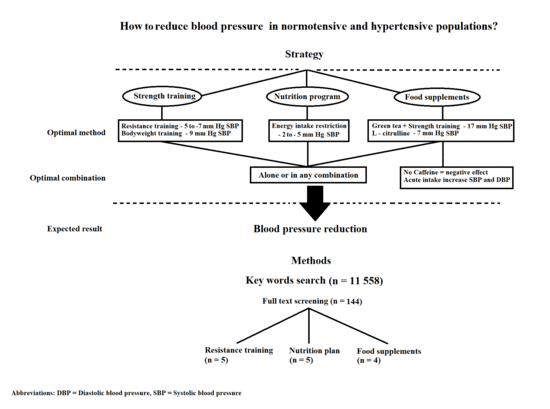
Role of Nutrition and Exercise Programs in Reducing Blood Pressure: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Types of Studies
2.3. Types of Outcomes
2.4. Data Extraction and Evaluation
3. Results
3.1. Study Characteristics
3.2. Strength Training Intervention
3.3. Nutrition Program
3.4. The Effectivity of Food Supplements
4. Discussion
4.1. Blood Pressure Reduced by Strength Training Alone
4.2. Blood Pressure was Reduced by the Nutrition Program
4.3. Effect of Supplements on Blood Pressure
4.4. Combined Effect of Strength Training, Nutrition Program and Supplementation on Blood Pressure
4.5. Changes in Body Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687. [Google Scholar] [CrossRef] [PubMed]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.W.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans: An AHA scientific statement from the council on high blood pressure research professional and public education subcommittee. J. Clin. Hypertens. 2005, 7, 102–109. [Google Scholar] [CrossRef]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T., Jr.; et al. Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamotte, M.; Niset, G.; Van De Borne, P. The effect of different intensity modalities of resistance training on beat-to-beat blood pressure in cardiac patients. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 12–17. [Google Scholar] [CrossRef]
- Meyer, K. Resistance exercise in chronic heart failure—Landmark studies and implications for practice. Clin. Investig. Med. 2006, 29, 166–169. [Google Scholar]
- Pescatello, L.S.; Franklin, B.A.; Fagard, R.; Farquhar, W.B.; Kelley, G.A.; Ray, C.A. American College of Sports Medicine position stand. Exercise and hypertension. Med. Sci. Sports Exerc. 2004, 36, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S.; American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef]
- Hamer, M. The anti-hypertensive effects of exercise: Integrating acute and chronic mechanisms. Sports Med. 2006, 36, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on Blood Pressure of Reduced Dietary Sodium and the Dietary Approaches to Stop Hypertension (DASH) Diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Stevens, V.J.; Obarzanek, E.; Cook, N.R.; Lee, I.M.; Appel, L.J.; West, D.S.; Milas, N.C.; Mattfeldt-Beman, M.; Belden, L.; Bragg, C.; et al. Long-term weight loss and changes in blood pressure: Results of the trials of hypertension prevention, phase II. Ann. Intern. Med. 2001, 134, 1–11. [Google Scholar] [CrossRef]
- LaMonte, M.J.; Yanowitz, F.G. Aerobic exercise for lowering blood pressure: A metaanalysis. Clin. J. Sport Med. 2002, 12, 407. [Google Scholar] [PubMed]
- Pescatello, L.S.; Kulikowich, J.M. The aftereffects of dynamic exercise on ambulatory blood pressure. Med. Sci. Sports Exerc. 2001, 33, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Whelton, S.P.; Chin, A.; Xin, X.; He, J. Effect of aerobic exercise on blood pressure: A meta-analysis of randomized, controlled trials. Ann. Intern. Med. 2002, 136, 493–503. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, P.E.C.P.; da Silva, V.S.; Camacho, L.A.B.; Vasconcelos, A.G.G. Effects of long-term resistance training on blood pressure: A systematic review. Revista Brasileira de Cineantropometria Desempenho Humano 2017, 19, 730–742. [Google Scholar]
- Inder, J.D.; Carlson, D.J.; Dieberg, G.; McFarlane, J.R.; Hess, N.C.; Smart, N.A. Isometric exercise training for blood pressure management: A systematic review and meta-analysis to optimize benefit. Hypertens. Res. 2016, 39, 88–94. [Google Scholar] [CrossRef]
- Lemes, I.R.; Ferreira, P.H.; Linares, S.N.; MacHado, A.F.; Pastre, C.M.; Netto, J. Resistance training reduces systolic blood pressure in metabolic syndrome: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016, 50, 1438–1442. [Google Scholar] [CrossRef] [PubMed]
- Wewege, M.A.; Thom, J.M.; Rye, K.A.; Parmenter, B.J. Aerobic, resistance or combined training: A systematic review and meta-analysis of exercise to reduce cardiovascular risk in adults with metabolic syndrome. Atherosclerosis 2018, 274, 162–171. [Google Scholar] [CrossRef]
