Biological Characterization of Cynara cardunculus L. Methanolic Extracts: Antioxidant, Anti-proliferative, Anti-migratory and Anti-angiogenic Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Total Phenolic Content
| Phenolic content (mg/g Cc DM) | EC50 Reducing capacity (mg/mL DM) | EC50 DPPH scavenging activity (mg/mL DM) | |||
|---|---|---|---|---|---|
| Leaves | Dry | Cultivated | 7.31 ± 1.01 | 5.79 ± 0.47 | 6.97 ± 1.28 |
| Wild | 5.22 ± 0.10 | 5.02 ± 0.18 | 6.95 ± 1.37 | ||
| Green | Cultivated | 9.25 ± 0.29 | 4.32 ± 0.14 | 3.44 ± 0.96 | |
| Wild | 5.56 ± 0.01 | 3.28 ± 0.29 | 15.78 ± 2.99 | ||
| Stalks | Dry | Cultivated | 2.86 ± 0.24 | 10.52 ± 0.57 | 13.63 ± 3.11 |
| Wild | 2.88 ± 0.24 | 11.04 ± 0.41 | 13.98 ± 2.85 | ||
| Green | Cultivated | 0.66 ± 0.07 | 15.70 ± 0.29 | 34.33 ± 7.46 | |
| Wild | 0.67 ± 0.12 | 18.17 ± 0.61 | 46.67 ± 2.46 | ||
| Inflorescences | Dry | Cultivated | 4.06 ± 0.14 | 8.39 ± 0.45 | 11.15 ± 1.88 |
| Wild | 3.58 ± 0.02 | 6.96 ± 0.32 | 9.16 ± 1.89 | ||
| Green | Cultivated | 2.62 ± 0.05 | 32.49 ± 1.28 | 15.76 ± 4.21 | |
| Wild | 1.20 ± 0.07 | 26.46 ± 0.53 | 66.08 ± 8.77 | ||

2.1.2. Reduction Capacity
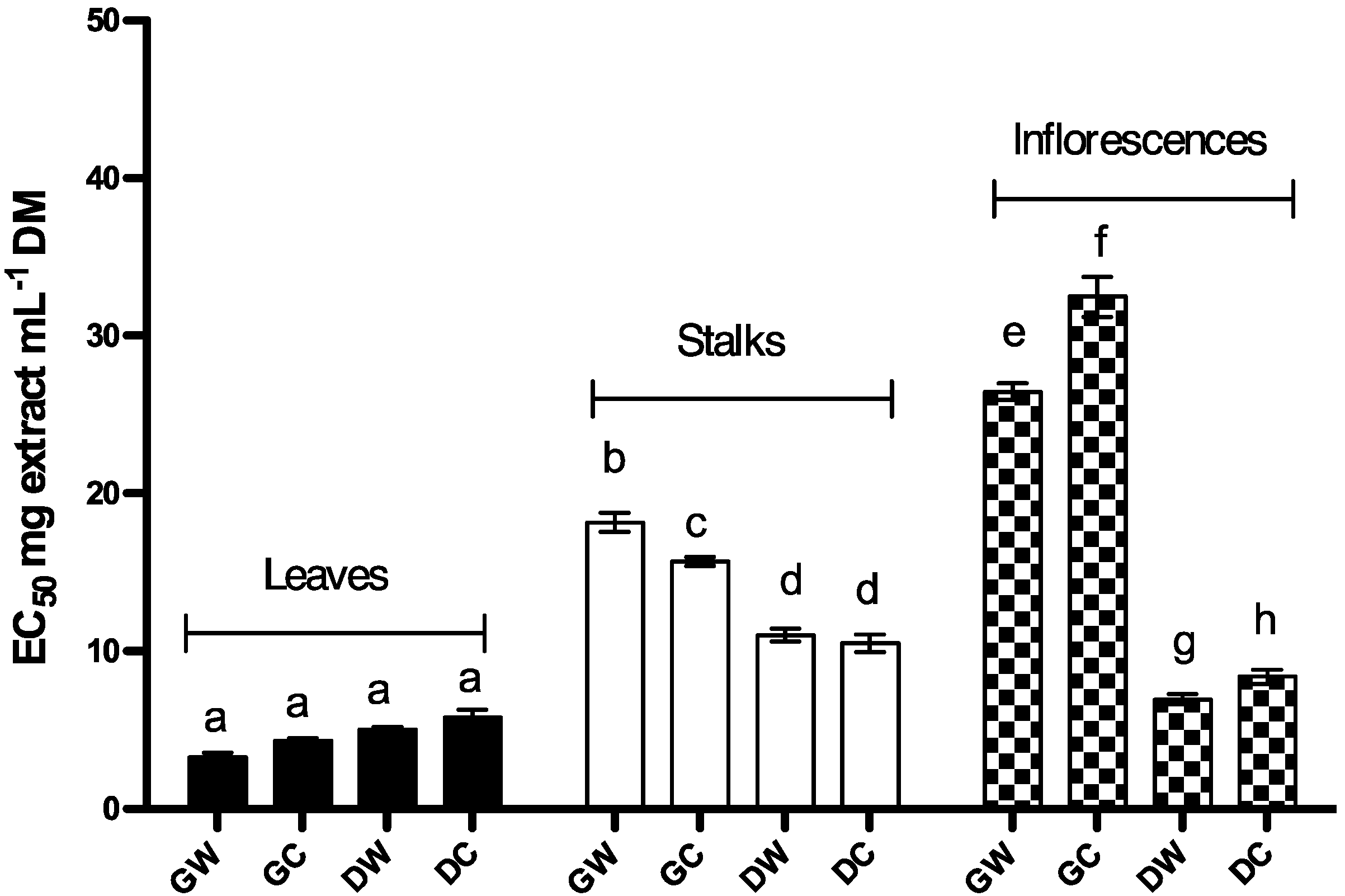
2.1.3. Free DPPH Radical-Scavenging Activity
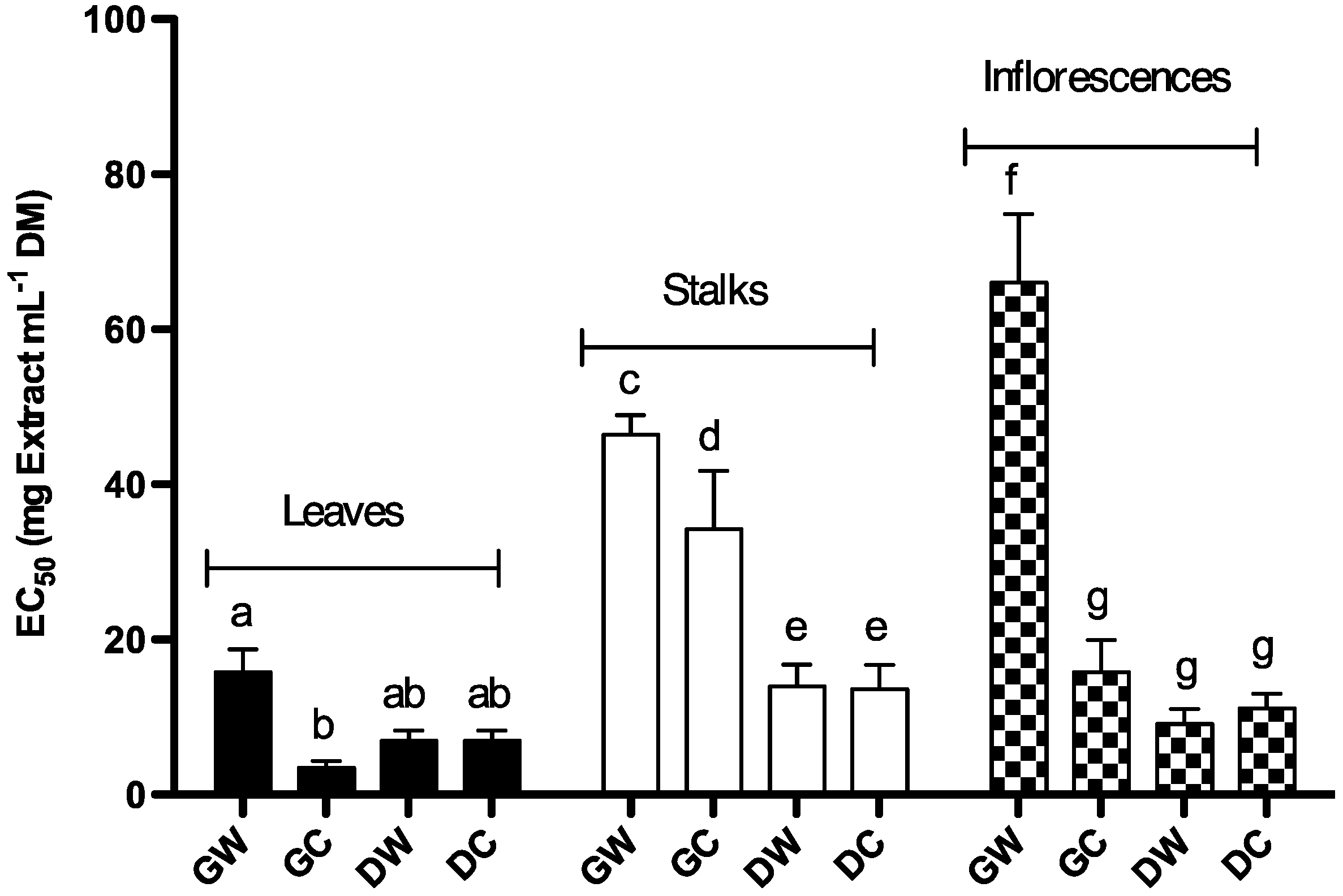
2.1.4. Protective Effect of Cc Extracts against H2O2 Radicals in Saccharomyces cerevisiae
