1. Introduction
Osmotic dehydration of fruits (apple, cherry, blueberry, cranberry
etc.) generates a liquid waste in the form of spent osmotic solutions (SOS), which needs to be disposed of in an environmentally benign manner. Osmotic dehydration is often used as an industrial process to reduce water activity (a measurement of the amount of water available for microbial growth), infuse solute into fruits to increase weight, and to dehydrate fruits. In the osmotic dehydration of fruits, solute (sugar) is mixed with water to prepare an osmotic solution (OS). The fruits are submerged into this osmotic solution under specific conditions of mass ratio of fruit:solution (1:10 to 1:2 [
1]), temperature (20–40 °C [
1]) and vacuum (5 to 10 kPa [
2]) for a prescribed short period of time (15 min [
2]) to facilitate water removal from fruit. After the prescribed period, partially dehydrated fruits are filtered out from the solution and placed in a dehydrator for further drying. The leftover solution is known as spent osmotic solution (SOS). Most of the industries reuse/recycle SOS several times but every time it is recycled, the solution becomes darker, lower in pH, and richer in extractives (pectin, and acids) from the fruit being processed [
1,
3]. It also accumulates undesirable fungi and bacteria during processing and/or storage [
1]. The SOS must be treated to regulatory standards before it can be discharged to land or surface/subsurface water [
4].
Current procedures for handling waste from the fruit and vegetable industry are documented in the United States Environmental Protection Agency’s document # EPA-625/3-77-0007 [
5]. The document explicitly explains wastewater treatment procedures. Similar regulatory documents are also adopted by various states. For example, the Michigan Department of Environmental Quality has documented its code and procedures under the title “Michigan Fruit and Vegetable Processor’s Guide to Environmental Regulations” [
6]. The fruit dehydration industry follows these regulatory standards for treating their waste. Currently, most of the fruit dehydration industries mix their spent osmotic solutions (SOS) with other wastewater, which includes leftover waste fruits, sanitizers/lubricants, waste from floor drains,
etc. This wastewater is usually treated at either an onsite wastewater treatment facility or a Publicly Owned Treatment Works (POTW). It is expected that at the end of the wastewater treatment, the effluent would meet either surface or ground water discharge standards.
Federal and state regulations suggest use of a combination of physical, chemical and biological treatments to achieve the effluent limitations presented in the
Table 1. Physical processes are screening, sedimentation (gravity settling) and filtration to remove large debris, grit and suspended solids. Chemical treatments are precipitation and chemical destruction systems to remove phosphorus. Biological treatments rely on living organisms to remove pollutants, particulates, and dissolved organic material from the wastewater. This includes processes such as waste-stabilization lagoons, trickling filters, rotating biological contactors, and activated sludge. Granholm and Chester [
6] and the EPA [
5] have documented all of the above processes in detail.
Table 1.
Potential Permit Limitations in the State of Michigan for effluent discharge into surface and ground water (Source: Granholm and Chester, [6]) and effluent limitations for BOD and Total Suspended Solids (TSS) (kg/1000kg of raw material processed) and for pH as listed in 40 CFR §407.
Table 1.
Potential Permit Limitations in the State of Michigan for effluent discharge into surface and ground water (Source: Granholm and Chester, [6]) and effluent limitations for BOD and Total Suspended Solids (TSS) (kg/1000kg of raw material processed) and for pH as listed in 40 CFR §407.
