Salinity-Induced Changes of Multiparametric Fluorescence Indices of Tomato Leaves
Abstract
:1. Introduction
2. Experimental Section
2.1. Genotypes and Growth Conditions
2.2. Treatments
2.3. Analytical Determinations
2.3.1. Mineral Concentrations
2.3.2. Proline Concentration
2.3.3. Chlorophyll Concentration
2.4. Fluorescence Measurements
2.4.1. Pulse-Amplitude-Modulated (PAM) Chlorophyll Fluorescence
2.4.2. Multiple Fluorescence Excitation and Fluorescence Ratios
2.5. Data Analysis
3. Results
3.1. Mineral, Proline and Chlorophyll Concentrations
| Tomato Cultivar | Treatments | Na [mg/g DM] | K [mg/g DM] | Mg [mg/g DM] | Proline [mg/g DM] | Chl a + b [mg/g DM] |
|---|---|---|---|---|---|---|
| Rio Grande | Control | 4.74 ± 0.20 * | 70.95 ± 1.44 * | 3.74 ± 0.14 * | 0.54 ± 0.04 * | 12.67 ± 0.26 *ns |
| NaCl | 20.95 ± 7.26 | 53.97 ± 2.35 | 3.20 ± 0.07 | 4.14 ± 0.18 | 13.69 ± 0.63 | |
| H-2274 | Control | 4.99 ± 0.21 * | 69.07 ± 1.84 * | 3.32 ± 0.12 * | 0.44 ± 0.07 * | 12.28 ± 0.28 *ns |
| NaCl | 16.56 ± 1.20 | 63.36 ± 1.97 | 2.87 ± 0.05 | 3.36 ± 0.24 | 12.40 ± 0.29 | |
| Harzfeuer | Control | 5.40 ± 0.13 * | 73.04 ± 2.40 * | 2.47 ± 0.07 * | 0.25 ± 0.03 * | 14.32 ± 0.35 * |
| NaCl | 33.79 ± 1.27 | 38.71 ± 1.87 | 1.83 ± 0.09 | 1.79 ± 0.21 | 12.54 ± 0.41 |
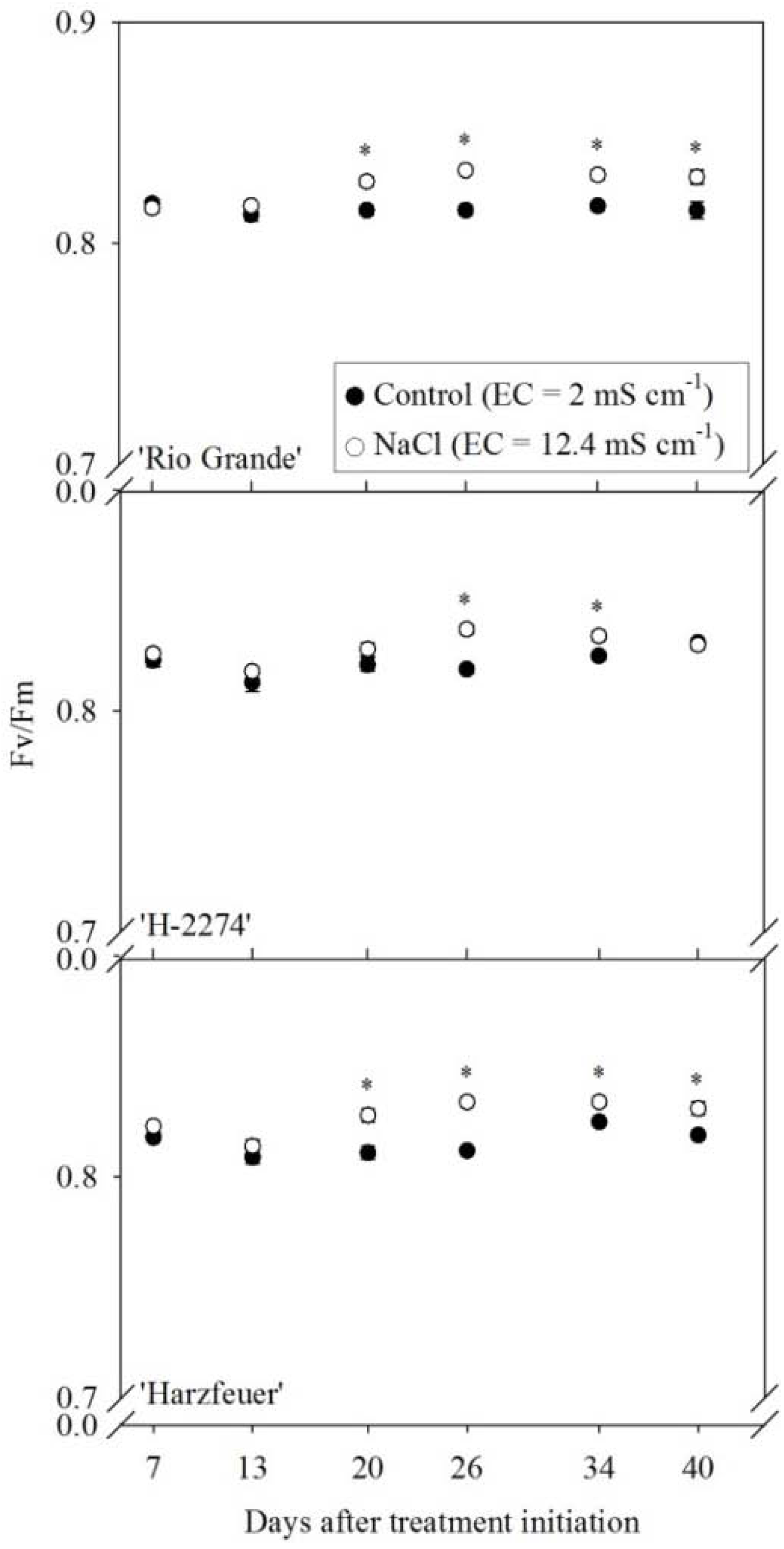
3.2. Maximum Quantum Efficiency of Photosystem II (Fv/Fm)
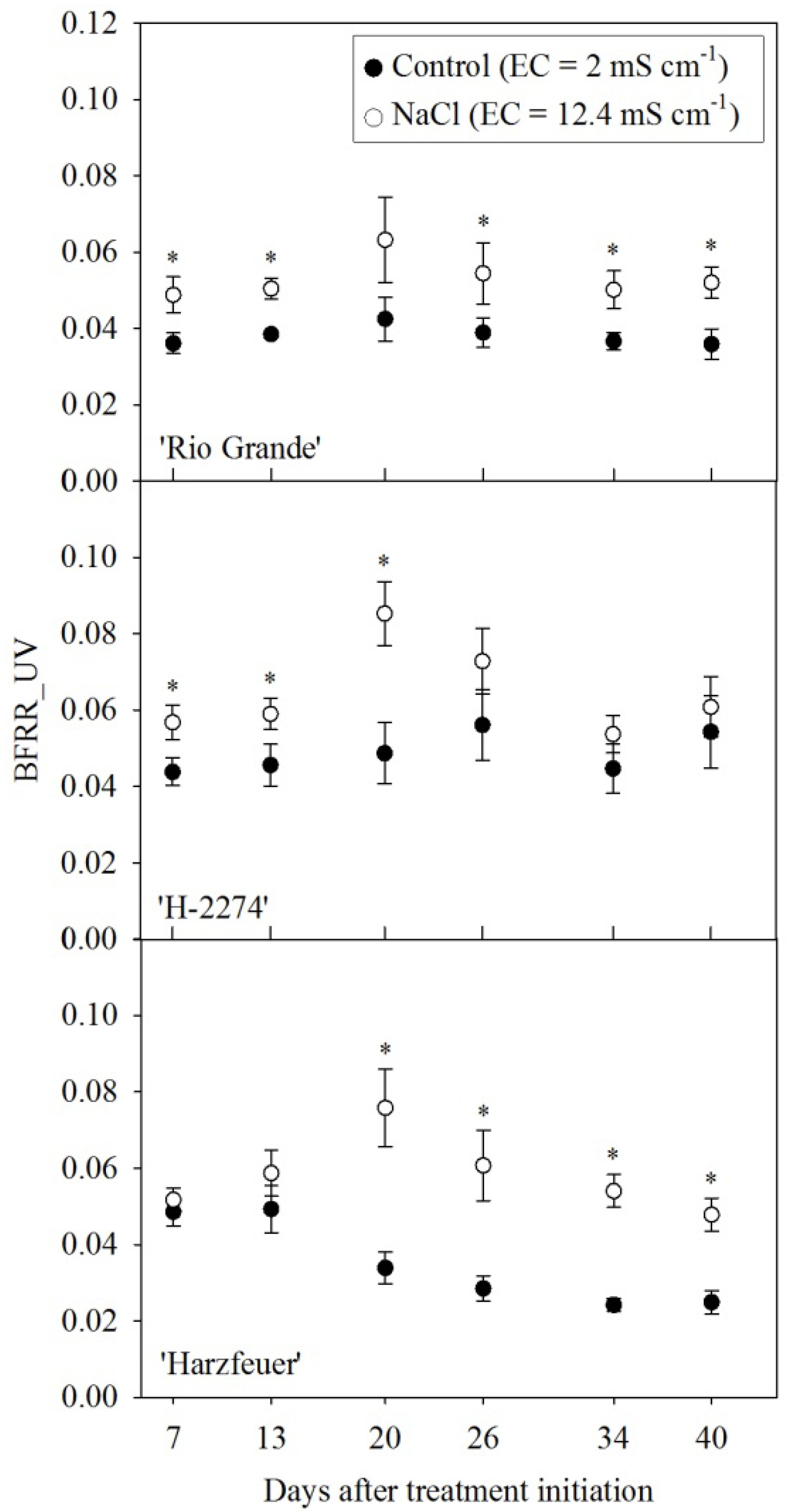
3.3. Blue-to-Far-Red Fluorescence Ratio (BFRR_UV)
3.4. Flavonol-Index (FLAV)

3.5. Nitrogen Balance Index (NBI_G)

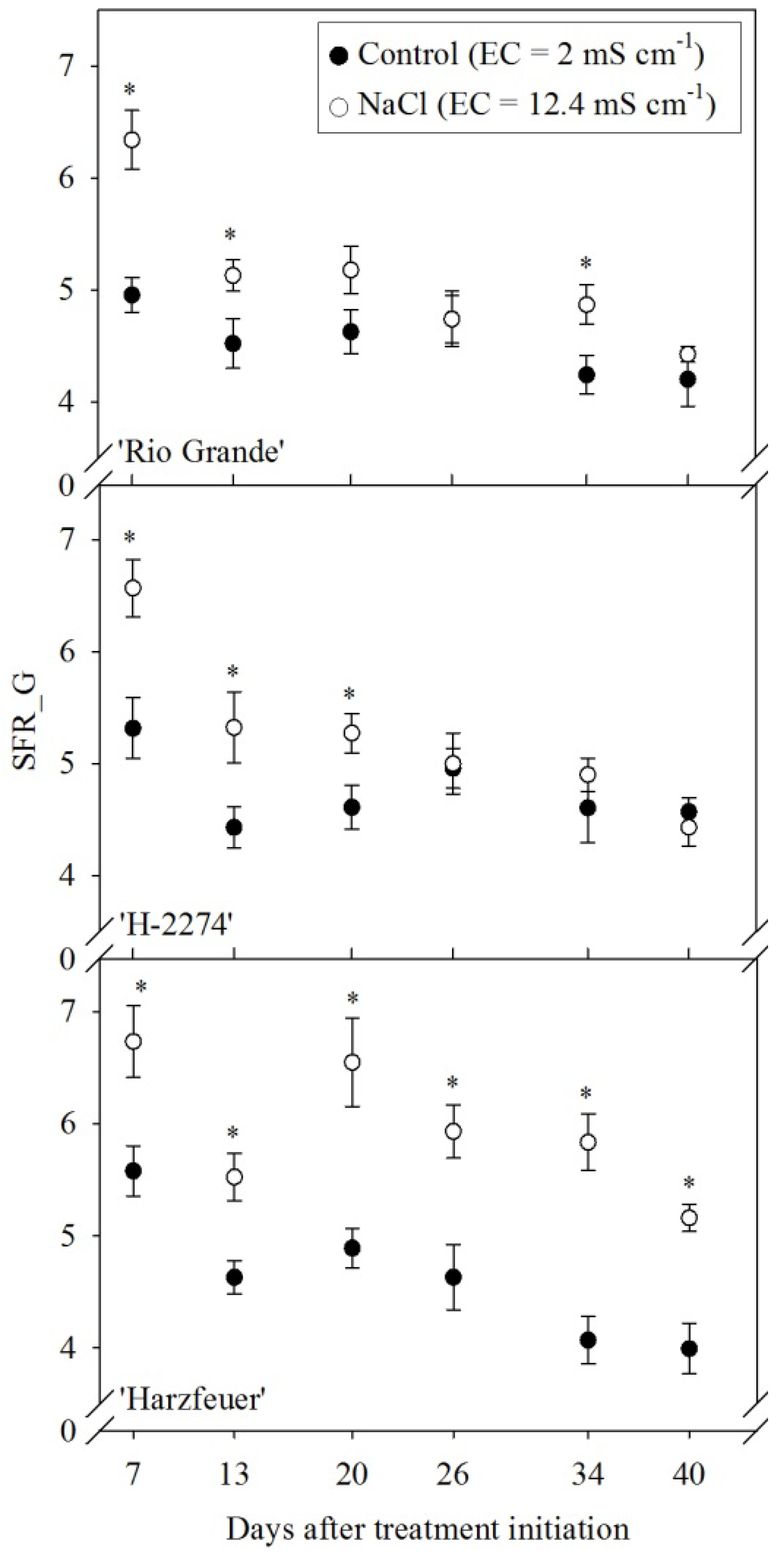
3.6. Simple Fluorescence Ratio (SFR_G)
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| BF | blue fluorescence |
| BFRR_UV | blue-to-far-red fluorescence ratio |
| Chl | chlorophyll |
| ChlF | chlorophyll fluorescence |
| G | green |