- Hovell, M.F. The experimental evidence for weight-loss treatment of essential hypertension: A critical review. Am. J. Public Health 1982, 72, 359–368. [Google Scholar] [CrossRef]
- Ndanuko, R.N.; Tapsell, L.C.; Charlton, K.E.; Neale, E.P.; Batterham, M.J. Dietary Patterns and Blood Pressure in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2016, 7, 76–89. [Google Scholar] [CrossRef]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A.; Azadbakht, L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: A systematic review and meta-analysis on randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1253–1261. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Chaimani, A.; Schwedhelm, C.; Toledo, E.; Pünsch, M.; Hoffmann, G.; Boeing, H. Comparative effects of different dietary approaches on blood pressure in hypertensive and pre-hypertensive patients: A systematic review and network meta-analysis. Crit. Rev. Food Sci Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Herrod, P.J.J.; Doleman, B.; Blackwell, J.E.M.; O’Boyle, F.; Williams, J.P.; Lund, J.N.; Phillips, B.E. Exercise and other nonpharmacological strategies to reduce blood pressure in older adults: A systematic review and meta-analysis. J. Am. Soc. Hypertens. 2018, 12, 248–267. [Google Scholar] [CrossRef] [PubMed]
- Aucott, L.; Poobalan, A.; Smith, W.C.S.; Avenell, A.; Jung, R.; Broom, J. Effects of weight loss in overweight/obese individuals and long-term hypertension outcomes: A systematic review. Hypertension 2005, 45, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Moore, L.L.; Visioni, A.J.; Qureshi, M.M.; Bradlee, M.L.; Ellison, R.C.; D’Agostino, R. Weight loss in overweight adults and the long-term risk of hypertension the framingham study. Arch. Intern. Med. 2005, 165, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Mulrow, C.D.; Chiquette, E.; Angel, L.; Cornell, J.; Summerbell, C.; Anagnostelis, B.; Grimm, R., Jr.; Brand, M.B. Dieting to reduce body weight for controlling hypertension in adults. Cochrane Database Syst. Rev. 2000, 2, CD000484. [Google Scholar]
- Sjostrom, M.; Karlsson, A.B.; Kaati, G.; Yngve, A.; Green, L.W.; Bygren, L.O. A four week residential program for primary health care patients to control obesity and related heart risk factors: Effective application of principles of learning and lifestyle change. Eur J. Clin. Nutr 1999, 53 (Suppl. 2), S72–S77. [Google Scholar] [CrossRef]
- Stevens, V.J.; Corrigan, S.A.; Obarzanek, E.; Bernauer, E.; Cook, N.R.; Hebert, P.; Mattfeldt-Beman, M.; Oberman, A.; Sugars, C.; Dalcin, A.T.; et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch. Intern. Med. 1993, 153, 849–858. [Google Scholar] [CrossRef]
- Figueiredo, T.; Rhea, M.R.; Peterson, M.; Miranda, H.; Bentes, C.M.; dos Reis, V.M.; Simao, R. Influence of number of sets on blood pressure and heart rate variability after a strength training session. J. Strength Cond. Res. 2015, 29, 1556–1563. [Google Scholar] [CrossRef]
- Moraes, M.R.; Bacurau, R.F.; Simoes, H.G.; Campbell, C.S.; Pudo, M.A.; Wasinski, F.; Pesquero, J.B.; Wurtele, M.; Araujo, R.C. Effect of 12 weeks of resistance exercise on post-exercise hypotension in stage 1 hypertensive individuals. J. Hum. Hypertens. 2012, 26, 533–539. [Google Scholar] [CrossRef]
- Scher, L.M.; Ferriolli, E.; Moriguti, J.C.; Scher, R.; Lima, N.K. The effect of different volumes of acute resistance exercise on elderly individuals with treated hypertension. J. Strength Cond. Res. 2011, 25, 1016–1023. [Google Scholar] [CrossRef]
- Tokmakidis, S.P.; Kalapotharakos, V.I.; Smilios, I.; Parlavantzas, A. Effects of detraining on muscle strength and mass after high or moderate intensity of resistance training in older adults. Clin. Physiol. Funct. Imaging 2009, 29, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Baum, K.; Rüther, T.; Essfeld, D. Reduction of blood pressure response during strength training through intermittent muscle relaxations. Int. J. Sports Med. 2003, 24, 441–445. [Google Scholar] [PubMed]
- Gjovaag, T.; Hjelmeland, A.K.; Øygard, J.B.; Vikne, H.; Mirtaheri, P. Acute hemodynamic and cardiovascular responses following resistance exercise to voluntary exhaustion. Effects of different loadings and exercise durations. J. Sports Med. Phys. Fit. 2016, 56, 616–623. [Google Scholar]