| Indicator | Potential Permit Limitations in the State of Michigan | Effluent limitations (40 CFR §407) |
|---|
| Limit for Surface Water Discharge | Limit for Ground Water Discharge |
|---|
| Biochemical Oxygen Demand (BOD) | 1.86 mg/g of raw material daily maximum (As listed in 40 CFR§ 407) | 45 mg/L daily maximum
30 mg/L monthly average | 1.86 kg/1000kg of raw material processed daily
1.13 kg/1000kg monthly
0.8 kg/1000kg yearly |
| Dissolved Oxygen | 4.0 to 8.0 mg/L (cold water)
3.0 to 6.0 mg/L (warm water) | No limit but lagoon should maintain 2.0 mg/L to prevent odor | |
| Nitrogen Compounds | 0.5 mg/L to 30 mg/L (Ammonia) | 5.0 mg/L (total inorganic nitrogen)
With 0.5 mg/L (nitrite limitation) | |
| Total Suspended Solids | (As listed in 40 CFR 407) | - | 3.34 kg/1000kg of raw material processed daily
2.32 kg/1000kg monthly
1.48 kg/1000kg yearly |
| Total Dissolved Solids (In-stream after discharge) | 750 mg/L as a daily maximum 500 mg/L as a monthly average | no standards | |
| Phosphorus | 1 mg/L | 1 mg/L at 1000 feet away from surface water | |
| pH | 6.5 to 9.0 | 6.5 to 9.0 | 6.0 to 9.5 |
While the final destiny of SOS is to dispose of it in a wastewater treatment facility, several management practices have been suggested to minimize its generation in the first place [
1,
4]. In addition, extractions of value-added compounds from certain fruit processing waste have also been proposed [
3,
7,
8]. Since sugar-based SOS is the main source of Biochemical Oxygen Demand (BOD), it is highly recommended to reduce its generation through optimization of the osmotic dehydration process and recycling of SOS several times. Current industrial osmotic dehydration processes involve submerging fruit into osmotic solutions to dewater the fruit. For these processes, the approximate food-to-solution ratio is recommended to be 1:5 to maintain fruit dewatering capacity of the solution [
1]. However, technical breakthroughs in new process equipment may actually reduce solution consumption to a ratio of 1:2. During dehydration, aromas, pigments, acids and proteins are leached from fruits into the SOS, which changes the chemical (organic matter), physical (pH, water activity, and viscosity), and sensory (color and flavor) characteristics of the SOS. Repeated use of the SOS results in darkening of the solution and degradation in the antioxidant content. Additionally, leaching of organic acids from fruit to solution reduces the pH from 6.0 to 4.0. Before repeated use, the SOS are concentrated by a combination of the following processes: evaporation, solute addition, membrane concentration, and cryoconcentration. However, controlling microbial contamination during SOS reuse is always a challenge. Research suggests that the concentrated spent solution must be sanitized in order to reduce the
Coliform bacteria to 12000 (total) and 12000 (fecal) and
Streptococcus faecalis to 2000 CFU (Colony Forming Units)/100 mL [
1].
SOS from fruit dehydration, which can no longer be recycled, are sometimes used for other food preparations, for example, syrup for fruit canning, fruit jams, additives to fruit juices and soft drinks, and as a source of by-products (flavoring). Several studies on the extraction of by-products have been reported [
3,
7,
8,
9]. Blueberry waste, to be specific, has been used to extract anthocyanins and polyphenolics at a lab-scale using various methods. For example, Paes
et al. [
7] used supercritical CO
2 and pressurized water whereas Nicoué
et al. [
9] used ethanol to extract anthocynanins. Lee and Wrolstad [
8] used citric acid, SO
2, and enzymes to extract polyphenolics and anthocynanins. Nevertheless, none of these processes achieved commercial success and SOS is currently considered as waste.
While biological treatment of industrial waste has been considered acceptable practice since the dawn of environmental regulations, significant technical breakthroughs in other thermochemical technologies, for example, pyrolysis, gasification, and hydrothermal liquefaction/carbonization, have the potential to not only reduce waste and greenhouse gas (GHG) emissions but also generate valuable bioproducts in a very short reaction time. Hydrothermal carbonization is defined as a mild form of pyrolysis using an aqueous feedstock [
10]. During hydrothermal carbonization, biomass is treated in hot compressed water yielding three product forms: solid hydrochar, aqueous compounds, and gases [
10]. Gupta
et al. [
11] state that hydrothermal carbonization is generally performed by heating biomass in subcritical water at 230–350 °C and 3.48 to 20.68 MPa to obtain insoluble carbon-rich (hydrochar) and water-soluble products (biocrude). A review article authored by Libra
et al. [
12] states that hydrothermal carbonization processes are performed up to process temperatures of 220 °C and corresponding pressures of up to approximately 2 MPa to transform organics into solid hydrochar and very little gases (1%–5%). In another review, Funke and Ziegler [
13] define hydrothermal carbonization as a combination of dehydration and decarboxylation reactions which are achieved by applying temperatures of 180–200 °C in a suspension of biomass and water at saturated pressure for several hours. It may seem that Gupta
et al. [
11], Libra
et al. [
12], and Funke and Ziegler [
13] reported different temperature and pressure conditions for hydrothermal carbonization; however, in all three cases water is kept in subcritical condition. Subcritical water is also called pressurized hot water, compressed hot water, or superheated water [
14]. Subcritical water is hot water maintained in the liquid state at temperatures between 100 °C and 374 °C under pressurized conditions. The critical temperature and pressure of water are 374 °C and 22.4 MPa, respectively [
14]. Wiboonsirikul and Adachi [
14] report that physiochemical properties of water, in particular its relative dielectric constant and ion product, change with increasing temperature. The ion product of water is defined as the product of the concentrations of hydrogen and hydroxyl ions [
14]. The relative dielectric constant, ability to dissolve both polar and non-polar substances, of water decreases from 80 at 25 °C to 27 at 250 °C [
14]. For comparison, solvents like methanol and ethanol have dielectric constants of 33 and 24, respectively, at 25 °C [
14]. Therefore, under subcritical conditions, hot compressed water develops an ability to dissolve natural compounds, such as phenolic, polycyclic aromatic compounds, and oils [
14] due to its low dielectric constant. In addition, it acts as acidic or basic catalysts due to, approximately, threefold higher dissociation constant for hydrogen and hydroxyl ions than normal water. Finally, subcritical water has a higher diffusion coefficient for solutes and a low viscosity and surface tension, which promote more mass transfer and penetration ability [
14].
