New Horizons in Early Dementia Diagnosis: Can Cerebellar Stimulation Untangle the Knot?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
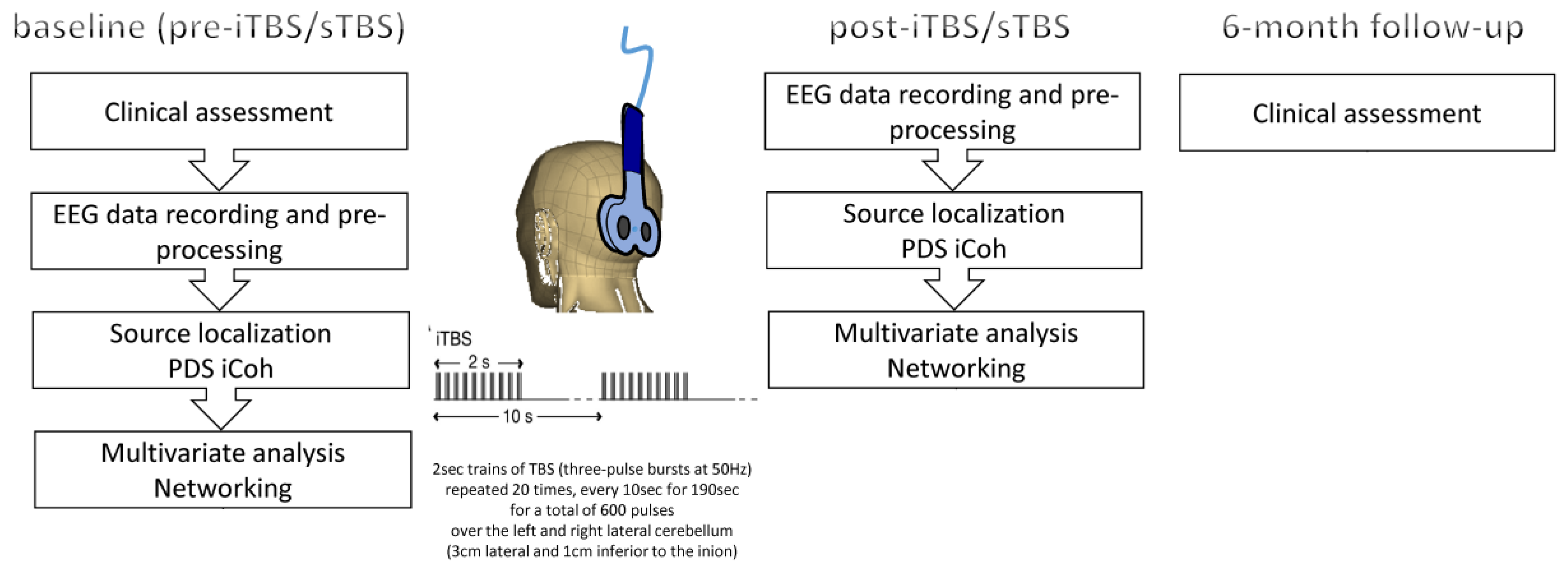
2.2. Experimental Procedure
2.3. EEG Recording and Analysis
2.4. Statistical Analysis
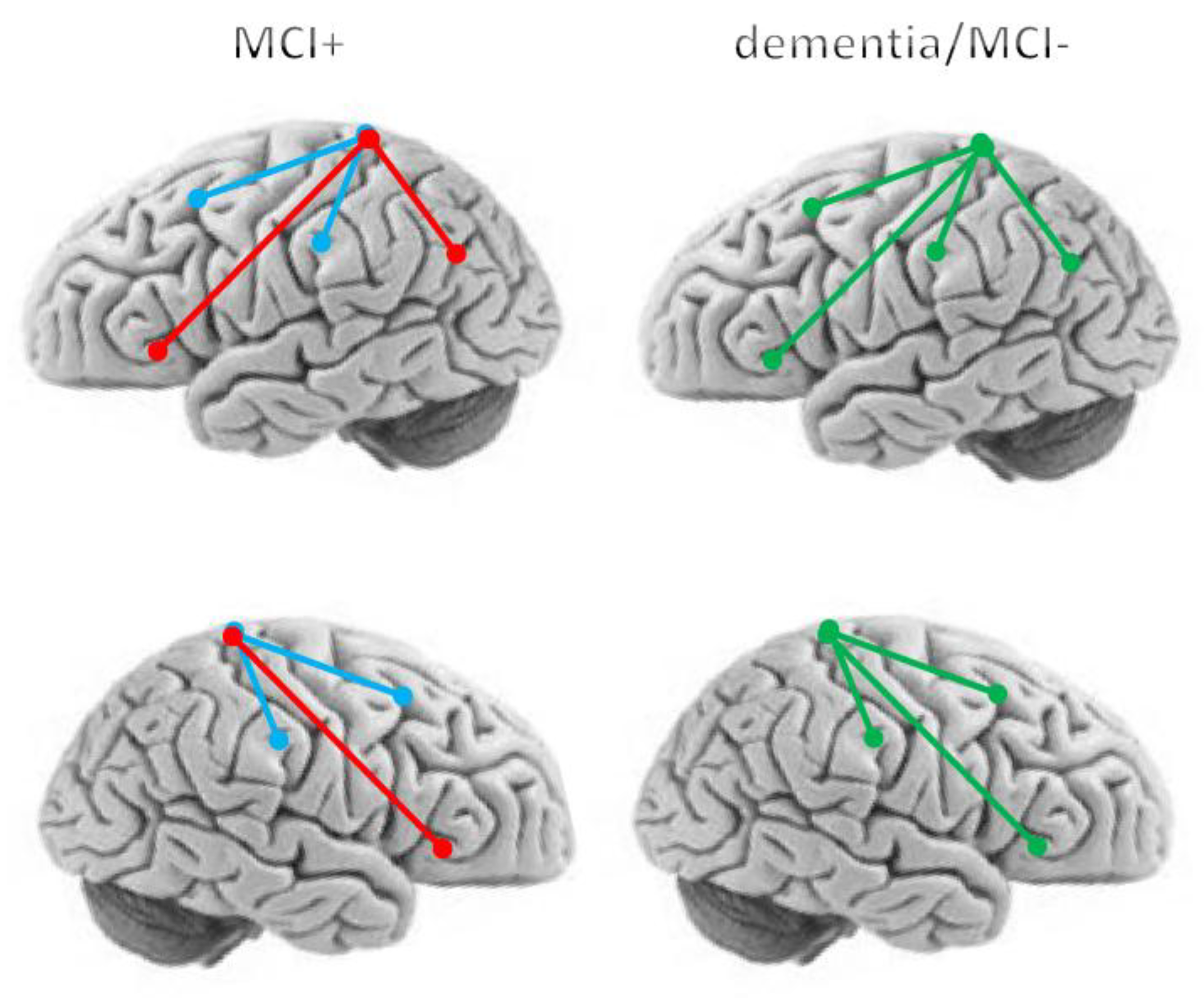
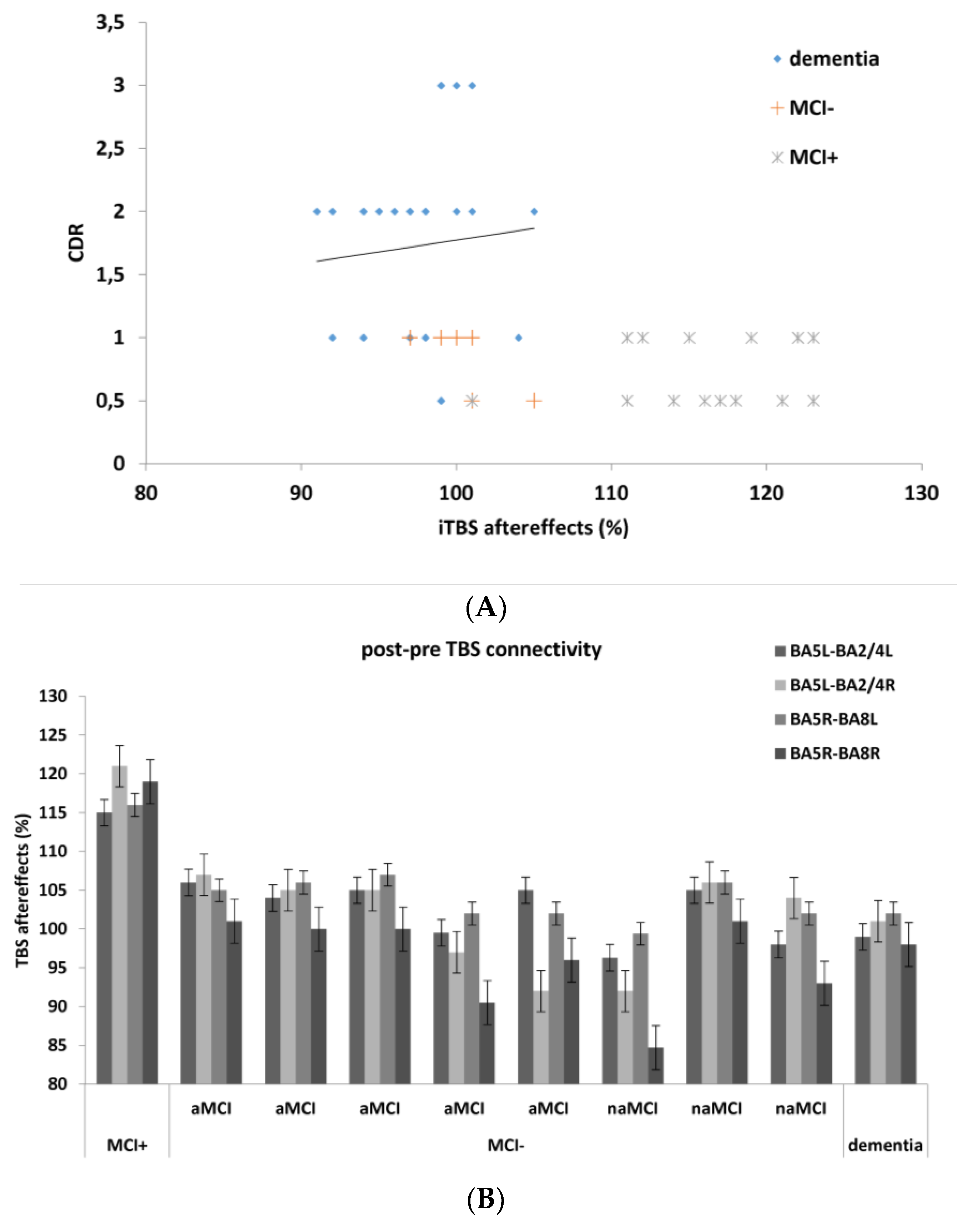
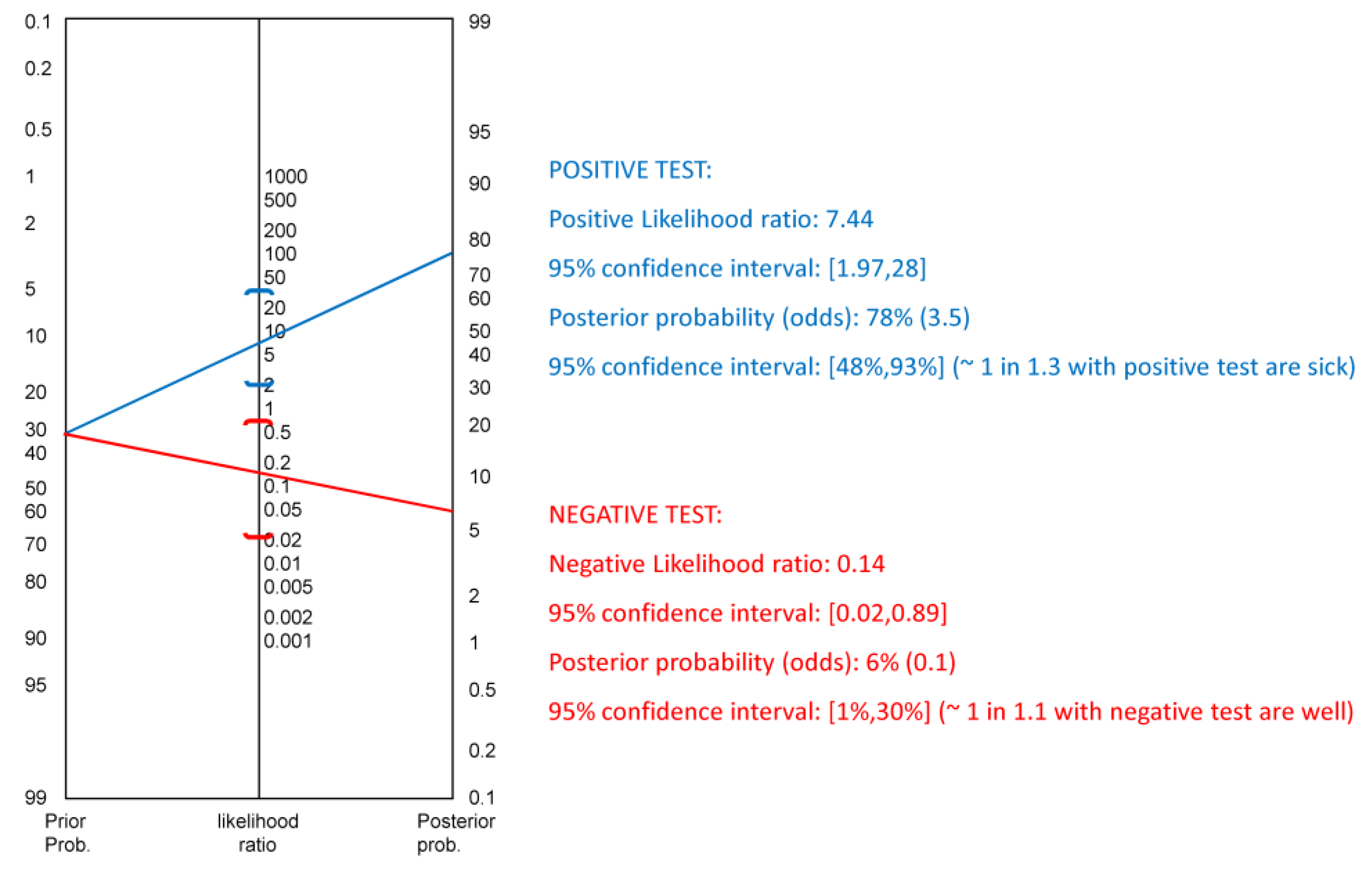
3. Results
4. Discussion
4.1. Neurophysiological Basis of Cerebellar iTBS Aftereffects
4.2. Limitations
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Mayeux, R.; Stern, Y. Epidemiology of Alzheimer disease. Cold Spring Harbor Perspect. Med. 2012, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Paterson, R.W.; Warren, J.D.; Zetterberg, H.; O’Brien, J.T.; Fox, N.C.; Halliday, G.M.; Schott, J.M. Biomarkers in dementia: Clinical utility and new directions. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Marra, A.; Naro, A.; Chillura, A.; Bramanti, A.; Maresca, G.; De Luca, R.; Manuli, A.; Bramanti, P.; Calabrò, R.S. Evaluating Peripersonal Space through the Functional Transcranial Doppler: Are We Paving the Way for Early Detecting Mild Cognitive Impairment to Dementia Conversion? J. Alzheimer’s Dis. 2018, 62, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Drago, V.; Babiloni, C.; Bartrés-Faz, D.; Caroli, A.; Bosch, B.; Hensch, T.; Didic, M.; Klafki, H.-W.; Pievani, M.; Jovicich, J.; et al. Disease Tracking Markers for Alzheimer’s Disease at the Prodromal (MCI) Stage. J. Alzheimer’s Dis. 2011, 26, 159–199. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Tardiff, S.; Dye, C.; Arrighi, H.M. Rate of Conversion from Prodromal Alzheimer’s Disease to Alzheimer’s Dementia: A Systematic Review of the Literature. Dement. Geriatr. Cognit. Disord. EXTRA 2013, 3, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.; Güntekin, B.; Tülay, E.; Yener, G.G. Evoked and event related coherence of Alzheimer patients manifest differentiation of sensory–cognitive networks. Brain Res. 2010, 1357, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Yener, G.G.; Başar, E. Brain oscillations as biomarkers in neuropsychiatric disorders. Suppl. Clin. Neurophysiol. 2013, 62, 343–363. [Google Scholar] [PubMed]
- Yener, G.G.; Başar, E. Sensory evoked and event related oscillations in Alzheimer’s disease: A short review. Cogn. Neurodyn. 2010, 4, 263–274. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef]
- Pievani, M.; De Haan, W.; Wu, T.; Seeley, W.W.; Frisoni, G.B. Functional Network Disruption in the Degenerative Dementias. Lancet Neurol. 2011, 10, 829–843. [Google Scholar] [CrossRef]
- Seeley, W.W.; Crawford, R.K.; Zhou, J.; Miller, B.L.; Greicius, M.D. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009, 62, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, M.; Dai, Z.; Liang, X.; Song, H.; He, Y.; Li, K. Differentially disrupted functional connectivity of the sub-regions of the inferior parietal lobule in Alzheimer’s disease. Brain Struct. Funct. 2015, 220, 745–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, C.; Zhao, C.; Qi, Z.; Zhou, W.; Lu, J.; He, Y.; Li, K. Spatial patterns of intrinsic brain activity in mild cognitive impairment and Alzheimer’s disease: A resting-state functional MRI study. Hum. Brain Mapp. 2011, 32, 1720–1740. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jia, X.; Liang, P.; Qi, Z.; Yang, Y.; Zhou, W.; Li, K. Changes in thalamus connectivity in mild cognitive impairment: Evidence from resting state fMRI. Eur. J. Radiol. 2012, 81, 277–285. [Google Scholar] [CrossRef]
- Wang, L.; Zang, Y.; He, Y.; Liang, M.; Zhang, X.; Tian, L.; Wu, T.; Jiang, T.; Li, K. Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: Evidence from resting state fMRI. Neuroimage 2006, 31, 496–504. [Google Scholar] [CrossRef]