| DAT | days after treatment induction |
| DM | dry matter; FLAV, flavonol-index |
| FRF | far-red fluorescence |
| Fo | ground fluorescence |
| Fm | maximum fluorescence |
| Fv/Fm | maximum quantum efficiency of photosystem II |
| H-2274 | Solanum lycopersicum L. var. H-2274 |
| Harzfeuer | Solanum lycopersicum L. F1 hybrid Harzfeuer |
| NBI | nitrogen balance index |
| PAM | pulse-amplitude-modulated chlorophyll fluorescence |
| RF | red fluorescence |
| SFR | simple fluorescence ratio |
| UV | ultraviolet |
| var. | variety |
Conflicts of Interest
References
- Cuartero, J.; Bolarín, M.C.; Asíns, M.J.; Moreno, V. Increasing salt tolerance in the tomato. J. Exp. Bot. 2006, 57, 1045–1058. [Google Scholar] [CrossRef]
- Hunsche, M.; Bürling, K.; Saied, A.S.; Schmitz-Eiberger, M.; Sohail, M.; Gebauer, J.; Noga, G.; Buerkert, A. Effects of NaCl on surface properties, chlorophyll fluorescence and light remission, and cellular compounds of Grewia tenax (Forssk.) fiori and Tamarindus inidica L. leaves. Plant Growth Regul. 2010, 61, 253–263. [Google Scholar] [CrossRef]
- Hunsche, M.; Lankes, C.; Hoffstall, H.; Noga, G. Vegetative performance, leaf water potential, and partitioning of minerals and soluble sugars: Traits for ranking the NaCl-tolerance of tomato genotypes. Plant Growth Regul. 2010, 62, 151–162. [Google Scholar] [CrossRef]
- Gautier, H.; Lopez-Lauri, F.; Massot, C.; Murshed, R.; Marty, I.; Grasselly, D.; Keller, C.; Sallanon, H.; Génard, M. Impact of ripening and salinity on tomato fruit ascorbate content and enzyme activities related to ascorbate recycling. Funct. Plant Sci. Biotechnol. 2010, 4, 66–75. [Google Scholar]
- Incerti, A.; Navari-Izzo, F.; Pardossi, A.; Mensuali, A.; Izzo, R. Effect of sea water on biochemical properties of fruit of tomato (Lycopersicon esculentum Mill.) genotypes differing for ethylene production. J. Sci. Agric. 2007, 87, 2528–2537. [Google Scholar] [CrossRef]