- Gjøvaag, T.; Hjelmeland, A.K.; Øygard, J.B.; Vikne, H.; Mirtaheri, P. Resistance exercise and acute blood pressure responses. J. Sports Med. Phys. Fit. 2016, 56, 616–623. [Google Scholar]
- Nascimento Dda, C.; Tibana, R.A.; Benik, F.M.; Fontana, K.E.; Ribeiro Neto, F.; Santana, F.S.; Santos-Neto, L.; Silva, R.A.; Silva, A.O.; Farias, D.L.; et al. Sustained effect of resistance training on blood pressure and hand grip strength following a detraining period in elderly hypertensive women: A pilot study. Clin. Interv. Aging 2014, 9, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Lamotte, M.; Fleury, F.; Pirard, M.; Jamon, A.; van de Borne, P. Acute cardiovascular response to resistance training during cardiac rehabilitation: Effect of repetition speed and rest periods. Eur. J. Prev. Cardiol. 2010, 17, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Paulo, A.C.; Tricoli, V.; Queiroz, A.C.C.; Laurentino, G.; Forjaz, C.L.M. Blood Pressure Response During Resistance Training of Different Work-to-Rest Ratio. J. Strength Cond. Res. 2019, 33, 399–407. [Google Scholar] [CrossRef]
- Veloso, J.; Polito, M.D.; Riera, T.; Celes, R.; Vidal, J.C.; Bottaro, M. Effects of rest interval between exercise sets on blood pressure after resistance exercises. Arquivos Brasileiros de Cardiologia 2010, 94, 482–487. [Google Scholar]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Westman, E.C.; McDuffie, J.R.; Grambow, S.C.; Jeffreys, A.S.; Bolton, J.; Chalecki, A.; Oddone, E.Z. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Arch. Intern. Med. 2010, 170, 136–145. [Google Scholar] [CrossRef]
- Burke, V.; Hodgson, J.M.; Beilin, L.J.; Giangiulioi, N.; Rogers, P.; Puddey, I.B. Dietary protein and soluble fiber reduce ambulatory blood pressure in treated hypertensives. Hypertension 2001, 38, 821–826. [Google Scholar] [CrossRef]
- Attina, D.A.; Cupelli, V.; Milicia, R.; Catalano, G.; Scavelli, A. Effects of physical exercise of different duration in hypertensive elderly subjects. Int. J. Sports Cardiol. 1996, 5, 67–70. [Google Scholar]
- Fekete, A.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey protein lowers blood pressure and improves endothelial function and lipid biomarkers in adults with prehypertension and mild hypertension: Results from the chronic Whey2Go randomized controlled trial. Am. J. Clin. Nutr. 2016, 104, 1534–1544. [Google Scholar] [CrossRef]
- Fekete, Á.A.; Giromini, C.; Chatzidiakou, Y.; Givens, D.I.; Lovegrove, J.A. Whey protein lowers systolic blood pressure and Ca-caseinate reduces serum TAG after a high-fat meal in mildly hypertensive adults. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Fekete, Á.A.; Ian Givens, D.; Lovegrove, J.A. Casein-derived lactotripeptides reduce systolic and diastolic blood pressure in a meta-analysis of randomised clinical trials. Nutrients 2015, 7, 659–681. [Google Scholar] [CrossRef]
- He, J.; Gu, D.; Wu, X.; Chen, J.; Duan, X.; Chen, J.; Whelton, P.K. Effect of soybean protein on blood pressure: A randomized, controlled trial. Ann. Intern. Med. 2005, 143, 1–9. [Google Scholar] [CrossRef]
- Pal, S.; Ellis, V. The chronic effects of whey proteins on blood pressure, vascular function, and inflammatory markers in overweight individuals. Obesity 2010, 18, 1354–1359. [Google Scholar] [CrossRef]
- Rebholz, C.M.; Friedman, E.E.; Powers, L.J.; Arroyave, W.D.; He, J.; Kelly, T.N. Dietary protein intake and blood pressure: A meta-analysis of randomized controlled trials. Am. J. Epidemiol. 2012, 176, S27–S43. [Google Scholar] [CrossRef]
- Al-Solaiman, Y.; Jesri, A.; Mountford, W.K.; Lackland, D.T.; Zhao, Y.; Egan, B.M. DASH lowers blood pressure in obese hypertensives beyond potassium, magnesium and fibre. J. Hum. Hypertens. 2010, 24, 237–246. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Green, T.; Harrison, T.N.; Reynolds, K. Dietary approaches to prevent hypertension. Curr. Hypertens. Rep. 2013, 15, 694–702. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors a Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Rees, K.; Hartley, L.; Flowers, N.; Clarke, A.; Hooper, L.; Thorogood, M.; Stranges, S. ‘Mediterranean’ dietary pattern for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2013, 2013. [Google Scholar] [CrossRef]