While solid hydrochar is main product of hydrothermal carbonization, another process known as hydrothermal liquefaction is more geared towards the production of liquid biocrude [
15,
16]. Toor
et al. [
15] state that hydrothermal liquefaction is carried out at 280–370 °C and between 10 and 25 MPa. It should be noted that both hydrothermal carbonization and hydrothermal liquefaction have overlapping operating conditions of temperature and pressure. Therefore, similar conditions of temperature and pressure might create hydrothermal carbonization reactions (dehydration and decarboxylation) for one type of feedstock and hydrothermal liquefaction reactions (complete degradation of feedstock into different chemical compounds) for another feedstock. For example, hydrothermal carbonization of glucose has been reported under 170 °C to 240 °C [
17] whereas its liquefaction has been reported between 300 °C to 400 °C and 25 to 40 MPa [
15]. Similarly, hydrothermal carbonization of fructose has been reported between 120 °C to 140 °C [
18] whereas its liquefaction has been reported between 200 °C to 320 °C [
15]. Therefore, process conditions of subcritical water together with the thermo-chemical decomposition behavior of the feedstock decide whether reaction conditions fall into hydrothermal liquefaction or carbonization.
The feedstock of interest in this study is SOS, a waste generated from osmotic dehydration of fruits. Hydrothermal carbonization has been extensively studied [
19,
20] for treatment of wastewater and sludge. Shanableh [
20] reports complete removal of chemical oxygen demand as a result of hydrothermal treatment of municipal sludge. Hydrothermal carbonization of municipal sludge from the food industry not only retains 75% of the feedstock carbon in the hydrochar but also generates wastewater rich in hydrolysis products with chemical oxygen demand, total organic carbon, and pH similar to the landfill leachate [
19]. Literature review reveals that the product of interest, hydrochar, generated from hydrothermal carbonization of sugar rich feedstock, collectively known as saccharides, is more valuable than that generated from treatment of municipal sludge. For example, hydrochars, which are also referred as carbon microspheres, were produced from hydrothermal carbonization of saccharides [
17,
18,
21,
22,
23]. To avoid confusion, the term “carbon microspheres” refers to hydrochars with various volatiles present at their surface and in their core, whereas term “carbon microparticles” refers to pure carbon free of volatiles. Carbon microspheres (hydrochar) produced from pure compounds have shown promise in nano-applications. Some researchers went a step further and tailored hydrochars into core/shell nanoparticles for optical nano-devices [
24,
25] nano-fibers [
26,
27] and nano-cables/nanotubes [
28,
29]. While pure saccharides have been used to produce carbon microspheres, no work has been reported on studying the thermo-chemical decomposition behavior of SOS and analyzing hydrochars produced from hydrothermal carbonization of SOS. SOS contains some acids, antioxidants, and soluble fiber, which should generate valuable carbon microspheres (hydrochars) with different morphology. Therefore, the main objectives of this research were the following:
to compare the thermo-chemical decomposition behavior of osmotic solution (OS) and spent osmotic solution (SOS) generated from osmotic dehydration of blueberries; and
to compare hydrochar yields and properties produced during hydrothermal carbonization of SOS and OS.
It was anticipated that hydrothermal carbonization of SOS would follow a reaction pattern similar to the one reported for the mixture of glucose and acrylic acid [
23], proteins [
30] and orange juice [
21]. Therefore, this research evaluates hydrothermal carbonization of SOS to produce carbon microspheres (hydrochar), carbon microparticles, and other valuable products.