- Cayuela, E.; Pérez-Alfocea, F.; Caro, M.; Bolarín, M.C. Priming of seeds with NaCl induces physiological changes in tomato plants grown under salt stress. Physiol. Plant. 1996, 96, 231–236. [Google Scholar] [CrossRef]
- Juan, M.; Rivero, R.M.; Romero, L.; Ruiz, J.M. Evaluation of some nutritional and biochemical indicators in selecting salt-resistant tomato cultivars. Environ. Exp. Bot. 2005, 54, 193–201. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 120, 425–432. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Lang, M.; Sowinska, M.; Heisel, F.; Miehé, J.A. Detection of vegetation stress via a new high resolution fluorescence imaging system. J. Plant Physiol. 1996, 148, 599–612. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Samson, G.; Morales, F.; Tremblay, N.; Moya, I. Ultraviolet-induced fluorescence for plant monitoring: Present state and prospects. Agronomic 1999, 19, 543–578. [Google Scholar] [CrossRef]
- Buschmann, C.; Lichtenthaler, H.K. Principles and characteristics of multi-colour fluorescence imaging of plants. J. Plant Physiol. 1998, 152, 297–314. [Google Scholar] [CrossRef]
- Morales, F.; Cerovic, Z.G.; Moya, I. Time-resolved blue-green fluorescence of sugar beet (Beta vulgaris L.) leaves. Spectroscopic evidence for the presence of ferulic acid as the main fluorophore of the epidermis. Biochim. Biophys. Acta 1996, 1273, 251–262. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Schweiger, J. Cell wall bound ferulic acid, the major substance of the blue-green fluorescence emission of plants. J. Plant Physiol. 1998, 152, 272–282. [Google Scholar] [CrossRef]