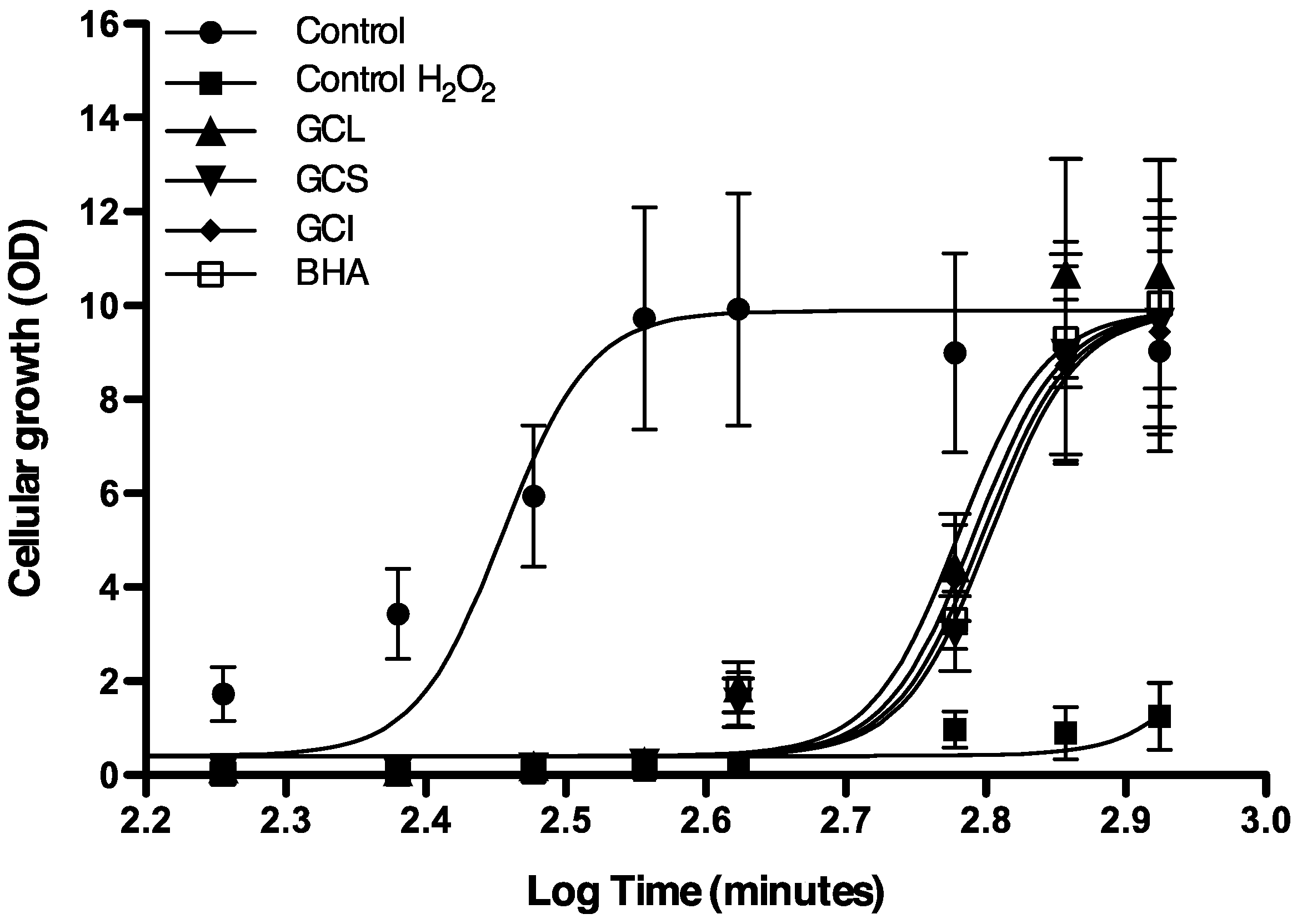

2.1.5. Effect of Cc Extracts in Tumor Cell Proliferation

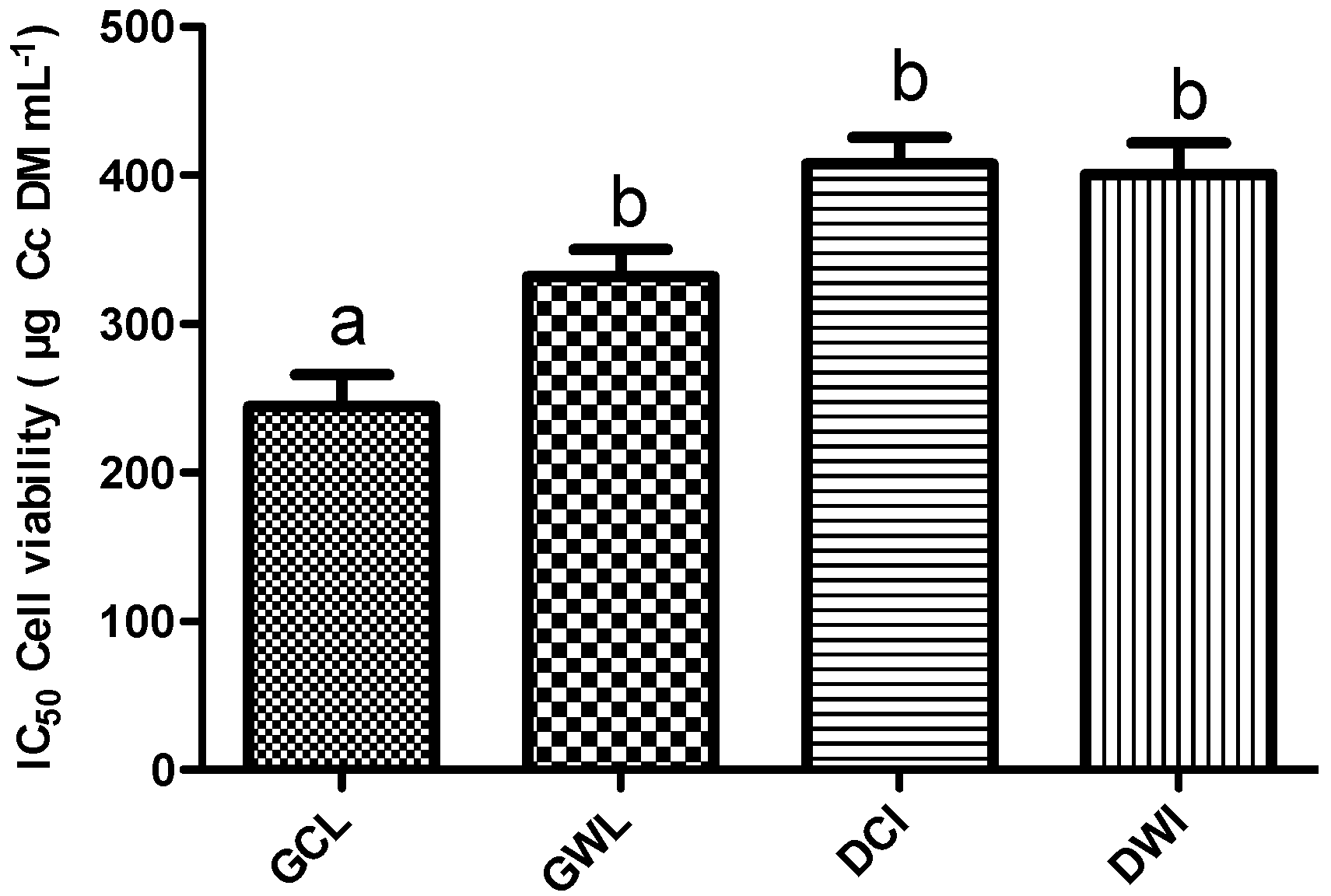
2.1.6. Effects on MDA-MB-231 Cells Migration Capability
2.1.7 In Vivo Anti-Angiogenic Activity
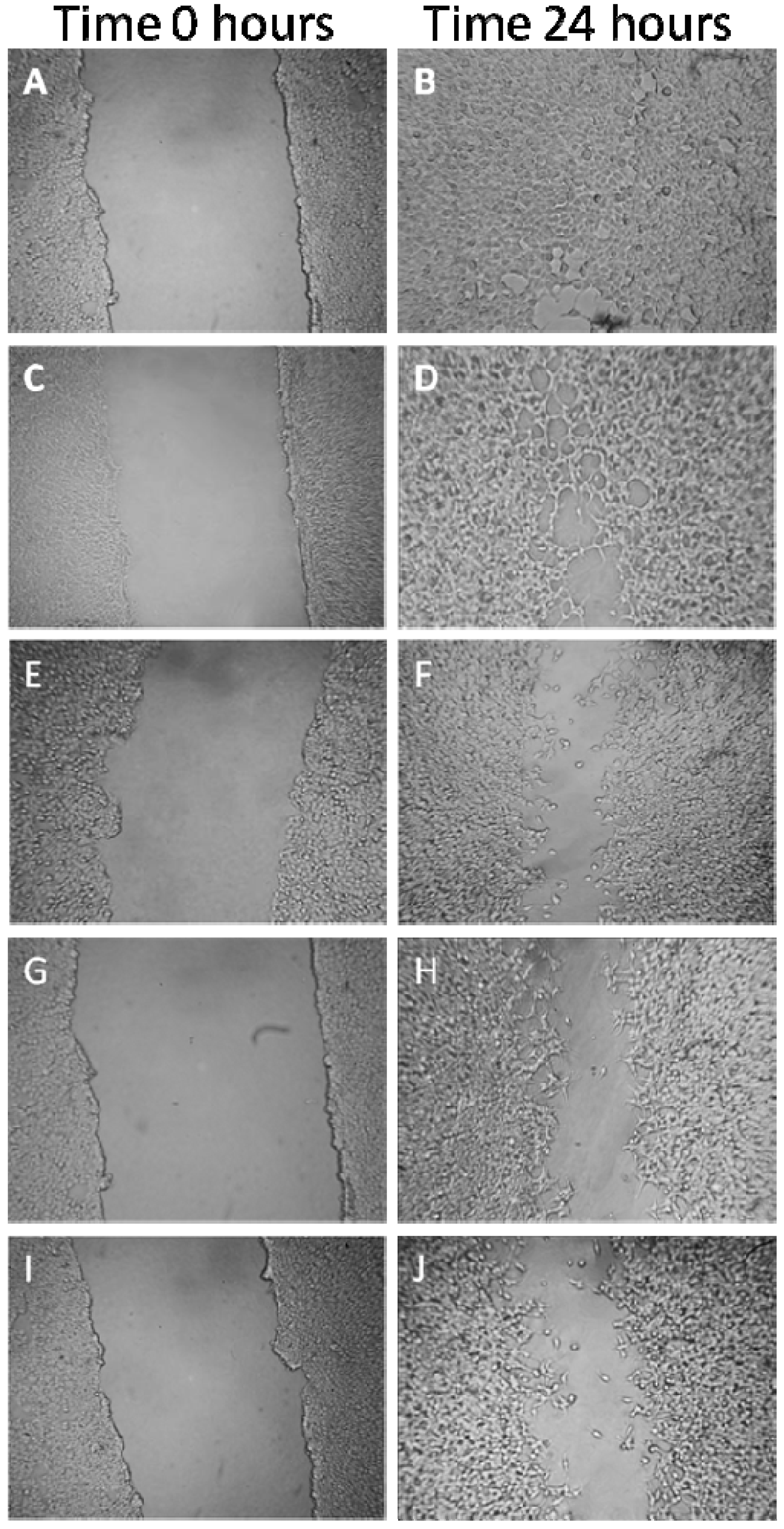
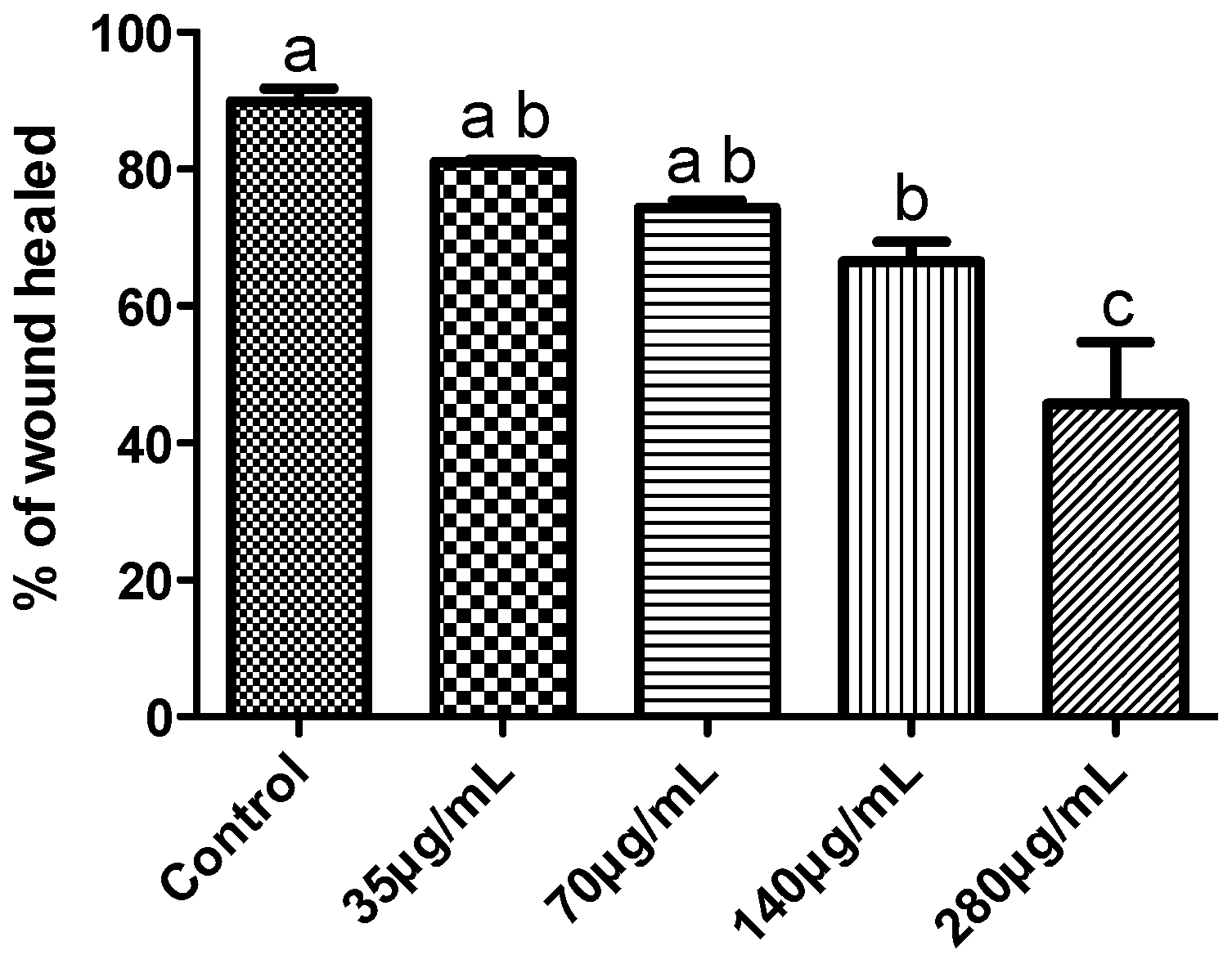

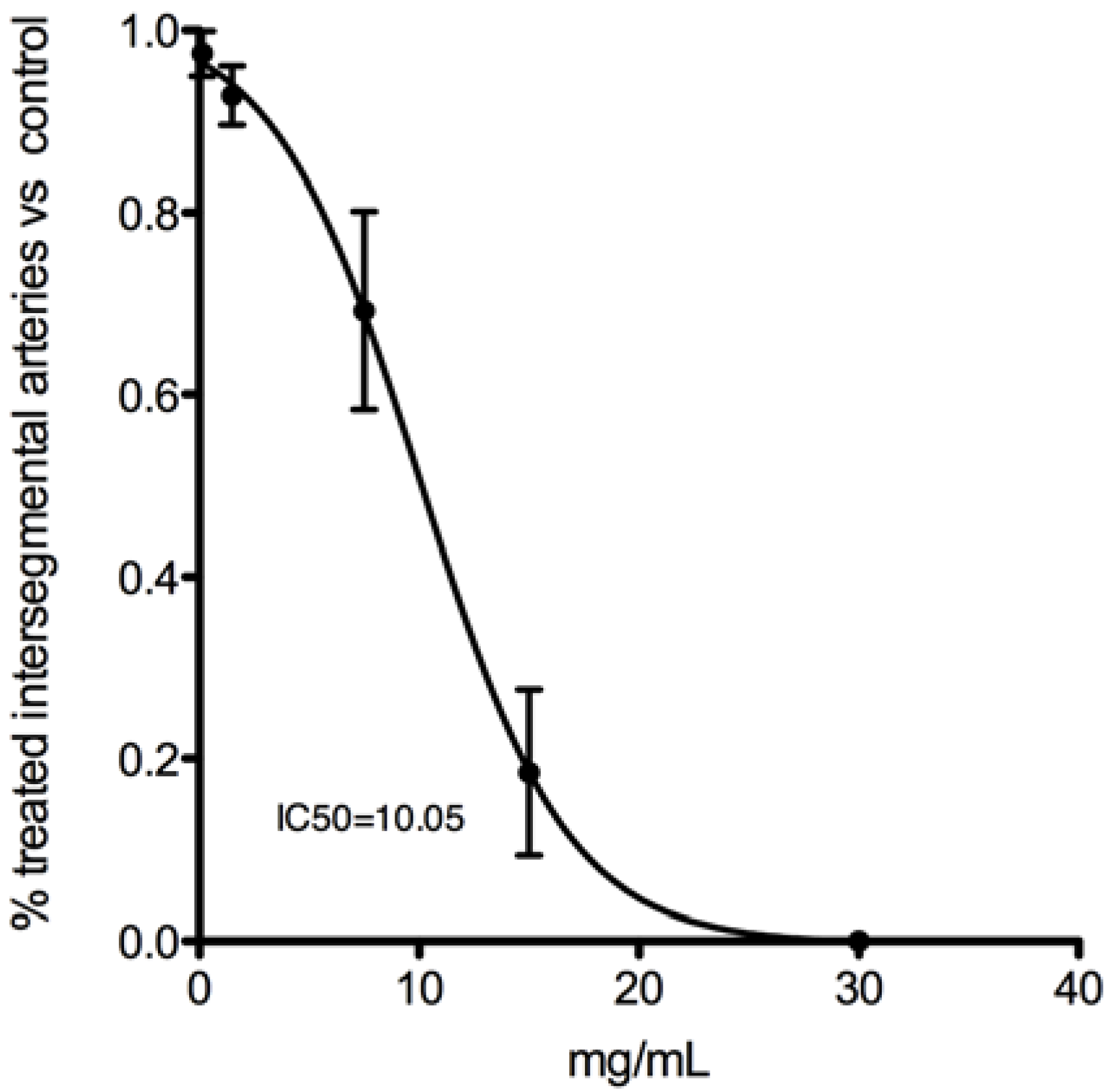
2.2. Discussion
3. Experimental Section
3.1. Chemicals
3.2. Plant Material
3.3. Sample Preparation
3.4. Measurement of Total Phenolic Compounds
3.5. Reducing Power Assay
3.6. DPPH Radical Scavenging Assay
3.7. Protective Effect against H2O2 Radicals in Saccharomyces cerevisiae
3.8. Cell Line and Culture
3.9. Analysis of Cell Viability
3.10. Wound Healing Assay
3.11. Animal Husbandry and Spawning
3.12. Zebrafish Model for in Vivo Angiogenesis
3.13. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Ksouri, R.; Ksouri, W.M.; Jallali, I.; Debez, A.; Magne, C.; Hiroko, I.; Abdelly, C. Medicinal halophytes: Potent source of health promoting biomolecules with medical, nutraceutical and food applications. Crit. Rev. Biotechnol. 2011, 32, 289–326. [Google Scholar]
- Benlloch-Gonzalez, M.; Fournier, J.M.; Ramos, J.; Benlloch, M. Strategies underlying salt tolerance in halophytes are present in cynara cardunculus. Plant Sci. 2005, 168, 653–659. [Google Scholar] [CrossRef]