- Wang, K.; Liang, M.; Wang, L.; Tian, L.; Zhang, X.; Li, K.; Jiang, T. Altered functional connectivity in early Alzheimer’s disease: A resting state fMRI study. Hum. Brain Mapp. 2007, 28, 967–978. [Google Scholar] [CrossRef]
- Zhang, H.-Y.; Wang, S.-J.; Liu, B.; Ma, Z.-L.; Yang, M.; Zhang, Z.-J.; Teng, G.-J. Resting Brain Connectivity: Changes during the Progress of Alzheimer Disease. Radiology 2010, 256, 598–606. [Google Scholar] [CrossRef]
- Bai, F.; Shu, N.; Yuan, Y.; Shi, Y.; Yu, H.; Wu, D.; Wang, J.; Xia, M.; He, Y.; Zhang, Z. Topologically Convergent and Divergent Structural Connectivity Patterns between Patients with Remitted Geriatric Depression and Amnestic Mild Cognitive Impairment. J. Neurosci. 2012, 32, 4307–4318. [Google Scholar] [CrossRef]
- Schmahmannm, J.D.; Pandya, D.N. Disconnection syndromes of basal ganglia, thalamus, and cerebro-cerebellar systems. Cortex 2008, 44, 1037–1066. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Marra, C.; Quaranta, D.; Vita, M.G.; Bramanti, P.; Rossini, P.M. Human brain networks in cognitive decline: A graph theoretical analysis of cortical connectivity from EEG data. J. Alzheimer’s Dis. 2014, 41, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, F.; Lizio, R.; Boccardi, M.; Prestia, A.; Frisoni, G.; Babiloni, C.; Rossini, P. P62: Occipital sources of resting state alpha rhythms are related to local gray matter density in subjects with amnesic mild cognitive impairment and Alzheimer’s disease. Clin. Neurophysiol. 2014, 125, S66. [Google Scholar] [CrossRef]
- Hsiao, F.J.; Chen, W.T.; Wang, Y.J.; Yan, S.H.; Lin, Y.Y. Altered source-based EEG coherence of resting-state sensorimotor network in early-stage Alzheimer’s disease compared to mild cognitive impairment. Neurosci. Lett. 2014, 558, 47–52. [Google Scholar] [CrossRef] [PubMed]
- McBride, J.; Zhao, X.; Munro, N.; Smith, C.; Jicha, G.; Jiang, Y. Resting EEG Discrimination of Early Stage Alzheimer’s Disease from Normal Aging Using Inter-Channel Coherence Network Graphs. Ann. Biomed. Eng. 2013, 41, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Moretti, D.V.; Frisoni, G.B.; Binetti, G.; Zanetti, O. Anatomical substrate and scalp EEG markers are correlated in subjects with cognitive impairment and Alzheimer’s disease. Front. Psychiatry 2011, 1, 152. [Google Scholar] [CrossRef] [PubMed]
- Rossini, P.; Del Percio, C.; Pasqualetti, P.; Cassetta, E.; Binetti, G.; Forno, G.D.; Ferreri, F.; Frisoni, G.B.; Chiovenda, P.; Miniussi, C.; et al. Conversion from mild cognitive impairment to Alzheimer’s disease is predicted by sources and coherence of brain electroencephalography rhythms. Neuroscience 2006, 143, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Bramanti, A.; Leo, A.; Manuli, A.; Sciarrone, F.; Russo, M.; Bramanti, P.; Calabrò, R.S. Effects of cerebellar transcranial alternating current stimulation on motor cortex excitability and motor function. Brain Struct. Funct. 2017, 222, 2891–2906. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Leo, A.; Russo, M.; Cannavò, A.; Milardi, D.; Bramanti, P.; Calabrò, R.S. Does Transcranial Alternating Current Stimulation Induce Cerebellum Plasticity? Feasibility, Safety and Efficacy of a Novel Electrophysiological Approach. Brain Stimul. 2016, 9, 388–395. [Google Scholar] [CrossRef]
- Naro, A.; Milardi, D.; Cacciola, A.; Russo, M.; Sciarrone, F.; La Rosa, G.; Bramanti, A.; Calabrò, R.S. What Do We Know About the Influence of the Cerebellum on Walking Ability? Promising Findings from Transcranial Alternating Current Stimulation. Cerebellum 2017, 16, 859–867. [Google Scholar] [CrossRef]
- Rastogi, A.; Cash, R.; Dunlop, K.; Vesia, M.; Kucyi, A.; Ghahremani, A.; Downar, J.; Chen, J.; Chen, R. Modulation of cognitive cerebello-cerebral functional connectivity by lateral cerebellar continuous theta burst stimulation. Neuroimage 2017, 158, 48–57. [Google Scholar] [CrossRef]