- Svetkey, L.P.; Simons-Morton, D.; Vollmer, W.M.; Appel, L.J.; Conlin, P.R.; Ryan, D.H.; Ard, J.; Kennedy, B.M. Effects of dietary patterns on blood pressure: Subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch. Intern. Med. 1999, 159, 285–293. [Google Scholar] [CrossRef]
- Barbeau, P.A.; Holloway, T.M.; Whitfield, J.; Baechler, B.L.; Quadrilatero, J.; van Loon, L.J.C.; Chabowski, A.; Holloway, G.P. α-Linolenic acid and exercise training independently, and additively, decrease blood pressure and prevent diastolic dysfunction in obese Zucker rats. J. Physiol. 2017, 595, 4351–4364. [Google Scholar] [CrossRef]
- Moradi, H.; Nikbakht, H.; Ebrahim, P.R.; Abed-Natanzi, H. The effects of 8 weeks of aerobic training and grape seed extract on some cardiovascular functional indexes in non-active men. J. Isfahan Med. Sch. 2017, 34, 1439–1444. [Google Scholar]
- Neto, M.M.; da Silva, T.F.; de Lima, F.F.; Siqueira, T.M.Q.; Toscano, L.T.; de Moura, S.; Silva, A.S. Whole Red Grape Juice Reduces Blood Pressure at Rest and Increases Post-exercise Hypotension. J. Am. Coll. Nutr. 2017, 36, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Puga, G.M.; De, P.; Novais, I.; Katsanos, C.S.; Zanesco, A. Combined effects of aerobic exercise and l-arginine ingestion on blood pressure in normotensive postmenopausal women: A crossover study. Life Sci. 2016, 151, 323–329. [Google Scholar] [CrossRef]
- Digiesi, V.; Cantini, F.; Brodbeck, B. Effect of coenzyme Q10 on essential arterial hypertension. Curr. Ther. Res. 1990, 47, 841–845. [Google Scholar]
- Digiesi, V.; Cantini, F.; Bisi, G.; Guarino, G.; Oradei, A.; Littarru, G. Mechanism of action of coenzyme Q10 in essential hypertension. Curr. Ther. Res. 1992, 51, 668–672. [Google Scholar]
- Dyckner, T.; Wester, P.O. Effect of magnesium on blood pressure. Br. Med. J. 1983, 286, 1847–1849. [Google Scholar] [CrossRef]
- Huo, Y.; Li, J.; Qin, X.; Huang, Y.; Wang, X.; Gottesman, R.F.; Tang, G.; Wang, B.; Chen, D.; He, M.; et al. Efficacy of folic acid therapy in primary prevention of stroke among adults with hypertension in China: The CSPPT randomized clinical trial. JAMA 2015, 313, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; O’Donnell, M.J.; Rangarajan, S.; McQueen, M.J.; Poirier, P.; Wielgosz, A.; Morrison, H.; Li, W.; Wang, X.; Di, C.; et al. Association of urinary sodium and potassium excretion with blood pressure. N. Engl. J. Med. 2014, 371, 601–611. [Google Scholar] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in health and disease and in growth and development. Am. J. Clin. Nutr. 1991, 54, 438–463. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.J.; Wang, P.Q.; Li, S.J.; Li, X.K.; Zhang, Y.Q.; Wang, J. Garlic for hypertension: A systematic review and meta-analysis of randomized controlled trials. Phytomedicine 2015, 22, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The, P.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLOS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [PubMed]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by delphi consensus. J. Clin. Epidemiol. 1999, 51, 1235–1241. [Google Scholar] [CrossRef]
- Moraes, W.; Santos, N.S.D.; Aguiar, L.P.; Sousa, L.G.O. Maintenance of exercise training benefits is associated with adequate milk and dairy products intake in elderly hypertensive subjects following detraining. Einstein 2017, 15, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, S.A.; Gagnon, D.; Adams, A.N.; Moralez, G.; Kouda, K.; Jaffery, M.F.; Cramer, M.N.; Crandall, C.G. Folic acid ingestion improves skeletal muscle blood flow during graded handgrip and plantar flexion exercise in aged humans. Am. J. Physiol.-Heart Circ. Physiol. 2017, 313, H658–H666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astorino, T.A.; Martin, B.J.; Schachtsiek, L.; Wong, K. Caffeine ingestion and intense resistance training minimize postexercise hypotension in normotensive and prehypertensive men. Res. Sports Med. 2013, 21, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Kim, J.Y.; Shim, E.; Hong, S.H.; Lee, M.; Jeon, J.Y.; Park, S. The effects of diet alone or in combination with exercise in patients with prehypertension and hypertension: A randomized controlled trial. Korean Circ. J. 2018, 48, 637–651. [Google Scholar] [CrossRef] [PubMed]