- Bürling, K.; Cerovic, Z.G.; Cornic, G.; Ducruet, J.-M.; Noga, G.; Hunsche, M. Fluorescence-based sensing of drought-induced stress in the vegetative phase of four contrasting wheat genotypes. Environ. Exp. Bot. 2013, 89, 51–59. [Google Scholar] [CrossRef]
- Leufen, G.; Noga, G.; Hunsche, M. Physiological response of sugar beet (Beta vulgaris) genotypes to a temporary water deficit, as evaluated with a multiparameter fluorescence sensor. Acta Physiol. Plant. 2013, 35, 1763–1774. [Google Scholar] [CrossRef]
- Kautz, B.; Noga, G.; Hunsche, M. Sensing drought- and salinity-imposed stresses on tomato leaves by means of fluorescence techniques. Plant Growth Regul. 2014. [Google Scholar] [CrossRef]
- Müller, V.; Lankes, C.; Schmitz-Eiberger, M.; Noga, G.; Hunsche, M. Estimation of flavonoid and centelloside accumulation in leaves of Centella asiatica L. Urban by multiparametric fluorescence measurements. Environ. Exp. Bot. 2013, 93, 27–34. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. Changes in carotenoids, tocopherols and diterpenes during drought and recovery, and the biological significance of chlorophyll loss in Rosmarinus officinalis plants. Planta 2000, 210, 925–931. [Google Scholar] [CrossRef]
- Bürling, K.; Hunsche, M.; Noga, G. Quantum yield of non-regulated energy dissipation in PSII (Y(NO)) for early detection of leaf rust (Puccinia triticina) infection in susceptible and resistant wheat (Triticum aestivum L.) cultivars. Precis. Agric. 2010, 11, 703–716. [Google Scholar] [CrossRef]
- Kishor, K.P.B.; Sreenivasulu, N. Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ. 2014, 37, 300–311. [Google Scholar] [CrossRef]
- Santa-Cruz, A.; Acosta, M.; Rus, A.; Bolarin, M.C. Short-term salt tolerance mechanisms in differentially salt tolerant tomato species. Plant Physiol. Biochem. 1999, 37, 65–71. [Google Scholar] [CrossRef]