3. Experimental Section
The experimental procedures for procurement of materials to be tested, preparation of samples, freeze-drying, thermo-gravimetric analysis, hydrothermal treatment, and analytical characterization are detailed in the following sections.
3.1. Materials and Sample Preparation
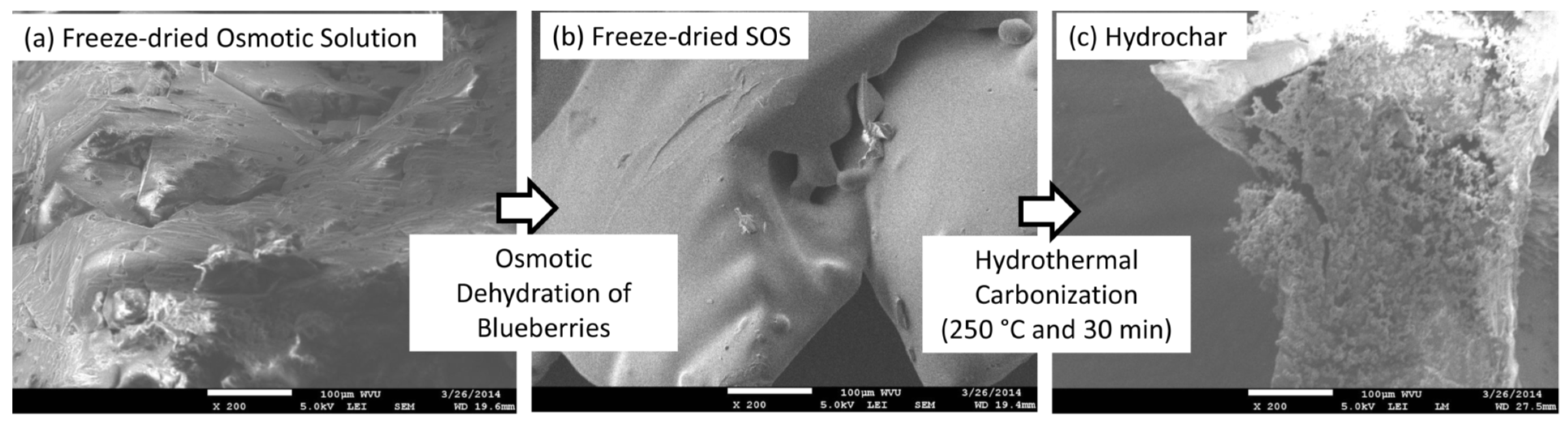
Spent osmotic solutions (SOS) and one control osmotic solution (OS) were procured from an ongoing project of the National Science Foundation WVU (West Virginia University, Morgantown, WV, USA) ADVANCE Sponsorship Program on osmotic dehydration of blueberries. Frozen blueberries were purchased from the Oceana Foods, Inc. (Shelby, MI, USA). In this project, an osmotic solution with a sucrose (off-the-shelf sugar) concentration of 65 °Brix was prepared at WVU. The OS and blueberries were mixed at a fruit-to-solution ratio of 1:3 g/g. To generate SOS, frozen blueberries were thawed to room temperature and then submerged in the osmotic solution for vacuum treatment. During this stage, a vacuum of 5 kPa (1.5 inches of Hg or 50 mbar) was applied for 15 min before incubation. Following vacuum treatment, incubation of fruit and solution was carried out at 50 °C for 300 min. After 300 min, the SOS was separated from the partially dehydrated blueberries. In addition, unused OS was used as a control. The SOS and OS were each divided into two sets of sub-samples. One set of sub-sample was subjected to freeze-drying to prepare a dried powder for thermo-gravimetric analysis (TGA), Attenuated Total Reflectance Infrared (ATR-IR) Spectroscopy, and elemental analysis. Another set of sub-sample was used for hydrothermal carbonization to produce hydrochars.