- Tremblay, S.; Austin, D.; Hannah, R.; Rothwell, J.C. Non-invasive brain stimulation as a tool to study cerebellar-M1 interactions in humans. Cereb. Ataxias 2016, 3, 957. [Google Scholar] [CrossRef] [PubMed]
- Habas, C.; Kamdar, N.; Nguyen, D.; Keller, K.; Beckmann, C.F.; Menon, V.; Greicius, M.D. Distinct Cerebellar Contributions to Intrinsic Connectivity Networks. J. Neurosci. 2009, 29, 8586–8594. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.I.L.; Hopkins, D.A.; Mayrhofer, H.C.; Bruner, E.; Van Leeuwen, F.W.; Raaijmakers, W.; Schmahmann, J.D. The cerebellum in Alzheimer’s disease: Evaluating its role in cognitive decline. Brain 2017, 141, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D.; Anderson, C.M.; Newton, N.; Ellis, R.D. The function of the cerebellum in cognition, affect and consciousness. Conscious. Emot. 2001, 2, 273–309. [Google Scholar] [CrossRef][Green Version]
- Schmahmann, J.D.; Pandya, D.N. The cerebrocerebellar system. In The Cerebellum and Cognition; Schmahmann, J.D., Ed.; Academic Press: San Diego, CA, USA, 1997; Volume 41, pp. 31–60. [Google Scholar]
- Larner, A.J. The Cerebellum in Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 1997, 8, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Sherman, J.C.; Schmahmann, J.D. The cerebellar cognitive affective syndrome. Brain 1998, 121, 561–579. [Google Scholar]
- Schmahmann, J.D.; Weilburg, J.B.; Sherman, J.C. The neuropsychiatry of the cerebellum—Insights from the clinic. Cerebellum 2007, 6, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Schmahmann, J.D. An emerging concept. The cerebellar contribution to higher function. Arch. Neurol. 1991, 48, 1178–1187. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Cerebellum in Alzheimer’s disease and frontotemporal dementia: Not a silent bystander. Brain 2016, 139, 1314–1318. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Disorders of the Cerebellum: Ataxia, Dysmetria of Thought, and the Cerebellar Cognitive Affective Syndrome. J. Neuropsychiatry Clin. Neurosci. 2004, 16, 367–378. [Google Scholar] [CrossRef]
- Schmahmann, J.D. Dysmetria of thought: Clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn. Sci. 1998, 2, 362–371. [Google Scholar] [CrossRef]
- Schmahmann, J.D. From movement to thought: Anatomic substrates of the cerebellar contribution to cognitive processing. Hum. Brain Mapp. 1996, 4, 174–198. [Google Scholar] [CrossRef]
- Schmahmann, J.D. The Cerebellum and Cognition; Academic: San Diego, CA, USA, 1997. [Google Scholar]
- Schwarz, C. The fate of spontaneous synchronous rhythms on the cerebro-cerebellar loop. Cerebellum 2010, 9, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.; Schmahmann, J. Functional topography in the human cerebellum: A meta-analysis of neuroimaging studies. NeuroImage 2009, 44, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Tzvi, E.; Zimmermann, C.; Bey, R.; Münte, T.F.; Nitschke, M.; Krämer, U.M. Cerebellar degeneration affects cortico-cortical connectivity in motor learning networks. NeuroImage Clin. 2017, 16, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Tan, R.; Hodges, J.R.; Hu, X.; Sami, S.; Hornberger, M. Network-selective vulnerability of the human cerebellum to Alzheimer’s disease and fronto-temporal dementia. Brain 2016, 139(Pt. 5), 1527–1538. [Google Scholar] [CrossRef] [PubMed]
- Castellazzi, G.; Palesi, F.; Casali, S.; Vitali, P.; Sinforiani, E.; Wheeler-Kingshott, C.A.M.; D’Angelo, E.U. A comprehensive assessment of resting state networks: Bidirectional modification of functional integrity in cerebro-cerebellar networks in dementia. Front. Mol. Neurosci. 2014, 8, 223. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, X.; Song, H.; Li, K.; Wang, Z. Altered Functional Connectivity of Cognitive-Related Cerebellar Subregions in Alzheimer’s Disease. Front. Aging Neurosci. 2017, 9, 143. [Google Scholar] [CrossRef]