- Lanteri, S.; Acquadro, A.; Saba, E.; Portis, E. Molecular fingerprinting and evaluation of genetic distances among selected clones of globe artichoke (Cynara cardunculus L. var. scolymus L.). J. Hortic. Sci. Biotechnol. 2004, 79, 863–870. [Google Scholar]
- Portis, E.; Acquadro, A.; Comino, C.; Mauromicale, G.; Saba, E.; Lanteri, S. Genetic structure of island populations of wild cardoon [Cynara cardunculus L. var. sylvestris (Lamk) Fiori] detected by AFLPs and SSRs. Plant Sci. 2005, 169, 199–210. [Google Scholar] [CrossRef]
- Acquadro, A.; Portis, E.; Lee, D.; Donini, P.; Lanteri, S. Development and characterization of microsatellite markers in Cynara cardunculus L. Genome 2005, 48, 217–225. [Google Scholar] [CrossRef]
- Sarmento, A.C.; Lopes, H.; Oliveira, C.S.; Vitorino, R.; Samyn, B.; Sergeant, K.; Debyser, G.; van Beeumen, J.; Domingues, P.; Amado, F.; et al. Multiplicity of aspartic proteinases from Cynara cardunculus L. Planta 2009, 230, 429–439. [Google Scholar] [CrossRef]
- Abrantes, S.; Amaral, M.E.; Costa, A.P.; Duarte, A.P. Cynara cardunculus L. alkaline pulps: Alternatives fibres for paper and paperboard production. Bioresour. Technol. 2007, 98, 2873–2878. [Google Scholar] [CrossRef]
