Na | Fe | S |
|---|---|---|---|---|---|---|---|---|
| raw woodchips | 0.61 ± 0.03 | 4.91 ± 0.13 | 4.57 ± 0.03 | 2.15 ± 0.15 | 0.49 ± 0.04 | 2.93 ± 0.64 | 0.69 ± 0.07 | |
| biochar | 250 | 0.32 ± 0.09 | 3.87 ± 0.39 | 2.57 ± 0.10 | 1.57 ± 0.04 | 0.33 ± 0.01 | - | 0.52 ± 0.0 |
| steam activated | 250 | 1.34 ± 0.35 | 12.7 ± 0.02 | 7.93 ± 0.75 | 4.37 ± 0.04 | 1.08 ± 0.06 | 4.37 ± 0.97 | 2.90 ± 0.18 |
| acid activated | 250 | 3.76 ± 0.29 | 0.36 ± 0.01 | 2.23 ± 0.63 | 0.71 ± 0.01 | 0.13 ± 0.03 | 4.03 ± 1.62 | 0.24 ± 0.01 |
| base activated | 250 | 0.64 ± 0.05 | 1.04 ± 0.01 | 11.9 ± 0.51 | 2.62 ± 0.08 | 0.09 ± 0.03 | 1.61 ± 0.10 | 0.80 ± 0.02 |
| biochar | 500 | 1.55 ± 0.19 | 12.1 ± 0.45 | 12.0 ± 0.52 | 5.06 ± 0.15 | 1.04 ± 0.07 | 9.66 ± 4.8 | 0.89 ± 0.01 |
| steam activated | 500 | 1.81 ± 0.14 | 17.5 ± 0.44 | 14.2 ± 0.84 | 5.97 ± 0.29 | 1.19 ± 0.21 | 6.03 ± 2.0 | 1.01 ± 0.02 |
| acid activated | 500 | 3.61 ± 0.52 | 0.22 ± 0.01 | 4.16 ± 0.02 | 1.56 ± 0.09 | 0.09 ± 0.05 | 7.45 ± 5.11 | 0.43 ± 0.0 |
| base activated | 500 | 2.75 ± 1.02 | 3.53 ± 0.12 | 24.3 ± 1.08 | 4.61 ± 0.15 | 0.17 ± 0.02 | 7.60 ± 3.62 | 1.12 ± 0.04 |
| raw chicken litter | 16.7 ± 1.5 | 39.3 ± 3.8 | 44.2 ± 3.3 | 10.1 ± 0.9 | 8.77 ± 0.64 | 2.77 ± 0.04 | 11.2 ± 1.3 | |
| biochar | 250 | 11.2 ± 2.6 | 42.5 ± 2.7 | 34.2 ± 8.8 | 8.20 ± 0.91 | 10.9 ± 0.3 | 4.86 ± 2.12 | 7.96 ± 0.38 |
| steam activated | 250 | 34.9 ± 1.0 | 130 ± 5.9 | 136 ± 4.7 | 24.8 ± 0.03 | 25.7 ± 2.5 | 10.0 ± 1.84 | 19.8 ± 1.8 |
| acid activated | 250 | 11.7 ± 0.7 | 0.45 ± 0.00 | 37.0 ± 3.5 | 0.78 ± 0.18 | 0.12 ± 0.04 | 8.37 ± 1.23 | 3.30 ± 0.19 |