- Guevara, M.A.; Corsi-Cabrera, M. EEG Coherence or EEG Correlation International. J. Psychophysiol. 1996, 23, 145–153. [Google Scholar] [CrossRef]
- Casula, E.; Ponzo, V.; Bassi, M.S.; Veniero, D.; Caltagiron, C.; Koch, G. P221 Cerebellar theta burst stimulation modulates the neural activity of interconnected parietal and motor areas. Clin. Neurophysiol. 2017, 128, e121–e122. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D. High-frequency brain activity and muscle artifacts in MEG/EEG: A review and recommendations. Front. Hum. Neurosci. 2013, 7, 138. [Google Scholar] [CrossRef]
- Dai, Z.; Yan, C.; Wang, Z.; Wang, J.; Xia, M.; Li, K.; He, Y. Discriminative analysis of early Alzheimer’s disease using multi-modal imaging and multilevel characterization with multi-classifier (M3). Neuroimage 2012, 59, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.I.; Radua, J.; Lückmann, H.C.; Sack, A.T. Meta-analysis of functional network alterations in Alzheimer’s disease: Toward a network biomarker. Neurosci. Biobehav. Rev. 2013, 37, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Van Dun, K.; Van Overwalle, F.; Manto, M.; Marien, P. Targeting the Cerebellum by Noninvasive Neurostimulation: A Review. Cerebellum 2017, 16, 695–741. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, G.; Argyropoulos, G.P.; Bastian, A.; Cortes, M.; Davis, N.J.; Edwards, D.J.; Ferrucci, R.; Fregni, F.; Galea, J.M.; Hamada, M.; et al. Cerebellar Transcranial Direct Current Stimulation (ctDCS). Neuroscience 2014, 22, 83–97. [Google Scholar] [CrossRef]
- Grimaldi, G.; Argyropoulos, G.P.; Boehringer, A.; Celnik, P.; Edwards, M.J.; Ferrucci, R.; Galea, J.M.; Groiss, S.J.; Hiraoka, K.; Kassavetis, P.; et al. Non-invasive cerebellar stimulation—A consensus paper. Cerebellum 2014, 13, 121–138. [Google Scholar] [CrossRef]
- Minks, E.; Kopickova, M.; Marecek, R.; Streitova, H.; Bares, M. Transcranial magnetic stimulation of the cerebellum. Biomed. Pap. 2010, 154, 133–139. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Rocchi, L.; Casula, E.; Tocco, P.; Berardelli, A.; Rothwell, J. Somatosensory Temporal Discrimination Threshold Involves Inhibitory Mechanisms in the Primary Somatosensory Area. J. Neurosci. 2016, 36, 325–335. [Google Scholar] [CrossRef]
- Koch, G.; Rothwell, J.; Mori, F.; Marconi, B.; Oliveri, M.; Caltagirone, C. THO10 Changes in intracortical circuits of the human motor cortex following theta burst stimulation of the lateral cerebellum. Clin. Neurophysiol. 2008, 119, S29–S30. [Google Scholar] [CrossRef]
- Hallett, M.; Di Iorio, R.; Rossini, P.M.; Park, J.E.; Chen, R.; Celnik, P.; Strafella, A.P.; Matsumoto, H.; Ugawa, Y. Contribution of transcranial magnetic stimulation to assessment of brain connectivity and networks. Clin. Neurophysiol. 2017, 128, 2125–2139. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Di Iorio, R.; Rossini, P.M.; Park, J.E.; Chen, R.; Celnik, P.; Strafella, A.P.; Matsumoto, H.; Ugawa, Y. Exploring the connectivity between the cerebellum and motor cortex in humans. J. Physiol. 2004, 557 Pt 2, 689–700. [Google Scholar]
- Ferrucci, R.; Bocci, T.; Cortese, F.; Ruggiero, F.; Priori, A. Cerebellar transcranial direct current stimulation in neurological disease. Cereb. Ataxias 2016, 3, 16. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.; Strigaro, G.; Murase, N.; Sadnicka, A.; Galea, J.M.; Edwards, M.J.; Rothwell, J.C. Cerebellar modulation of human associative plasticity. J. Physiol. 2012, 590, 2365–2374. [Google Scholar] [CrossRef] [PubMed]
- Popa, D.; Spolidoro, M.; Proville, R.D.; Guyon, N.; Belliveau, L.; Léna, C. Functional Role of the Cerebellum in Gamma-Band Synchronization of the Sensory and Motor Cortices. J. Neurosci. 2013, 33, 6552–6556. [Google Scholar] [CrossRef]