- Villani, R.G.; Gornall, J. Short-Term resistance exercise does not alter the hypotensive effect of low-energy dieting. J. Strength Cond. Res. 1999, 13, 286–288. [Google Scholar]
- Sales, A.R.; Silva, B.M.; Neves, F.J.; Rocha, N.G.; Medeiros, R.F.; Castro, R.R.; Nobrega, A.C. Diet and exercise training reduce blood pressure and improve autonomic modulation in women with prehypertension. Eur. J. Appl. Physiol. 2012, 112, 3369–3378. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Vicil, F.; Sanchez-Gonzalez, M.A.; Wong, A.; Ormsbee, M.J.; Hooshmand, S.; Daggy, B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am. J. Hypertens. 2013, 26, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Samami, N.; Kheirkhah, J.; Taati, B. The effect of three weeks green tea extract consumption on blood pressure, heart rate responses to a single bout resistance exercise in hypertensive women. High Blood Press. Cardiovasc. Prev. 2014, 21, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Alvarez-Alvarado, S.; Jaime, S.J.; Kinsey, A.W.; Spicer, M.T.; Madzima, T.A.; Figueroa, A. Combined whole-body vibration training and l-citrulline supplementation improves pressure wave reflection in obese postmenopausal women. Appl. Physiol. Nutr. Metab. 2016, 41, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Arazi, H.; Ghiasi, A.; Asgharpoor, S. A comparative study of cardiovascular responses to two rest intervals between circuit resistance exercises in normotensive woman. Revista Brasileira de Medicina do Esporte 2013, 19, 176–180. [Google Scholar] [CrossRef]
- Pescatello, L.S.; MacDonald, H.V.; Lamberti, L.; Johnson, B.T. Exercise for Hypertension: A Prescription Update Integrating Existing Recommendations with Emerging Research. Curr. Hypertens. Rep. 2015, 17, 87. [Google Scholar] [CrossRef]
- Castaneda, C.; Gordon, P.L.; Uhlin, K.L.; Levey, A.S.; Kehayias, J.J.; Dwyer, J.T.; Fielding, R.A.; Roubenoff, R.; Singh, M.F. Resistance Training To Counteract the Catabolism of a Low-Protein Diet in Patients with Chronic Renal Insufficiency: A Randomized, Controlled Trial. Ann. Intern. Med. 2001, 135, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B. Blood Pressure as a Cardiovascular Risk Factor: Prevention and Treatment. JAMA 1996, 275, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.E.; Fiatarone, M.A.; Morganti, C.M.; Trice, I.; Greenberg, R.A.; Evans, W.J. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: A randomized controlled trial. JAMA 1994, 272, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Skelton, D.A.; Young, A.; Greig, C.A.; Malbut, K.E. Effects of Resistance Training on Strength, Power, and Selected Functional Abilities of Women Aged 75 and Older. J. Am. Geriatr. Soc. 1995, 43, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Clements, K.M.; Fiatarone, M.A. A randomized controlled trial of progressive resistance training in depressed elders. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1997, 52, M27–M35. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, V.A.; Fagard, R.H.; Coeckelberghs, E.; Vanhees, L. Impact of resistance training on blood pressure and other cardiovascular risk factors: A meta-analysis of randomized, controlled trials. Hypertension 2011, 58, 950–958. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef] [Green Version]
- Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Arch. Intern. Med. 1997, 157, 2413–2446. [Google Scholar] [CrossRef]
- Reisin, E.; Frohlich, E.D.; Messerli, F.H.; Dreslinski, G.R.; Dunn, F.G.; Jones, M.M.; Batson, H.M. Cardiovascular changes after weight reduction in obesity hypertension. Ann. Intern. Med. 1983, 98, 315–319. [Google Scholar] [CrossRef]