| base activated | 250 | 13.0 ± 1.8 | 15.7 ± 2.47 | 63.3 ± 4.5 | 15.1 ± 0.92 | 2.47 ± 0.46 | 8.58 ± 0.74 | 7.08 ± 0.30 |
| biochar | 480 | 29.5 ± 6.0 | 96.0 ± 1.05 | 102 ± 29 | 18.2 ± 1.0 | 23.8 ± 0.4 | 7.89 ± 2.84 | 14.9 ± 0.6 |
| steam activated | 480 | 29.5 ± 8.7 | 118 ± 28.8 | 106 ± 33 | 19.7 ± 3.8 | 25.1 ± 7.8 | 10.6 ± 4.78 | 13.5 ± 2.5 |
| acid activated | 480 | 24.8 ± 6.6 | 6.89 ± 0.12 | 58.3 ± 1.3 | 4.72 ± 0.09 | 2.59 ± 0.00 | 10.1 ± 0.49 | 4.62 ± 0.98 |
| base activated | 480 | 16.8 ± 6.2 | 27.3 ± 3.0 | 63.2 ± 2.6 | 21.0 ± 2.3 | 6.10 ± 0.66 | 10.1 ± 3.13 | 7.57 ± 0.02 |
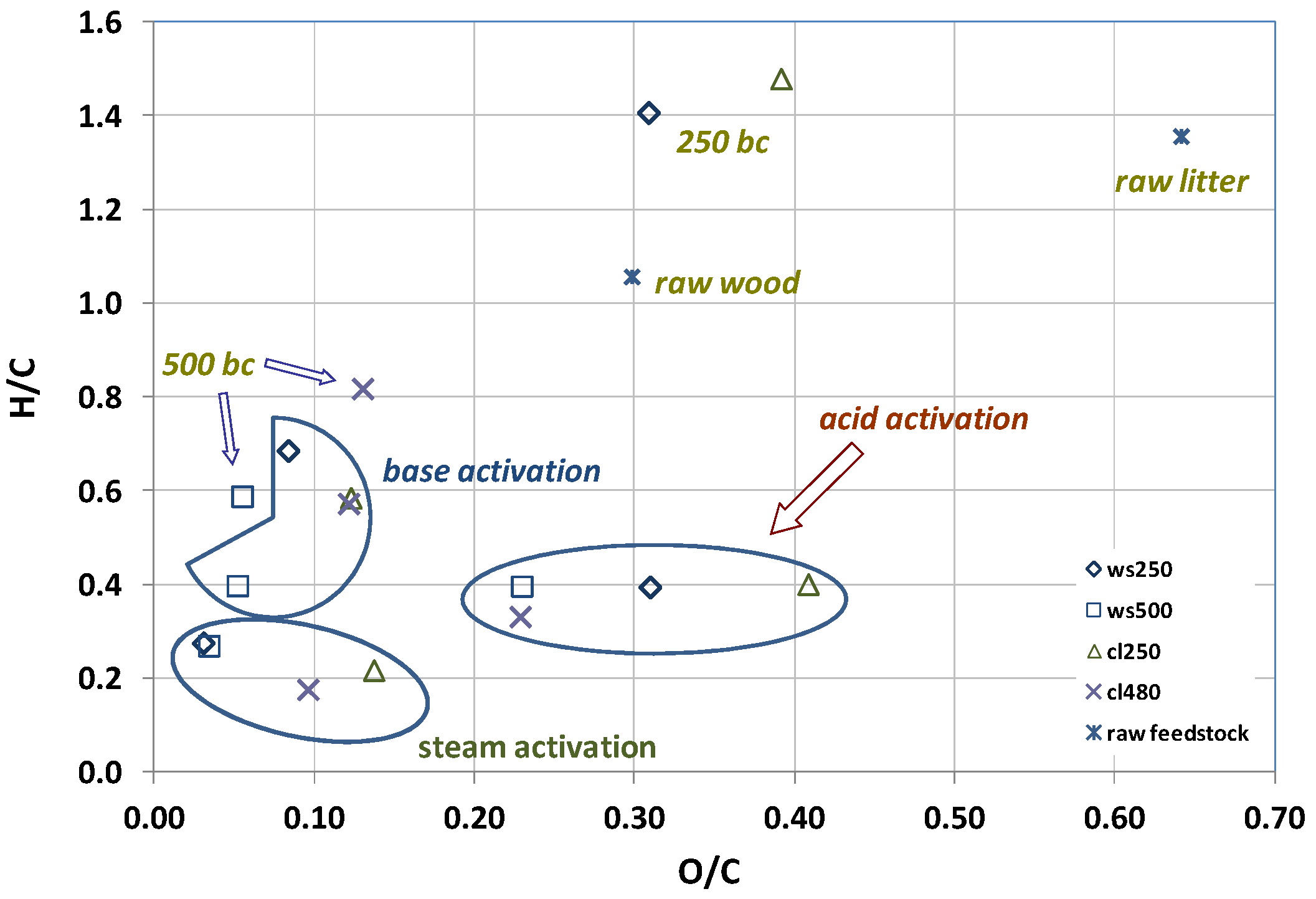
4. Conclusions
| Feedstock Source | Sample | As Adsorption % | As Adsorption mg/g | SC meq OH−/g | Cu Adsorption % | Cu Adsorption mg/g |
|---|---|---|---|---|---|---|
| WS 250 °C | BC | - | - | 0.00 | 15.5 | 1.08 |
| BC SA | - | - | 0.00 | 41.0 | 2.87 | |
| BC AA | 1.4 | 0.001 | 0.04 | 99.4 | 6.95 | |
| BC BA | 1.6 | 0.002 | 0.17 | 6.6 | 0.34 | |
| WS 500 °C | BC | - | - | 0.00 | 11.9 | 0.77 |
| BC SA | 1.4 | 0.001 | 0.15 | 34.5 | 2.25 | |
| BC AA | 1.2 | 0.001 | 0.20 | 98.7 | 6.43 | |
| BC BA | 1.7 | 0.002 | 0.46 | 30.1 | 1.50 | |
| CL 250 °C | BC | 0.6 | 0.001 | 0.00 | 67.8 | 3.68 |
| BC SA | 1.3 | 0.001 | 0.00 | 98.2 | 5.32 | |
| BC AA | 1.0 | 0.001 | 0.00 | 98.7 | 7.56 | |
| BC BA | 4.8 | 0.006 | 0.65 | 82.9 | 5.18 | |
| CL 480 °C | BC | - | - | 0.07 | 61.1 | 3.88 |
| BC SA | - | - | 0.00 | 95.0 | 5.96 | |
| BC AA | - | - | 0.00 | 96.5 | 7.39 | |
| BC BA | 4.9 | 0.006 | 0.56 | 82.4 | 5.23 |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hazrat, A.; Khan, E.; Saja, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar]