- Popa, T.; Velayudhan, B.; Hubsch, C.; Pradeep, S.; Roze, E.; Vidailhet, M.; Meunier, S.; Kishore, A. Cerebellar processing of sensory inputs primes motor cortex plasticity. Cereb. Cortex 2013, 23, 305–314. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Martorana, A.; Ponzo, V.; Bonnì, S.; D’Angelo, E.U.; Caltagirone, C.; Koch, G. Cerebellar theta burst stimulation modulates short latency afferent inhibition in Alzheimer’s disease patients. Front. Aging Neurosci. 2013, 5, 2. [Google Scholar] [CrossRef]
- Hintzen, A.; Pelzer, E.A.; Tittgemeyer, M. Thalamic interactions of cerebellum and basal ganglia. Brain Struct. Funct. 2018, 223, 569–587. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Albert, M.S.; Knopman, D.S.; McKhann, G.M.; Sperling, R.A.; Carrillo, M.C.; Thies, B.; Phelps, C.H. Introduction to the recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 257–262. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current vision and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. B Boil. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Fuchs, M.; Kastner, J.; Wagner, M.; Hawes, S.; Ebersole, J.S. A standardized boundary element method volume conductor model. Clin. Neurophysiol. 2002, 113, 702–712. [Google Scholar] [CrossRef]
- Brett, M.; Johnsrude, I.S.; Owen, A.M. The problem of functional localization in the human brain. Nat. Rev. Neurosci. 2002, 3, 243–249. [Google Scholar] [CrossRef]
- Brunet, D.; Murray, M.M.; Michel, C.M. Spatiotemporal Analysis of Multichannel EEG: CARTOOL. Comput. Intell. Neurosci. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Vincent, J.L.; Kahn, I.; Snyder, A.Z.; Raichle, M.E.; Buckner, R.L. Buckner Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008, 100, 3328–3342. [Google Scholar] [CrossRef]
- Hata, M.; Kazui, H.; Tanaka, T.; Ishii, R.; Canuet, L.; Pascual-Marqui, R.D.; Aoki, Y.; Ikeda, S.; Kanemoto, H.; Yoshiyama, K.; et al. Functional connectivity assessed by resting state EEG correlates with cognitive decline of Alzheimer’s disease—An eLORETA study. Clin. Neurophysiol. 2016, 127, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Jurcak, V.; Tsuzuki, D.; Dan, I. 10/20, 10/10, and 10/5 systems revisited: Their validity as relative head-surface-based positioning systems. NeuroImage 2007, 34, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Douw, L.; Nieboer, D.; Stam, C.J.; Tewarie, P.; Hillebrand, A. Consistency of magnetoencephalographic functional connectivity and network reconstruction using a template versus native MRI for co-registration. Hum. Brain Mapp. 2018, 39, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Mehrkanoon, S.; Breakspear, M.; Boonstra, T.W. Low-dimensional dynamics of resting-state cortical activity. Brain Topogr. 2014, 27, 338–352. [Google Scholar] [CrossRef] [PubMed]
- Mehrkanoon, S.; Breakspear, M.; Daffertshofer, A.; Boonstra, T.W. Non-identical smoothing operators for estimating time-frequency interdependence in electrophysiological recordings. EURASIP J. Adv. Signal Process. 2013, 2013, 73. [Google Scholar] [CrossRef]
- Nolte, G.; Bai, O.; Wheaton, L.; Mari, Z.; Vorbach, S.; Hallett, M. Identifying true brain interaction from EEG data using the imaginary part of coherency. Clin. Neurophysiol. 2004, 115, 2292–2307. [Google Scholar] [CrossRef] [PubMed]
- Drakesmith, M.; El-Deredy, W.; Welbourne, S. Reconstructing Coherent Networks from Electroencephalography and Magnetoencephalography with Reduced Contamination from Volume Conduction or Magnetic Field Spread. PLoS ONE 2015, 8, e81553. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, A.R.; Lobaugh, N.J. Partial least squares analysis of neuroimaging data: Applications and advances. NeuroImage 2004, 23, S250–S263. [Google Scholar] [CrossRef]
- Langdon, A.J.; Boonstra, T.W.; Breakspear, M. Multi-frequency phase locking in human somatosensory cortex. Prog. Biophys. Mol. Boil. 2011, 105, 58–66. [Google Scholar] [CrossRef]