- Botella, M.A.; Rosado, A.; Breassan, R.A.; Hasegawa, P.M. Plant adaptive responses to salinity stress. In Plant Abiotic Stress; Jenks, M.A., Hasegawa, P.M., Eds.; Blackwell Publishing Ltd.: Berlin, Germany, 2005; pp. 37–70. [Google Scholar]
- Chinnusamy, V.; Zhu, J.-K. Plant salt tolerance. In Plant Responses to Abiotic Stress; Hirt, H., Shinozaki, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 241–270. [Google Scholar]
- Takagi, H. Proline as a stress protectant in yeast: Physiological functions, metabolic regulations, and biotechnological applications. Appl. Microbiol. Biotechnol. 2008, 81, 211–223. [Google Scholar] [CrossRef]
- Kumar, S.G.; Reddy, A.; Sudhakar, C. NaCl effects on proline metabolism in two high yielding genotypes of mulberry (Morus alba L.) with contrasting salt tolerance. Plant Sci. 2003, 165, 1245–1251. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crop Prod. 2010, 31, 13–19. [Google Scholar]
- Havaux, M. Stress tolerance of photosystem II in vivo. Plant Physiol. 1992, 100, 424–432. [Google Scholar] [CrossRef]
- Lu, C.; Qiu, N.; Lu, Q.; Wang, B.; Kuang, T. Does salt stress lead to increased susceptibility of photosystem II to photoinhibition and changes in photosynthetic pigment composition in halophyte Suaeda salsa grown outdoors. Plant Sci. 2002, 163, 1063–1068. [Google Scholar] [CrossRef]
- Buschmann, C.; Langsdorf, G.; Lichtenthaler, H.K. Imaging of the blue, green, and red fluorescence emission of plants: An overview. Photosynthetica 2000, 38, 483–491. [Google Scholar] [CrossRef]
- Bilger, W.; Veit, M.; Schreiber, L.; Schreiber, U. Measurement of leaf epidermal transmittance of UV radiation by chlorophyll fluorescence. Physiol. Plant. 1997, 101, 754–763. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Buschmann, C.; Lichtenthaler, H.K. The chlorophyll fluorescence ratio F735/F700 as an accurate measure of the chlorophyll content in plants. Remote Sens. Environ. 