- Wang, Y.; Björn, L.O. Heavy metal pollution in Guangdong Province, China, and the strategies to manage the situation. Front. Environ. Sci. 2014, 2, 1–12. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Jegan, J.R.; Palanivelu, K.; Velan, M. Copper removal from aqueous solution by marine green alga Ulva reticulata. Electron. J. Biotechnol. 2004, 7, 61–71. [Google Scholar]
- USEPA. National Primary Drinking Water Regulations; US Environmental Protection Agency: Washington, DC, USA, 2011.
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Matovic, D. Biochar as a viable carbon sequestration option: Global and Canadian perspective. Energy 2011, 36, 2011–2016. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A synthesis of its agronomic impact beyond carbon sequestration. J. Environ. Qual. 2011, 41, 973–989. [Google Scholar] [CrossRef] [PubMed]
- Ladanai, S.; Vinterbäck, J. Global Potential of Sustainable Biomass for Energy; Swedish Agricultural University: Uppsala, Sweden, 2009. [Google Scholar]
- McKendry, P. Energy production from biomass (Part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Spurr, S.H.; Vaux, H.J. Timber: Biological and economic potential. Science 1976, 191, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of wood/biomass for bio-oil: A critical review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- USEPA. Poultry—Production and Value 2014 Summary; United States Department of Agriculture, National Agricultural Statistics Service: Washington, DC, USA, 2015.