- Fernández, J.; Curt, M.D.; Aguado, P.L. Industrial applications of Cynara cardunculus L. for energy and other uses. Ind. Crops Prod. 2006, 24, 222–229. [Google Scholar] [CrossRef]
- Fratianni, F.; Tucci, M.; de Palma, M.; Pepe, R.; Nazzaro, F. Polyphenolic composition in different parts of some cultivars of globe artichoke (Cynara cardunculus L. var. scolymus (L.) Fiori). Food Chem. 2007, 104, 1282–1286. [Google Scholar] [CrossRef]
- Valentão, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J. Agric. Food Chem. 2002, 50, 4989–4993. [Google Scholar] [CrossRef]
- Trajtemberg, S.P.; Apóstolo, N.M.; Fernández, G. Calluses of Cynara cardunculus var. cardunculus cardoon (Asteraceae): Determination of cynarine and chlorogenic acid by automated high-preformance capillary electrophoresis. In Vitro Cell. Dev. Biol. Plant 2006, 42, 534–537. [Google Scholar] [CrossRef]
- Pinelli, P.; Agostini, F.; Comino, C.; Lanteri, S.; Portis, E.; Romani, A. Simultaneous quantification of caffeoyl esters and flavonoids in wild and cultivated cardoon leaves. Food Chem. 2007, 105, 1695–1701. [Google Scholar] [CrossRef]
- Bezáková, L.; Grancai, D.; Oblozinsky, M.; Vanko, M.; Holková, I.; Pauliková, I.; Garaj, V.; Gáplovský, M. Effects of flavonoids and cynarine from Cynara cardunculus L. on lipoxygenase activity. Acta Fac. Pharm. Univ. Comen. 2007, Tomus LIV, 48–52. [Google Scholar]