- Langford, H.G.; Blaufox, M.D.; Oberman, A.; Hawkins, C.M.; Curb, J.D.; Cutter, G.R.; Wassertheil-Smoller, S.; Pressel, S.; Babcock, C.; Abernethy, J.D. Dietary therapy slows the return of hypertension after stopping prolonged medication. JAMA 1985, 253, 657–664. [Google Scholar] [CrossRef]
- Stamler, R.; Stamler, J.; Gosch, F.C.; Civinelli, J.; Fishman, J.; McKeever, P.; McDonald, A.; Dyer, A.R. Primary prevention of hypertension by nutritional-hygienic means: Final report of a randomized, controlled trial. JAMA 1989, 262, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Stamler, R.; Stamler, J.; Grimm, R.; Gosch, F.C.; Elmer, P.; Dyer, A.; Berman, R.; Fishman, J.; Van Heel, N.; Civinelli, J. Nutritional therapy for high blood pressure: Final report of a four-year randomized controlled trial—the Hypertension Control Program. JAMA 1987, 257, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Happonen, P.; Voutilainen, S.; Salonen, J.T. Coffee drinking is dose-dependently related to the risk of acute coronary events in middle-aged men. J. Nutr. 2004, 134, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J.; Grgic, I.; Pickering, C.; Schoenfeld, B.J.; Bishop, D.J.; Pedisic, Z. Wake up and smell the coffee: Caffeine supplementation and exercise performance—an umbrella review of 21 published meta-analyses. Br. J. Sports Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Glade, M.J. Caffeine—not just a stimulant. Nutrition 2010, 26, 932–938. [Google Scholar] [CrossRef]
- Ali, A.; O’Donnell, J.; Foskett, A.; Rutherfurd-Markwick, K. The influence of caffeine ingestion on strength and power performance in female team-sport players. J. Int. Soc. Sports Nutr. 2016, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; He, J.; Whelton, P.K.; Suh, I.; Klag, M.J. The Effect of Chronic Coffee Drinking on Blood Pressure. Hypertension 1999, 33, 647–652. [Google Scholar] [CrossRef] [Green Version]
- Creager, M.A.; Cooke, J.P.; Mendelsohn, M.E.; Gallagher, S.J.; Coleman, S.M.; Loscalzo, J.; Dzau, V.J. Impaired vasodilation of forearm resistance vessels in hypercholesterolemic humans. J. Clin. Investig. 1990, 86, 228–234. [Google Scholar] [CrossRef]
- Mayhan, W.G. Role of nitric oxide in disruption of the blood-brain barrier during acute hypertension. Brain Res. 1995, 686, 99–103. [Google Scholar] [CrossRef]
- Rector, T.S.; Bank, A.J.; Mullen, K.A.; Tschumperlin, L.K.; Sih, R.; Pillai, K.; Kubo, S.H. Randomized, double-blind, placebo-controlled study of supplemental oral L-arginine in patients with heart failure. Circulation 1996, 93, 2135–2141. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Lu, F.-H.; Wu, J.-S.; Wu, C.-H.; Chang, C.-J. The protective effect of habitual tea consumption on hypertension. Arch. Intern. Med. 2004, 164, 1534–1540. [Google Scholar] [CrossRef]
- Stensvold, I.; Tverdal, A.; Solvoll, K.; Foss, O.P. Tea consumption. Relationship to cholesterol, blood pressure, and coronary and total mortality. Prev. Med. 1992, 21, 546–553. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Kono, S.; Shinchi, K.; Honjo, S.; Todoroki, I.; Sakurai, Y.; Umeda, T.; Imanishi, K.; Yoshizawa, N. Habitual coffee consumption and blood pressure: A study of self-defense officials in Japan. Eur. J. Epidemiol. 1998, 14, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, E.; Campos-Toimil, M.; Justiniano-Basaran, H.; Lugnier, C.; Orallo, F. Study of the mechanisms involved in the vasorelaxation induced by (−)-epigallocatechin-3-gallate in rat aorta. Br. J. Pharmacol. 2006, 147, 269–280. [Google Scholar] [CrossRef]
- Meschia, J.F.; Bushnell, C.; Boden-Albala, B.; Braun, L.T.; Bravata, D.M.; Chaturvedi, S.; Creager, M.A.; Eckel, R.H.; Elkind, M.S.V.; Fornage, M.; et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3754–3832. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.M.; Bowman, L.; Clarke, R.J.; Wallendszus, K.; Bulbulia, R.; Rahimi, K.; Haynes, R.; Parish, S.; Sleight, P.; Peto, R. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: A randomized trial. JAMA 2010, 303, 2486–2494. [Google Scholar]
- Lonn, E. Heart Outcomes Prevention Evaluation (HOPE) 2 Investigators. Homocysteine lowering with folic acid and B vitamins in vascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 414–415. [Google Scholar]