- Mehrkanoon, S.; Boonstra, T.W.; Breakspear, M.; Hinder, M.; Summers, J.J. Upregulation of cortico-cerebellar functional connectivity after motor learning. NeuroImage 2016, 128, 252–263. [Google Scholar] [CrossRef]
- Dong, J.; Jing, B.; Ma, X.; Liu, H.; Mo, X.; Li, H. Hurst Exponent Analysis of Resting-State fMRI Signal Complexity across the Adult Lifespan. Front. Mol. Neurosci. 2018, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Hurst, H.E. Long term storage capacity of reservoirs. Trans. Am. Soc. Civil. Eng. 1951, 116, 776–808. [Google Scholar]
- Deeks, J.J.; Altman, D.G. Diagnostic tests 4: likelihood ratios. BMJ 2004, 329, 168–169. [Google Scholar] [CrossRef] [PubMed]
- Maroco, J.; Silva, D.; Rodrigues, A.; Guerreiro, M.; Santana, I.; De Mendonça, A. Data mining methods in the prediction of Dementia: A real-data comparison of the accuracy, sensitivity and specificity of linear discriminant analysis, logistic regression, neural networks, support vector machines, classification trees and random forests. BMC Res. Notes 2011, 4, 299. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.rad.jhmi.edu/jeng/javarad/roc/JROCFITi.html.
- Kujala, J.; Pammer, K.; Cornelissen, P.; Roebroeck, A.; Formisano, E.; Salmelin, R. Phase coupling in a cerebro-cerebellar network at 8–13 Hz during reading. Cereb. Cortex 2007, 17, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Pollok, B.; Krause, V.; Butz, M.; Schnitzler, A. Modality specific functional interaction in sensorimotor synchronization. Hum. Brain Mapp. 2009, 30, 1783–1790. [Google Scholar] [CrossRef]
- Ben-Soussan, T.D.; Glicksohn, J.; Berkovich-Ohana, A. From Cerebellar Activation and Connectivity to Cognition: A Review of the Quadrato Motor Training. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Castellanos, N.P.; Paúl, N.; Ordóñez, V.E.; Demuynck, O.; Bajo, R.; Campo, P.; Bilbao, A.; Ortiz, T.; Del-Pozo, F.; Maestú, F. Reorganization of functional connectivity as a correlate of cognitive recovery in acquired brain injury. Brain 2010, 133, 2365–2381. [Google Scholar] [CrossRef]
- Garcés, P.; Pereda, E.; Hernández-Tamames, J.A.; Del-Pozo, F.; Maestú, F.; Pineda-Pardo, J. Ángel Multimodal description of whole brain connectivity: A comparison of resting state MEG, fMRI, and DWI. Hum. Brain Mapp. 2015, 37, 20–34. [Google Scholar] [CrossRef]
- Ponsen, M.M.; Stam, C.J.; Bosboom, J.L.W.; Berendse, H.W.; Hillebrand, A. A three dimensional anatomical view of oscillatory resting-state activity and functional connectivity in Parkinson’s disease related dementia: An MEG study using atlas-based beamforming. Neuroimage Clin. 2013, 2, 95–102. [Google Scholar] [CrossRef]
- Başar, E.; Düzgün, A. The CLAIR model: Extension of Brodmann areas based on brain oscillations and connectivity. Int. J. Psychophysiol. 2016, 103, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Bookheimer, S.Y.; Strojwas, M.H.; Cohen, M.S.; Saunders, A.M.; Pericak-Vance, M.A.; Mazziotta, J.C.; Small, G.W. Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 2000, 343, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Haegens, S.; Osipova, D.; Oostenveld, R.; Jensen, O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum. Brain Mapp. 2010, 31, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Klimesch, W.; Sauseng, P.; Hanslmayr, S. EEG alpha oscillations: The inhibition—Timing hypothesis. Brain Res. Rev. 2007, 53, 63–88. [Google Scholar] [CrossRef] [PubMed]
- Naro, A.; Corallo, F.; De Salvo, S.; Marra, A.; Di Lorenzo, G.; Muscarà, N.; Russo, M.; Marino, S.; De Luca, R.; Bramanti, P.; et al. Promising Role of Neuromodulation in Predicting the Progression of Mild Cognitive Impairment to Dementia. J. Alzheimer’s Dis. 2016, 53, 1375–1388. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, E.; Casali, S. Seeking a unified framework for cerebellar function and dysfunction: From circuit operations to cognition. Front. Neural Circuits 2016, 6, 116. [Google Scholar] [CrossRef] [PubMed]