- Sevcikova, P.; Glatz, Z.; Slanina, J. Analysis of artichoke (Cynara cardunculus L.) extract by means of micellar electrokinetic capillary chromatography. Electrophoresis 2002, 23, 249–252. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef]
- Gebhardt, R.; Fausel, M. Antioxidant and hepatoprotective effects of artichoke extracts and constituents in cultured rat hepatocytes. Toxicol. in Vitro 1997, 11, 669–672. [Google Scholar] [CrossRef]
- Krizkova, L.; Mucaji, P.; Nagy, M.; Krajcovic, J. Triterpenoid cynarasaponins from Cynara cardunculus L. reduce chemically induced mutagenesis in vitro. Phytomedicine 2004, 11, 673–678. [Google Scholar] [CrossRef]
- Zi, X.; Agarwal, R. Modulation of mitogen-activated protein kinase activation and cell cycle regulators by the potent skin cancer preventive agent silymarin. Biochem. Biophys. Res. Commun. 1999, 263, 528–536. [Google Scholar] [CrossRef]
- Varghese, L.; Agarwal, C.; Tyagi, A.; Singh, R.P.; Agarwal, R. Silibinin efficacy against human hepatocellular carcinoma. Clin. Cancer Res. 2005, 11, 8441–8448. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Carrington, M.F.; Walsh, N.A. Extracts from dulse (Palmaria palmata) are effective antioxidants and inhibitors of cell proliferation in vitro. Food Chem. Toxicol. 2005, 43, 1073–1081. [Google Scholar] [CrossRef]