- Toole, J.F.; Malinow, M.R.; Chambless, L.E.; Spence, J.D.; Pettigrew, L.C.; Howard, V.J.; Sides, E.G.; Wang, C.-H.; Stampfer, M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA 2004, 291, 565–575. [Google Scholar] [CrossRef]
- Bønaa, K.H.; Njølstad, I.; Ueland, P.M.; Schirmer, H.; Tverdal, A.; Steigen, T.; Wang, H.; Nordrehaug, J.E.; Arnesen, E.; Rasmussen, K. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N. Engl. J. Med. 2006, 354, 1578–1588. [Google Scholar] [CrossRef]
- Hernández-Díaz, S.; Werler, M.M.; Louik, C.; Mitchell, A.A. Risk of gestational hypertension in relation to folic acid supplementation during pregnancy. Am. J. Epidemiol. 2002, 156, 806–812. [Google Scholar] [CrossRef]
- Najjar, S.S.; Scuteri, A.; Shetty, V.; Wright, J.G.; Muller, D.C.; Fleg, J.L.; Spurgeon, H.P.; Ferrucci, L.; Lakatta, E.G. Pulse wave velocity is an independent predictor of the longitudinal increase in systolic blood pressure and of incident hypertension in the Baltimore Longitudinal Study of Aging. J. Am. Coll. Cardiol. 2008, 51, 1377–1383. [Google Scholar] [CrossRef] [PubMed]



| Authors | Subjects | Aim | Results |
|---|---|---|---|
| Villani and Gornall (1999) [74] | Premenopausal women NP-only group: n = 10, mean age (y) = 33, weight (kg) = 75.78 ± 3.2 NP + Strength training group: n = 10, mean age (y) = 33, weight (kg) = 79.50 ± 2.86 | The aim of the study was to investigate the combined influences of very-low-kilojoule diets and strength training on BP. | Resistance exercise did not significantly alter the BP reduction observed with short-term severe dieting. |
| Sales et al. (2012) [75] | Women with prehypertension: n = 10, age (y) = 39 ± 6, weight (kg) = 71.5 ± 10.7 Women with normotension n = 10, age (y) = 35 ± 11, weight (kg) = 66.5 ± 6.8 | The aim of the study was to investigate the effect of diet and exercise training on BP and autonomic modulation in women with prehypertension. | Diet and exercise training reduced SBP in women with prehypertension, and this was associated with parasympathetic augmentation and probably reduction in sympathetic cardiac modulation. |
| Astorino and Martin (2013) [72] | Hypertensive men: n = 7, age (y) = 23.9 ± 4.6, height (m) = 1.8 ± 0.1, mass (kg) = 89 ± 16.2 Normotensive men: n = 7, age (y) = 22.4 ± 4.0, height (m) = 1.8 ± 0.1, weight (kg) = 77.9 ± 6.4 | The primary aim of the study was to compare changes in BP in normotensive and prehypertensive men completing resistance exercise following caffeine ingestion. | Post-exercise hypotension did not occur in either treatment, suggesting that intense resistance training with or without caffeine intake may mitigate the BP-lowering effect of resistance exercise. |
| Figueroa et al. (2013) [76] | Postmenopausal women LIRET: n = 14, age (y) = 54 ± 1, height (m) = 1.66 ± 0.02, weight (kg) = 88.4 ± 4.6 Postmenopausal women NP: n = 13, age (y) = 54 ± 1, height (m) = 1.62 ± 0.02, weight (kg) = 89.0 ± 4.4 Postmenopausal women NP + LIRET: n = 14, age (y) = 54 ± 1, height (m) = 1.63 ± 0.02, weight (kg) = 86.7 ± 2.7 | The aim of the study was to evaluate the independent and combined effects of a hypocaloric diet and LIRET with slow movement on PWV and body composition. | A hypocaloric diet decreases baPWV mainly by reducing legPWV, and this reduction was related to the loss of truncal fat. Although LIRET alone does not affect PWV or body composition, LIRET combined with diet improves baPWV and muscle strength while preventing loss of lean body mass in obese postmenopausal women. |
| Arazi et al. (2014) [77] | Middle-aged women: n = 24, age (y) = 46.4 ± 6.3, height (m) = 1.66 ± 4.2, weight (kg) = 66.6 ± 9.2 kg | The aim of the study was to investigate the effects of green tea extract on BP, HR, and RPP responses to low-intensity resistance exercise in hypertensive women. | Three weeks of green tea extract ingestion did not influence SBP, DBP or HR but may be have a favorable effect on MAP and RPP responses to an acute resistance exercise during a 1-h exercise recovery. |