- Collins, E.R., Jr.; Barker, J.C.; Carr, L.E.; Brodie, H.L.; Martin, J.H., Jr. Poultry Waste Management Handbook; Natural Resource, Agriculture, and Engineering Service: Ithaca, NY, USA, 1999. [Google Scholar]
- Qiu, Y.; Cheng, H.; Xu, C.; Sheng, G.D. Surface characteristics of crop-residue-derived black carbon and lead(II) adsorption. Water Res. 2008, 42, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gómez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmedna, M.; Marshall, W.E.; Rao, R.M. Production of granular activated carbons from select agricultural by-products and evaluation of their physical, chemical and adsorption properties. Bioresour. Technol. 2000, 71, 113–123. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Zhang, K.; Cheung, W.H.; Valix, M. Roles of physical and chemical properties of activated carbon in the adsorption of lead ions. Chemosphere 2005, 60, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Agblevor, F.A. Pyrolysis characteristics and kinetics of chicken litter. Waste Manag. 2007, 27, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Shinogi, Y.; Kanri, Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 2003, 90, 241–247. [Google Scholar] [CrossRef]
- Qiu, G.; Guo, M. Quality of poultry litter-derived granular activated carbon. Bioresour. Technol. 2010, 101, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Risse, L.M.; Das, K.C.; Worley, J.; Thompson, S. Pyrolysis of poultry litter fractions for bio-char and bio-oil production. J. Agric. Sci. Appl. 2012, 1, 37–44. [Google Scholar]
- Lima, I.M.; Marshall, W.E. Utilization of turkey manure as granular activated carbon: Physical, chemical and adsorptive properties. Waste Manag. 2005, 25, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.; Marshall, W.E. Adsorption of selected environmentally important metals by poultry manure-based granular activated carbons. J. Chem. Technol. Biotechnol. 2005, 80, 1054–1061. [Google Scholar] [CrossRef]
- Xu, X.; Cao, X.; Zhao, L. Comparison of rice husk- and dairy manure-derived biochars for simultaneously removing heavy metals from aqueous solutions: Role of mineral components in biochars. Chemosphere 2013, 92, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Moscoso, J.L.G.; Kumar, S.; Cao, X.; Mao, J.; Schafran, G. Removal of copper and cadmium from aqueous solutions using switchgrass biochars produced via hydrothermal caronization process. J. Environ. Manag. 2012, 109, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lima, I.M.; Boykin, D.L.; Klasson, K.T.; Uchimiya, M. Influence of post-treatment strategies on the properties of activated chars from broiler manure. Chemosphere 2014, 95, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Some considerations of the origins of porosity in carbons from chemically activated wood. Carbon 1993, 31, 1185–1192. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels production through biomass pyrolysis—A technological review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Sekiguchi, Y.; Shafizadeh, F. The effect of inorganic additives on the formation, composition, and combustion of cellulosic char. J. Appl. Polym. Sci. 1984, 29, 1267–1286. [Google Scholar] [CrossRef]
- Jensen, P.A.; Sander, B.; Dam-Johansen, K. Pretreatment of straw for power production by pyrolysis and char wash. Biomass Bioenergy 2001, 20, 431–446. [Google Scholar] [CrossRef]
- Fahmi, R.; Bridgwater, A.V.; Darvell, L.I.; Jones, J.M.; Yates, N.; Thain, S.; Donnison, I.S. The effect of alkali metals on combustion and pyrolysis of Lolium and Festuca grasses, switchgrass and willow. Fuel 2007, 86, 1560–1569. [Google Scholar] [CrossRef]
- Lima, I.M.; Boateng, A.A.; Klasson, K.T. Pyrolysis of broiler manure: char and product gas characterization. Ind. Eng. Chem. Res. 2009, 48, 1292–1297. [Google Scholar]
- Johns, M.M.; Marshall, W.E.; Toles, C.A. The effect of activation method on the properties of pecan shell-activated carbons. J. Chem. Technol. Biotechnol. 1999, 74, 1037–1044. [Google Scholar] [CrossRef]
- Klasson, K.T.; Uchimiya, M.; Lima, I.M. Characterization of narrow micropores in almond shell biochars by nitrogen, carbon dioxide, and hydrogen adsorption. Ind. Crop. Prod. 2015, 67, 33–40. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Influence of mineral matter on biomass pyrolysis characteristics. Fuel 1995, 74, 1812–1822. [Google Scholar] [CrossRef]