- Guggisberg, A.G.; Dalal, S.S.; Findlay, A.M.; Nagarajan, S.S. High-Frequency Oscillations in Distributed Neural Networks Reveal the Dynamics of Human Decision Making. Front. Hum. Neurosci. 2008, 1, 14. [Google Scholar] [CrossRef]
- Baumann, O.; Borra, R.J.; Bower, J.M.; Cullen, K.E.; Habas, C.; Ivry, R.B.; Leggio, M.; Mattingley, J.B.; Molinari, M.; Moulton, E.A.; et al. Consensus Paper: The Role of the Cerebellum in Perceptual Processes. Cerebellum 2014, 14, 197–220. [Google Scholar] [CrossRef]
- Kellermann, T.; Regenbogen, C.; De Vos, M.; Mößnang, C.; Finkelmeyer, A.; Habel, U. Effective connectivity of the human cerebellum during visual attention. J. Neurosci. 2012, 32, 11453–11460. [Google Scholar] [CrossRef]
- Medina, J.F.; Mauk, M.D. Computer simulation of cerebellar information processing. Nat. Neurosci. 2000, 3, 1205–1211. [Google Scholar] [CrossRef]
- Steriade, M.; Deschenes, M. The thalamus as a neuronal oscillator. Brain Res. Rev. 1984, 8, 1–63. [Google Scholar] [CrossRef]
- Fonov, V.; Coupé, P.; Eskildsen, S.; Collins, L. Atrophy specific MRI brain template for Alzheimer’s disease and Mild Cognitive Impairment. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2011, 7, S58. [Google Scholar] [CrossRef]
- Puce, A.; Hämäläinen, M.S. A Review of Issues Related to Data Acquisition and Analysis in EEG/MEG Studies. Brain Sci. 2017, 7, 58. [Google Scholar] [CrossRef] [PubMed]


| Group | Age (y) | Education (y) | dd (y) | CDR | Brain Volume | Medications | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donepezil (mg/day) | SSRI | tzD | AED | TA | ATA | Other Drugs | ||||||
| aMCI (n = 13) | 75 | 6 | 4 | 0.5 | 0.71 | x | x | |||||
| 75 | 5 | 4 | 0.5 | 0.74 | x | x | ||||||
| 66 | 11 | 5 | 1 | 0.77 | x | |||||||
| 74 | 16 | 3 | 1 | 0.8 | x | x | ||||||
| 70 | 9 | 5 | 0.5 | 0.75 | ||||||||
| 70 | 16 | 2 | 1 | 0.75 | ||||||||
| 69 | 11 | 2 | 1 | 0.7 | x | x | x | |||||
| 69 | 15 | 3 | 0.5 | 0.71 | x | x | ||||||
| 68 | 16 | 3 | 0.5 | 0.74 | x | x | ||||||
| 68 | 17 | 3 | 1 | 0.77 | x | |||||||
| 71 | 5 | 3 | 1 | 0.71 | x | |||||||
| 67 | 14 | 4 | 1 | 0.74 | x | x | ||||||
| 72 | 12 | 2 | 1 | 0.76 | x | x | ||||||
| mean s.d. | 70 | 12 | 3 | 0.7 | 0.74 | |||||||
| 3 | 4 | 1 | 0.3 | 0.03 | ||||||||
| naMCI (n = 12) | 69 | 10 | 3 | 1 | 0.78 | |||||||
| 67 | 12 | 5 | 0.5 | 0.78 | x | x | x | |||||
| 74 | 11 | 3 | 0.5 | 0.79 | x | |||||||
| 65 | 15 | 4 | 1 | 0.78 | ||||||||
| 72 | 17 | 5 | 1 | 0.79 | x | |||||||
| 71 | 9 | 2 | 0.5 | 0.76 | x | |||||||
| 68 | 14 | 4 | 1 | 0.76 | x | |||||||
| 69 | 16 | 3 | 1 | 0.78 | ||||||||
| 67 | 17 | 4 | 0.5 | 0.75 | ||||||||
| 65 | 17 | 4 | 1 | 0.69 | ||||||||
| 62 | 8 | 5 | 1 | 0.71 | x | x | x | |||||
| 62 | 11 | 4 | 0.5 | 0.75 | x | |||||||
| mean s.d. | 68 | 13 | 4 | 0.8 | 0.76 | |||||||
| 4 | 3 | 1 | 0.2 | 0.03 | ||||||||
| grand mean s.d. | 69 | 12 | 4 | 1 | 0.75 | |||||||
| 1.9 | 1 | 0.3 | 0.03 | 0.01 | ||||||||
| AD (n = 12) | 68 | 16 | 4 | 2 | 0.7 | 13 | x | x | ||||
| 67 | 14 | 2 | 1 | 0.69 | 18 | x | x | x | x | |||
| 72 | 11 | 4 | 3 | 0.72 | 11 | x | x | x | x | |||
| 71 | 7 | 1 | 2 | 0.7 | 12 | x | x | |||||
| 71 | 7 | 4 | 2 | 0.69 | 13 | x | x | x | x | |||
| 74 | 15 | 3 | 3 | 0.6 | 17 | x | x | |||||
| 79 | 9 | 3 | 3 | 0.6 | 15 | x | x | |||||
| 75 | 8 | 3 | 3 | 0.62 | 15 | x | x | x | x | |||
| 72 | 5 | 4 | 0.5 | 0.7 | 17 | x | x | x | x | |||
| 77 | 12 | 3 | 1 | 0.64 | 17 | x | x | x | x | |||
| 71 | 15 | 1 | 1 | 0.61 | 11 | x | x | |||||
| 68 | 13 | 3 | 2 | 0.63 | 17 | x | x | x | x | |||
| mean s.d. | 72 | 11 | 3 | 2 | 0.66 | 15 | ||||||
| 4 | 4 | 1 | 1 | 0.04 | 3 | |||||||
| VaD (n = 12) | 73 | 17 | 1 | 1 | 0.75 | 12 | x | x | x | x | ||
| 73 | 13 | 2 | 2 | 0.65 | 13 | x | x | |||||
| 69 | 12 | 4 | 2 | 0.77 | 13 | x | x | |||||
| 71 | 9 | 2 | 2 | 0.75 | 13 | x | x | x | x | |||
| 71 | 8 | 1 | 2 | 0.65 | 13 | x | x | |||||