- Aruoma, O.I. Nutrition and health aspects of free radicals and antioxidants. Food Chem. Toxicol. 1994, 32, 671–683. [Google Scholar] [CrossRef]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Barrett, J.C.; Wiseman, R.W. Cellular and molecular mechanisms of multistep carcinogenesis: Relevance to carcinogen risk assessment. Environ. Health Perspect. 1987, 76, 65–70. [Google Scholar] [CrossRef]
- Valentao, P.; Fernandes, E.; Carvalho, F.; Andrade, P.B.; Seabra, R.M.; Bastos, M.L. Antioxidative properties of cardoon (Cynara cardunculus L.) infusion against superoxide radical, hydroxyl radical, and hypochlorous acid. J. Agric. Food Chem. 2002, 50, 4989–4993. [Google Scholar] [CrossRef]
- Shen, Q.; Dai, Z.; Lu, Y. Rapid determination of caffeoylquinic acid derivatives in Cynara scolymus L. by ultra-fast liquid chromatography/tandem mass spectrometry based on a fused core C18 column. J. Sep. Sci. 2010, 33, 3152–3158. [Google Scholar] [CrossRef]
- Wider, B.; Pittler, M.H.; Thompson-Coon, J.; Ernst, E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst. Rev. 2009. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of tunisian halophytes. Compte Rendues de Biologies 2008, 331, 865–873. [Google Scholar] [CrossRef]
- Sengul, M.; Yildiz, H.; Gungor, N.; Okcu, Z. Total phenolic content, antioxidant activity, some physical and chemical properties of pestil. Asian J. Chem. 2010, 22, 448–454. [Google Scholar]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef]