3.2. Thermo-Gravimetric Analysis
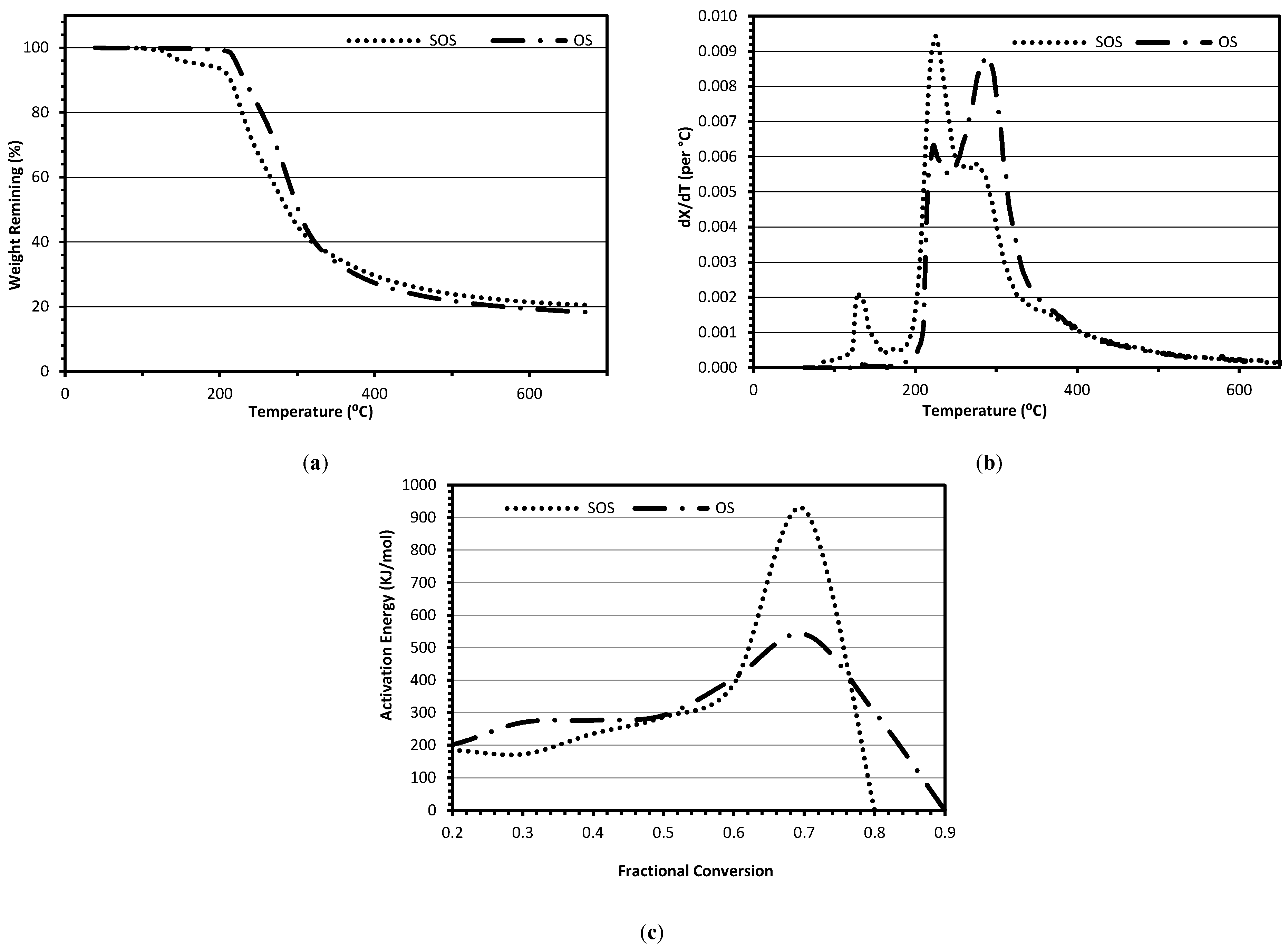
The thermo-chemical decomposition behavior of the solid freeze-dried samples was assessed using a thermo-gravimetric analyzer (TGA) (Model: Q50, TA Instruments, Schaumburg, IL, USA). The TGA experiments were performed by heating approximately 8–12 mg sample from 50 to 700 °C at three heating rates of 10, 30, and 50 °C/min under a nitrogen flow of 50 cm
3/min. The TGA data (percent weight lost as function of temperature) were processed to generate a derivative curve (dtg: rate of fractional conversion as function of temperature) to identify various reaction zones. The TGA data were also used to calculate the activation energy for decomposition using the isoconversion method [
35].
3.3. Hydrothermal Carbonization
Hydrothermal carbonization of the OS and SOS samples was performed by placing 50 mL pre-weighed liquid sample (m
feed) inside a one-liter sealed pressure reactor (Model: 4500, Parr Instrument Company, Moline, IL, USA) at a constant stirrer speed (rpm) and zero cold nitrogen pressure as shown in
Figure 5. The current model is not capable of logging stirrer rpm and changes in pressure.