- Tseng, R. Physical and chemical properties and adsorption type of activated carbon prepared from plum kernels by NaOH activation. J. Hazard. Mater. 2007, 147, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, E.; Jakab, E.; Varhegyi, G.; Bourke, J.; Manley-Harris, M.; Nunoura, T.; Antal, M.J. Do all carbonized charcoals have the same chemical structure? 1. Implications of thermogravimetry-mass spectrometry measurements. Ind. Eng. Chem. Res. 2007, 46, 5943–5953. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizao, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D.; Engelhard, M.H. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006, 37, 1477–1488. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, D.; Zhu, L. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures. Environ. Sci. Technol. J. 2008, 42, 5137–5143. [Google Scholar] [CrossRef]
- Mukherjee, A.; Zimmerman, A.R.; Harris, W. Surface chemistry variations among a series of laboratory-produced biochars. Geoderma 2011, 163, 247–255. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Klasson, K.T.; Wartelle, L.H.; Rodgers, J.E.; Lima, I.M. Copper (II) adsorption by activated carbons from pecan shells: Effect of oxygen level during activation. Ind. Crop. Prod. 2009, 30, 72–77. [Google Scholar] [CrossRef]
- Jaramillo, J.; Gómez-Serrano, V.; Alvarez, P.M. Enhanced adsorption of metal ions onto functionalized granular activated carbons prepared from cherry stones. J. Hazard. Mater. 2009, 161, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Polo, M.S.; Utrilla, J.R. Adsorbent-adsorbate interactions in the adsorption of Cd (II) and Hg (II) on ozonized activated carbons. Environ. Sci. Technol. 2002, 36, 3850–3854. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: Sterling, VA, USA, 2012; pp. 53–63. [Google Scholar]
- Van Krevelen, D.W. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Yan, Q.; Toghiani, H.; Yu, F.; Cai, Z.; Zhang, J. Effects of pyrolysis conditions on yield of bio-chars from pine chips. Forest Prod. J. 2011, 61, 367–371. [Google Scholar] [CrossRef]
- Zieliňska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Shindo, H. Elementary composition, humus composition, and decomposition in soil of charred grassland plants. Soil Sci. Plant Nutr. 1991, 37, 651–657. [Google Scholar] [CrossRef]
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (red pine) wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Chen, S.; Liao, W.; Liu, C.; Wen, Z.; Kincaid, R.L.; Harison, J.H.; Elliott, D.C.; Brown, D.C.; Solana, A.E.; Stevens, D.J. Value-Added Chemicals from Animal Manure; Pacific Northwest National Laboratory: Richland, WA, USA, 2003. [Google Scholar]
- Das, K.C.; Garcia-Perez, M.; Bibens, B.; Melear, N. Slow pyrolysis of poultry litter and pine woody biomass: Impact of chars and bio-oils on microbial growth. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2008, 43, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Linsen, B.G. The texture and surface chemistry of carbons. In Physical and Chemical Aspects of Adsorbents and Catalyst; Academic Press: London, UK, 1970. [Google Scholar]
- Chilton, N.; Marshall, W.E.; Rao, RM.; Bansode, R.R.; Losso, J.N. Activated carbon from pecan shell: Process description and economic analysis. Ind. Crop. Prod. 2003, 17, 209–217. [Google Scholar]
- Toles, C.A.; Marshall, W.E.; Johns, M.M.; Wartelle, L.H.; McAloon, A. Acid-activated carbons from almond shells: Physical, chemical and adsorptive properties and estimated cost of production. Bioresour. Technol. 2000, 71, 87–92. [Google Scholar] [CrossRef]
- Lima, I.M.; McAloon, A.; Boateng, A.A. Activated carbon from broiler litter: Process description and cost of production. Biomass Bioenergy 2008, 32, 568–572. [Google Scholar] [CrossRef]
- Toles, C.A.; Marshall, W.E.; Wartelle, L.H.; McAloon, A. Steam- or carbon dioxide-activated carbons from almond shells: Physical, chemical and adsorptive properties and estimated cost of production. Bioresour. Technol. 2000, 75, 197–203. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, I.M.; Ro, K.S.; Reddy, G.B.; Boykin, D.L.; Klasson, K.T. Efficacy of Chicken Litter and Wood Biochars and Their Activated Counterparts in Heavy Metal Clean up from Wastewater. Agriculture 2015, 5, 806-825. https://doi.org/10.3390/agriculture5030806
Lima IM, Ro KS, Reddy GB, Boykin DL, Klasson KT. Efficacy of Chicken Litter and Wood Biochars and Their Activated Counterparts in Heavy Metal Clean up from Wastewater. Agriculture. 2015; 5(3):806-825. https://doi.org/10.3390/agriculture5030806
Chicago/Turabian StyleLima, Isabel M., Kyoung S. Ro, G. B. Reddy, Debbie L. Boykin, and Kjell T. Klasson. 2015. "Efficacy of Chicken Litter and Wood Biochars and Their Activated Counterparts in Heavy Metal Clean up from Wastewater" Agriculture 5, no. 3: 806-825. https://doi.org/10.3390/agriculture5030806