- Swerdlow, A.J.; dos Santos Silva, I.; Reid, A.; Qiao, Z.; Brewster, D.H.; Arrundale, J. Trends in cancer incidence and mortality in scotland: Description and possible explanations. Br. J. Cancer 1998, 77, 1–54. [Google Scholar]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Bhat, T.A.; Singh, R.P. Tumor angiogenesis-a potential target in cancer chemoprevention. Food Chem. Toxicol. 2008, 46, 1334–1345. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Szabo, M.R.; Iditoiu, C.; Chambre, D.; Lupea, A.X. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicryl-hydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Motic Images Plus 2.0 Software, version 2.0, Motic Spain, S.L.U.: Barcelona, Spain, 2010.
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Tran, T.C.; Sneed, B.; Haider, J.; Blavo, D.; White, A.; Aiyejorun, T.; Baranowski, T.C.; Rubinstein, A.L.; Doan, T.N.; Dingledine, R.; et al. Automated, quantitative screening assay for antiangiogenic compounds using transgenic zebrafish. Cancer Res. 2007, 67, 11386–11392. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Velez, Z.; Campinho, M.A.; Guerra, Â.R.; García, L.; Ramos, P.; Guerreiro, O.; Felício, L.; Schmitt, F.; Duarte, M. Biological Characterization of Cynara cardunculus L. Methanolic Extracts: Antioxidant, Anti-proliferative, Anti-migratory and Anti-angiogenic Activities. Agriculture 2012, 2, 472-492. https://doi.org/10.3390/agriculture2040472
Velez Z, Campinho MA, Guerra ÂR, García L, Ramos P, Guerreiro O, Felício L, Schmitt F, Duarte M. Biological Characterization of Cynara cardunculus L. Methanolic Extracts: Antioxidant, Anti-proliferative, Anti-migratory and Anti-angiogenic Activities. Agriculture. 2012; 2(4):472-492. https://doi.org/10.3390/agriculture2040472
Chicago/Turabian StyleVelez, Zélia, Marco A. Campinho, Ângela R. Guerra, Laura García, Patricia Ramos, Olinda Guerreiro, Laura Felício, Fernando Schmitt, and Maria Duarte. 2012. "Biological Characterization of Cynara cardunculus L. Methanolic Extracts: Antioxidant, Anti-proliferative, Anti-migratory and Anti-angiogenic Activities" Agriculture 2, no. 4: 472-492. https://doi.org/10.3390/agriculture2040472
APA StyleVelez, Z., Campinho, M. A., Guerra, Â. R., García, L., Ramos, P., Guerreiro, O., Felício, L., Schmitt, F., & Duarte, M. (2012). Biological Characterization of Cynara cardunculus L. Methanolic Extracts: Antioxidant, Anti-proliferative, Anti-migratory and Anti-angiogenic Activities. Agriculture, 2(4), 472-492. https://doi.org/10.3390/agriculture2040472