Figure 5.
Experimental set-up for hydrothermal carbonization experiments.
Figure 5.
Experimental set-up for hydrothermal carbonization experiments.
The difference in the weight of the empty reactor and the weight of the reactor with sample was used to calculate the actual sample weight. After that, the reactor was sealed, the weight of the sealed reactor was measured and air was removed from the headspace. To remove air from the sealed reactor, nitrogen gas was purged before the reaction for about 15 min to make sure that the gas composition in the reactor headspace was 99.5% nitrogen (verified from gas composition analysis). After that, the reactor was heated to 250 °C at the heating rate 10 °C/min, held at 250 °C for 30 min, and then cooled to room temperature. After cooling, gasses from the reactor headspace were sampled by airtight syringes (Model: 1000 series, Hamilton, NV, USA), and analyzed by gas chromatography (Model: Clarus GC 580, Perk Elmer Waltham, MA, USA). Upon completely releasing the headspace gasses, the sealed reactor was reweighed (m
slurry). The difference in the weight of the sealed reactor before and after heating accounted for the gases produced. After taking the weight, the reactor was opened and most of the contents of the reactor (hydrochar and some liquid) were collected and weighed on a drying pan. The drying pan with collected contents was placed inside an oven at 104 °C to evaporate the liquids. After 24 h of evaporation, the dried solid moisture-free hydrochar was weighed and the weight loss during the drying process was accounted for as the percent liquid content of the slurry (m
liquid). This value was later used to calculate the product yields (hydrochar, liquid, and gas yields) on a wet-feed basis and on dry-feed matter basis. The product yields on a wet-feed basis were calculated as follows:
To calculate product yields on a dry-feed matter basis, the following equations were used:
An example of calculation of product yields is given as follows: In an OS experiment, approximately, 59.40 g of feed sample (mfeed) was used, which contained 39.35 g soluble-solids (mdry-feed) and remaining water (20.05 g). Hydrothermal carbonization reaction produced 53.10 g slurry (mslurry) and 6.30 g gaseous products (mslurry−mfeed). The slurry, upon oven drying, resulted on 51.54% moisture (mliquid). Therefore, the hydrochar, liquid, and gas yields, on wet-feed basis, are calculated to be 43.32%, 46.07%, and 10.61%, respectively. In addition, the hydrochar, liquid, and gas yields, on dry-feed matter basis, are calculated to be 65.39%, 18.61%, and 16.01%, respectively.
The hydrochar was tested for elemental composition, proximate analysis, Brunauer-Emmett-Teller (BET) surface area and scanning electron microscopy (SEM). It is important to mention that the 250 °C reaction temperature and 30 min holding time was selected after reviewing the literature [
17,
21,
22,
23]. Sevilla [
17] has reported using holding times of 4.5 h for 190 °C, 1.0 h for 230 °C, and 0.5 h for 240 °C. Wang [
22] used a holding time of 5 h for 190 °C and Demir-Cakan [
23] had performed experiments at 150 °C with 16 h holding times. Therefore, literature suggested holding times of 30 min might be used for high reaction temperature (250 °C).