1999, 69, 296–302. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. Crit. Rev. Anal. Chem. 1998, 19, 29–85. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Vegetation stress: An introduction to the stress concept in plants. J. Plant Physiol. 1996, 148, 4–14. [Google Scholar] [CrossRef]
- Buschmann, C. Variability and application of the chlorophyll fluorescence emission ratio red/far-red of leaves. Photosynth. Res. 2007, 92, 261–271. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Goutouly, J.-P.; Hilbert, G.; Destrac-Irvine, A.; Martinon, M.; Moise, N. Mapping winegrape quality attributes using portable fluorescence-based sensors. In Proceedings of the FRUTIC 09, Conception, Chile, Progap INIA, Santiago, Chile, January 2009; Best, S., Ed.; Instituto de Investigaciones Agropecuarias: Santiago, Chile, 2009; pp. 301–310. [Google Scholar]
- Bhivare, V.N.; Nimbalkar, J.D. Salt stress effects on growth and mineral nutrition of French beans. Plant Soil 1984, 80, 91–98. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; de Pascale, S. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kautz, B.; Hunsche, M.; Noga, G. Salinity-Induced Changes of Multiparametric Fluorescence Indices of Tomato Leaves. Agriculture 2014, 4, 132-146. https://doi.org/10.3390/agriculture4020132
Kautz B, Hunsche M, Noga G. Salinity-Induced Changes of Multiparametric Fluorescence Indices of Tomato Leaves. Agriculture. 2014; 4(2):132-146. https://doi.org/10.3390/agriculture4020132
Chicago/Turabian StyleKautz, Burkard, Mauricio Hunsche, and Georg Noga. 2014. "Salinity-Induced Changes of Multiparametric Fluorescence Indices of Tomato Leaves" Agriculture 4, no. 2: 132-146. https://doi.org/10.3390/agriculture4020132