| 68 | 17 | 2 | 1 | 0.65 | 18 | x | x | |||||
| 70 | 6 | 4 | 2 | 0.67 | 12 | x | x | x | x | |||
| 75 | 8 | 3 | 2 | 0.63 | 15 | x | x | x | x | |||
| 74 | 7 | 3 | 2 | 0.66 | 15 | x | x | |||||
| 70 | 12 | 2 | 0.5 | 0.65 | 17 | x | x | |||||
| 70 | 17 | 1 | 0.5 | 0.63 | 8 | x | x | |||||
| 77 | 11 | 2 | 1 | 0.73 | 12 | x | x | x | x | |||
| mean s.d. | 72 | 11 | 2 | 1 | 0.68 | 13 | ||||||
| 3 | 4 | 1 | 1 | 0.05 | 3 | |||||||
| grand mean s.d. | 72 | 11 | 3 | 2 | 0.67 | 14 | ||||||
| 0.2 | 0.3 | 0.4 | 0.3 | 0.02 | 1 | |||||||
| Pre- and Post-iTBS Phase Spectra (π/2) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | W_p | B_p | Pre | Post | W_p | B_p | ||||||
| theta | alpha | ||||||||||||
| BA5L-BA2/4L | D/MCI- | 0.24 | ±0.04 | 0.22 | ±0.04 | 0.02 | 0.009 | 0.29 | ±0.04 | 0.38 | ±0.03 | 0.04 | 0.06 |
| MCI+ | 0.33 | ±0.04 | 0.35 | ±0.06 | 0.005 | 0.44 | ±0.06 | 0.01 | ±0.01 | 0.006 | |||
| alpha | beta | ||||||||||||
| BA5L-BA2/4R | D/MCI- | 0.26 | ±0.05 | 0.28 | ±0.05 | 0.03 | 0.05 | 0.26 | ±0.05 | 0.45 | ±0.02 | 0.003 | 0.05 |
| MCI+ | 0.34 | ±0.05 | 0.33 | ±0.05 | 0.006 | 0.32 | ±0.05 | 0.02 | ±0.01 | 0.008 | |||
| theta | alpha | ||||||||||||
| BA5R-BA8L | D/MCI- | 0.21 | ±0.04 | 0.21 | ±0.04 | 0.01 | 0.01 | 0.28 | ±0.05 | 0.63 | ±0.03 | 0.37 | 0.7 |
| MCI+ | 0.34 | ±0.04 | 0.38 | ±0.05 | 0.008 | 0.41 | ±0.05 | 0.01 | ±0.01 | 0.006 | |||
| alpha | Beta | ||||||||||||
| BA5R-BA8R | D/MCI- | 0.3 | ±0.05 | 0.28 | ±0.05 | 0.004 | 0.03 | 0.29 | ±0.05 | 0.23 | ±0.03 | <0.001 | 0.02 |
| MCI+ | 0.31 | ±0.05 | 0.37 | ±0.06 | 0.008 | 0.35 | ±0.05 | 0.03 | ±0.01 | 0.005 | |||
| pre- and post-iTBS Hurst exponent | |||||||||||||
| theta | Alpha | ||||||||||||
| BA5L-BA2/4L | D/MCI- | 0.92 | ±0.16 | 0.79 | ±0.16 | 0.05 | 0.006 | 0.3 | ±0.05 | 0.02 | ±0.01 | 0.004 | 0.06 |
| MCI+ | 0.82 | ±0.13 | 0.83 | ±0.12 | 0.006 | 0.28 | ±0.05 | 0.02 | ±0.01 | 0.005 | |||
| alpha | beta | ||||||||||||
| BA5L-BA2/4R | D/MCI- | 0.3 | ±0.06 | 0.31 | ±0.05 | 0.02 | 0.001 | 0.13 | ±0.02 | 0.44 | ±0.01 | 0.02 | 0.07 |
| MCI+ | 0.36 | ±0.05 | 0.33 | ±0.06 | 0.002 | 0.13 | ±0.02 | 0.03 | ±0.01 | 0.003 | |||
| theta | alpha | ||||||||||||
| BA5R-BA8L | D/MCI- | 0.83 | ±0.17 | 0.99 | ±0.17 | 0.03 | 0.006 | 0.34 | ±0.06 | 0.07 | ±0.17 | 0.002 | 0.00 |
| MCI+ | 0.93 | ±0.15 | 0.88 | ±0.14 | <0.001 | 0.41 | ±0.05 | 0.02 | ±0.01 | 0.006 | |||
| alpha | beta | ||||||||||||
| BA5R-BA8R | D/MCI- | 0.31 | ±0.05 | 0.28 | ±0.05 | 0.02 | 0.03 | 0.13 | ±0.02 | 0.4 | ±0.01 | 0.01 | 0.50 |
| MCI+ | 0.28 | ±0.04 | 0.33 | ±0.05 | 0.01 | 0.12 | ±0.02 | 0.04 | ±0.01 | 0.002 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naro, A.; Marra, A.; Billeri, L.; Portaro, S.; De Luca, R.; Maresca, G.; La Rosa, G.; Lauria, P.; Bramanti, P.; Calabrò, R.S. New Horizons in Early Dementia Diagnosis: Can Cerebellar Stimulation Untangle the Knot? J. Clin. Med. 2019, 8, 1470. https://doi.org/10.3390/jcm8091470
Naro A, Marra A, Billeri L, Portaro S, De Luca R, Maresca G, La Rosa G, Lauria P, Bramanti P, Calabrò RS. New Horizons in Early Dementia Diagnosis: Can Cerebellar Stimulation Untangle the Knot? Journal of Clinical Medicine. 2019; 8(9):1470. https://doi.org/10.3390/jcm8091470
Chicago/Turabian StyleNaro, Antonino, Angela Marra, Luana Billeri, Simona Portaro, Rosaria De Luca, Giuseppa Maresca, Gianluca La Rosa, Paola Lauria, Placido Bramanti, and Rocco Salvatore Calabrò. 2019. "New Horizons in Early Dementia Diagnosis: Can Cerebellar Stimulation Untangle the Knot?" Journal of Clinical Medicine 8, no. 9: 1470. https://doi.org/10.3390/jcm8091470
APA StyleNaro, A., Marra, A., Billeri, L., Portaro, S., De Luca, R., Maresca, G., La Rosa, G., Lauria, P., Bramanti, P., & Calabrò, R. S. (2019). New Horizons in Early Dementia Diagnosis: Can Cerebellar Stimulation Untangle the Knot? Journal of Clinical Medicine, 8(9), 1470. https://doi.org/10.3390/jcm8091470