3.4. Analytical Characterization
The OS and SOS were characterized for pH and total solids. The pH was measured using a pH meter (Model: AB15, Fisher Scientific, USA). To measure total solids, a known amount of sample was evaporated in a drying oven at 104 °C for 24 h. In addition, the OS and SOS were tested for chemical functional groups and elemental composition. The analysis of the chemical functional groups was performed using Attenuated Total Reflectance Infrared (ATR-IR) Spectroscopy (Model: Nicolet iS10 Smart iTR, Thermo Scientific). The elemental composition was measured using a CHNS analyzer (Model: Series II CNHS/O Analyzer 2400, PerkinElmer, Waltham, MA, USA). The elemental composition was used in an empirical equation to determine the higher heating value (HHV) of the samples [
31]. In addition, proximate analysis (moisture, volatile matter, fixed carbon and ash) was carried out using a proximate analyzer (Model: LECO TGA 701, LECO Corporation, St. Joseph, MI, USA) following the ASTM D3174 standard [
36]. Scanning Electron Microscopy was performed on a JEOL JSM-7600F Scanning Electron Microscope (Peabody, MA). The SEM sputter had a target of Gold/Palladium (60%/40%) and the chamber was maintained under a vacuum of 9.6 × 10
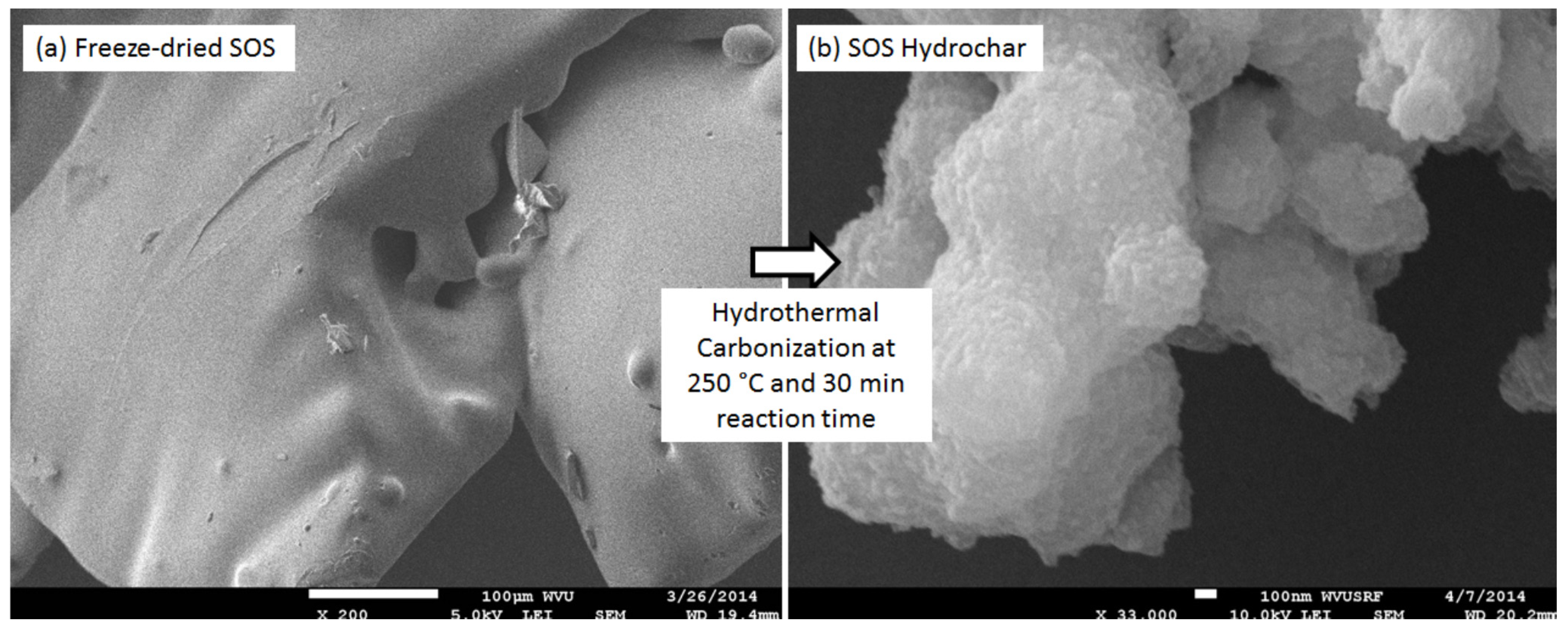
−5 Pa. Several SEM images with spherical particles of hydrochar were printed and spherical particles were numbered on the printed pictures. After that, the diameter of each numbered particle was manually measured with fifty spherical particles constituting the measured set. Brunauer-Emmett-Teller (BET) surface area was measured for the devolatilized hydrochars with nitrogen using a surface area analyzer (Model: ASAP 2020: Surface Area and Porosity Analyzer, Norcross, GA, USA). Devolatilization of hydrochars was performed by heating the hydrochars in the crucibles of the LECO TGA 701 up to 600 °C for 30 min under nitrogen. During BET surface area measurements, the samples were degassed at 300 °C under a vacuum for 12 h.
3.5. Statistical Experiment Design
Two treatments OS and SOS were used in a completely randomized experimental design with three replications. There were a total of six hydrothermal experiments performed.
4. Conclusions
In this paper, OS and SOS samples were characterized for their thermo-chemical decomposition behavior and for hydrothermal carbonization as a means of generating experimental data to develop a hydrothermal treatment to produce value-added hydrochar. SOS and OS were different in terms of total solid content and acidity (pH value). OS had a total solid content of 66.25% and a pH of 6.61 while SOS had total solid content of 59.00% and a pH of 3.61.