| Wong et al. (2016) [78] | Postmenopausal women whole-body vibration training + Placebo: n = 14, age (y) = 58 ± 4.0, height (m) = 1.6 ± 0.06, weight (kg) = 89.5 ± 10.6 Postmenopausal women L-citrulline: n = 14, age (y) = 58 ± 4.0, height (m) = 1.6 ± 0.05, weight (kg) = 83.8 ± 8.4 Postmenopausal women WBVT + L-citrulline: n = 13, age (y) = 58 ± 3.0, height (m) = 1.62 ± 0.05, weight (kg) = 88.3 ± 3.9 | The aim of the study was to examine the combined and independent effects of whole-body vibration training and L-citrulline supplementation on aortic hemodynamics and plasma nitric oxide metabolites in postmenopausal women. | This study supports the effectiveness of whole-body vibration training + L-citrulline as a potential intervention for the prevention of hypertension-related cardiac diseases in obese postmenopausal women. |
| Moraes et al. (2017) [70] | Low milk intake group: n = 16, age (y) = 70.2 ± 4.9 and weight (kg) = 70.1 ± 7.6 High milk intake group: n = 12, age (y) = 70.3 ± 5.0, weight (kg) = 68.6 ± 7.7 | The aim of the study was to investigate whether the maintenance of exercise training benefits are associated with adequate milk and dairy product intake in elderly hypertensive subjects after detraining. | Maintenance of exercise training benefits related to pressure levels, lower extremity strength and aerobic capacity is associated with adequate milk and dairy product intake in hypertensive elderly subjects following six weeks of detraining. |
| Romero et al. (2017) [71] | Adults (men and women): n = 9, age (y) = 68 ± 5, height (m) = 1.65 ± 5, weight (kg) = 70 ± 8 | The purpose of this study was to test the hypothesis that folic acid ingestion improves skeletal muscle blood flow in aged adults performing graded handgrip and plantar flexion exercise via increased vascular conductance. | Folic acid ingestion increases blood flow to active skeletal muscle primarily via improved local vasodilation in aged adults. |
| Lee et al. (2018) [73] | Adults (men and women) Advice-only comparison group: n = 28, age (y) = 43.4 ± 14.5, weight (kg) = 69.9 ± 9.2 Diet education group: n = 30, age (y) = 43.0 ± 13.5, weight (kg) = 72.8 ± 15.2 Diet and exercise education group: n = 27, age (y) 49.1 ± 10.1, weight (kg) = 76.6 ± 10.7 | The aim of this study was to evaluate the effectiveness of a home-based lifestyle modification intervention on BP management. | Lifestyle modification emphasizing both diet and exercise was effective for lowering BP and should be favored over diet-only modifications. |
| Authors | Sets | Rest Between Sets | Repetitions | Intensity | Frequency | Strength Training Methods | Number of Exercises |
|---|---|---|---|---|---|---|---|
| Villani and Gornall (1999) [74] | 3 | 1–2 min | 10 | 10 RM | 3x per week | Resistance training | 6 |
| Sales et al. (2012) [75] | 3 | - | 10 | 45–85% 1RM | 3x per week | Resistance + aerobic training | 10 |
| Astorino and Martin (2013) [72] | 4 | 2 min | As much as possible | 70–80% 1RM | 2 measurements | Resistance free weight training | 4 |
| Figueroa et al. (2013) [76] | 2–3 | - | 18–22x | - | 3x per week | Resistance machine training | 4 |
| Arazi et al. (2014) [77] | 2 | 2 min | 6–10 | 50% 1 RM | 1 measurement | Resistance training | 6 |
| Wong et al. (2016) [78] | 1–5 | 0.5–1 min | 0.5–1 min | 25–40 Hz | 3x per week | Bodyweight training | 8 |
| Moraes et al. (2017) [70] | - | - | - | - | 2x per week | Resistance + endurance + flexibility + stability training | - |
| Romero et al. (2017) [71] | 2 | 20 min | 1 | 3, 6, 9 kg | 2 measurements | Isometric training | 2 |
| Lee et al. (2018) [73] | 3 | - | - | Mild–moderate intensity | Every day | Circuit training | 5–7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurik, R.; Stastny, P. Role of Nutrition and Exercise Programs in Reducing Blood Pressure: A Systematic Review. J. Clin. Med. 2019, 8, 1393. https://doi.org/10.3390/jcm8091393
Jurik R, Stastny P. Role of Nutrition and Exercise Programs in Reducing Blood Pressure: A Systematic Review. Journal of Clinical Medicine. 2019; 8(9):1393. https://doi.org/10.3390/jcm8091393
Chicago/Turabian StyleJurik, Roman, and Petr Stastny. 2019. "Role of Nutrition and Exercise Programs in Reducing Blood Pressure: A Systematic Review" Journal of Clinical Medicine 8, no. 9: 1393. https://doi.org/10.3390/jcm8091393