Thermo-gravimetric experiments showed that the SOS sample lost 3.86, 28.66, 30.90, and 13.66% (weight) in the temperature zones of 120–160 °C; 180–250 °C, 250–350 °C, and 350–600 °C, respectively. In contrast, the OS sample showed weight loss of 0.18, 18.76, 47.29, and 14.18% (weight), respectively in the same temperature zones. Derivative thermo-gravimetric results showed that the spent osmotic solutions had one extra decomposition peak at 130 °C due to decomposition of antioxidants. In addition, both samples had two fixed peaks at 225 and 280 °C. The only difference between the SOS and OS in peaks was that the 225 °C peak was larger in the SOS due to the presence of higher amounts of non-sucrose sugars.
In addition, both samples produced, approximately, 40%–42% (on wet-feed basis) hydrochar with atomic O/C and H/C contents of 0.25 and 1.04, respectively. Both hydrochars had a higher heating value of approximately 30 MJ/kg; however, the hydrochar produced from the SOS sample had higher BET surface area (49.06 m2/g) than that produced from the OS sample (16.62 m2/g). In addition, the OS sample produced a hydrochar composed of smooth-surfaced spherical particles of 1.79 ± 1.30 μm diameter whereas the SOS sample produced hydrochar particles with no definite shape but with a raspberry like surface.
5. Implications
The results presented in this paper have broad technical and economic implications for the fruit dehydration industry. For example, anaerobic digestion is the most prevalent method for waste treatment to meet effluent requirements of reduced BOD and TSS. Food-dehydration systems are currently regulated for liquid waste disposal and very soon, all of them will be subjected to regulations for greenhouse gas emission. In some cases, fruit processing capacity is limited by the regulatory limitation for BOD, which is 1.86, 1.12, and 0.8 kg/1000 kg of fruit processed daily, monthly and yearly maximum, respectively, after utilizing the best wastewater treatment. The fruit-dehydration industry cannot operate without wastewater treatment because with the current processing methods, it generates approximately 1.91 kg BOD for every kg of fruit (apples) processed, just from SOS. Extrapolating this number, a medium-sized processing plant (≤10,000 ton/year processing capacity) would generate BOD of 19.1 tons per year, which must be reduced to 8.0 tons per year of operation. Discharging 19.1 tons of BOD without wastewater treatment would require the addition of approximately 168 billion gallons of water every year (based on 30 mg/L effluent limitation given in
Table 1). However, after wastewater treatment (combination of processes- physical, chemical, and biological treatment) [
5,
6] this dilution would require, under current practices, only 0.16 billion gallons of water every year. If practical data are to be considered, the state of Michigan processes 263,000 tons apples, 17,000 tons of cherries, and 24,300 tons of blueberries every year [
37], which generates 3.4, 1.3, and 0.22 billion gallons of wastewater every year, respectively. These numbers include the fruit dehydration industry. It is expected that the BOD requirement due to SOS will be reduced to zero with the proposed hydrothermal carbonization process, thereby, saving billions of gallons of water required for dilution and the cost associated with the wastewater treatment in addition to the reduction in environmental pollution/GHG emissions.
Wastewater treatment has associated costs as presented by the EPA [
5], which are 7.1, 31.1, 48.2, 120, 17.8, and 5 cents per 1000 gallons for flow measurement and screening, neutralization of the aerated lagoon, activated sludge with sludge concentration, filtration, and chlorination, respectively. Therefore, a wastewater treatment plant using all of these operations will cost $2.29 (in 1975) per 1000 gallons, which would be approximately $10 in the year 2014 after adjusting for inflation. This translates into $49.2 million spent by the state of Michigan fruit processors on wastewater treatment operations. It is expected that the proposed technology would not only eliminate most of this cost but it will also generate hydrochar and carbon microparticles, a value-added sequestered carbon. Additionally, hydrothermal carbonization would capture at least 46% [
17] and at most, 75% [
19] of the carbon present in the feedstock, which would translate into saving nearly 1.26 million tons of CO
2 every year. This calculation is based on 2.74 kg fructose in SOS/kg of apples, 10,000 tons production capacity, and 75% carbon capture through hydrothermal carbonization. Therefore, it is expected that the hydrothermal treatment of SOS will enhance sustainability of osmotic dehydration of fruits by saving billions of gallons of freshwater needed for BOD dilution, savings in cost of wastewater treatment, and savings in millions of tons of greenhouse gas emissions.