P2Y12 Inhibitor Pre-Treatment in Non-ST-Elevation Acute Coronary Syndrome: A Decision-Analytic Model
Abstract
:1. Introduction
2. Experimental Section
2.1. Model Assumptions and Data Sources
2.2. Baseline Assumptions of Patient Risk
2.3. Procedural Rates, Timing and Impact on Risk
2.4. Modification of Risks Associated with Initiation of P2Y12 Inhibition
2.5. Sensitivity Analysis
3. Results
3.1. Sensitivity Analysis
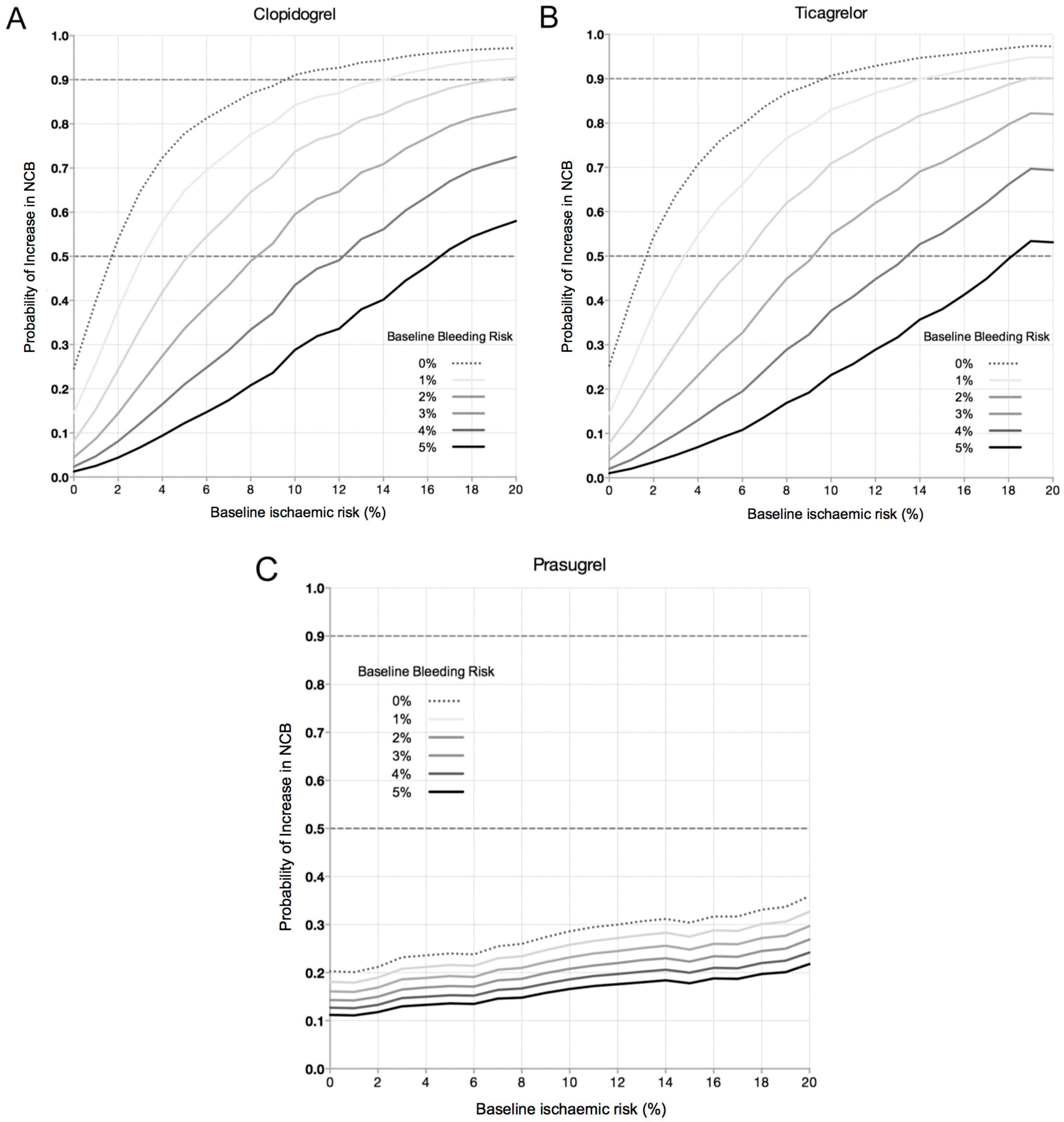
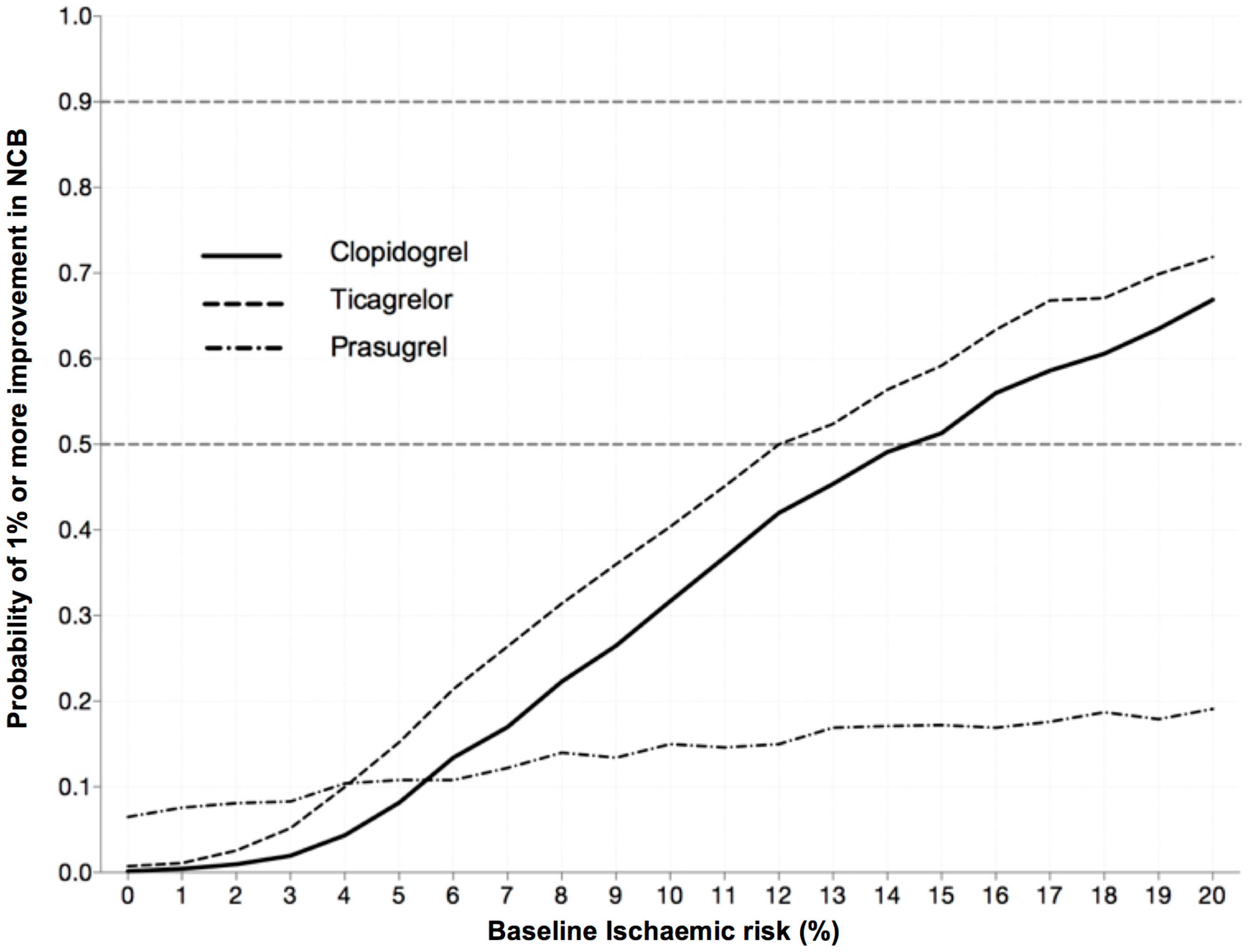
3.2. Increase in Net Clinical Benefit (NCB) with Pre-Treatment
3.3. Time to Coronary Angiography and Institutional CABG Rate
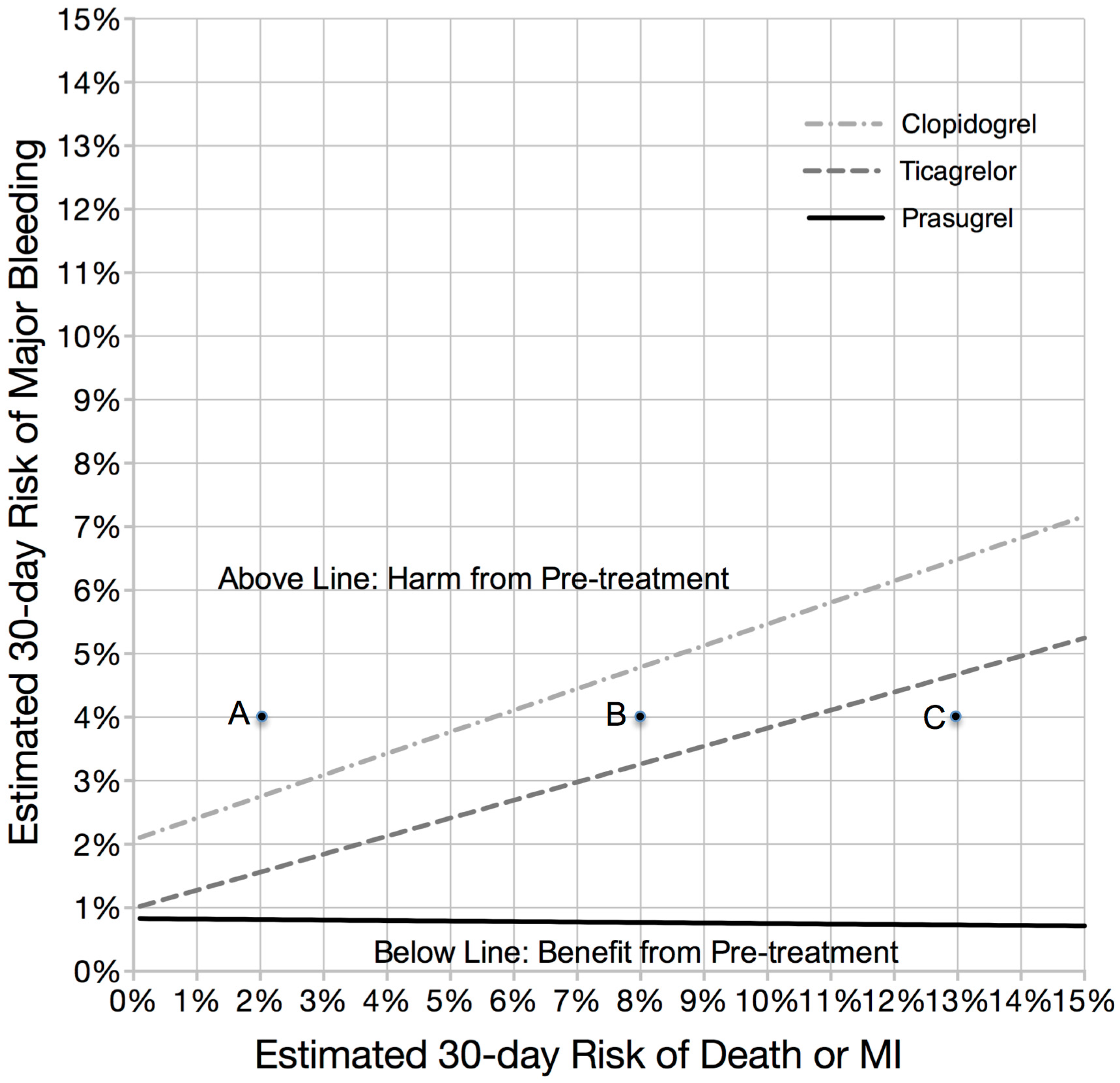
3.4. Magnitude of Effect: The Likelihood Providing a Significant Clinical Benefit from Pre-Treatment
4. Discussion
Limitations
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DAPT | Dual anti-platelet therapy |
| CABG | Coronary artery bypass graft |
| PCI | Percutaneous coronary intervention |
References
- Collet, J.P.; Silvain, J.; Bellemain-Appaix, A.; Montalescot, G. Pretreatment with P2Y12 inhibitors in non-st-segment-elevation acute coronary syndrome: An outdated and harmful strategy. Circulation 2014, 130, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M. Pretreatment with P2Y12 inhibitors in non-ST-segment-elevation acute coronary syndrome is clinically justified. Circulation 2014, 130, 1891–1903. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Clopidogrel in unstable angina to prevent recurrent events trial, I. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar] [PubMed]
- Biancari, F.; Airaksinen, K.E.; Lip, G.Y. Benefits and risks of using clopidogrel before coronary artery bypass surgery: Systematic review and meta-analysis of randomized trials and observational studies. J. Thorac. Cardiovasc. Surg. 2012, 143, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Widimsky, P.; Motovska, Z.; Simek, S.; Kala, P.; Pudil, R.; Holm, F.; Petr, R.; Bilkova, D.; Skalicka, H.; Kuchynka, P.; et al. Clopidogrel pre-treatment in stable angina: For all patients >6 h before elective coronary angiography or only for angiographically selected patients a few minutes before PCI? A randomized multicentre trial prague-8. Eur. Heart J. 2008, 29, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Steinhubl, S.R.; Berger, P.B.; Mann, J.T., 3rd; Fry, E.T.; DeLago, A.; Wilmer, C.; Topol, E.J. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: A randomized controlled trial. JAMA 2002, 288, 2411–2420. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Cannon, C.P.; Gibson, C.M.; Lopez-Sendon, J.L.; Montalescot, G.; Theroux, P.; Lewis, B.S.; Murphy, S.A.; McCabe, C.H.; Braunwald, E.; et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: The PCI-clarity study. JAMA 2005, 294, 1224–1232. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; van’t Hof, A.W. Prehospital ticagrelor in ST-segment elevation myocardial infarction. N. Engl. J. Med. 2014, 371, 2339. [Google Scholar] [CrossRef] [PubMed]
- Montalescot, G.; Bolognese, L.; Dudek, D.; Goldstein, P.; Hamm, C.; Tanguay, J.F.; ten Berg, J.M.; Miller, D.L.; Costigan, T.M.; Goedicke, J.; et al. Pretreatment with prasugrel in non-ST-segment elevation acute coronary syndromes. N. Engl. J. Med. 2013, 369, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Roffi, M.; Patrono, C.; Collet, J.P.; Mueller, C.; Valgimigli, M.; Andreotti, F.; Bax, J.J.; Borger, M.A.; Brotons, C.; Chew, D.P.; et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the european society of cardiology (ESC). Eur. Heart J. 2016, 37, 267–315. [Google Scholar] [PubMed]
- Chew, D.P.; Amerena, J.V.; Coverdale, S.G.; Rankin, J.M.; Astley, C.M.; Soman, A.; Brieger, D.B. Invasive management and late clinical outcomes in contemporary australian management of acute coronary syndromes: Observations from the acacia registry. Med. J. Aust. 2008, 188, 691–697. [Google Scholar] [PubMed]
- Chew, D.P.; French, J.; Briffa, T.G.; Hammett, C.J.; Ellis, C.J.; Ranasinghe, I.; Aliprandi-Costa, B.J.; Astley, C.M.; Turnbull, F.M.; Lefkovits, J.; et al. Acute coronary syndrome care across australia and new zealand: The snapshot acs study. Med. J. Aust. 2013, 199, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.R.; Yusuf, S.; Peters, R.J.; Bertrand, M.E.; Lewis, B.S.; Natarajan, M.K.; Malmberg, K.; Rupprecht, H.; Zhao, F.; Chrolavicius, S.; et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-cure study. Lancet 2001, 358, 527–533. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E., Jr.; Ganiats, T.G.; Holmes, D.R., Jr.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: A report of the american college of cardiology/american heart association task force on practice guidelines. J. Am. Coll. Cardiol. 2014, 64, e139–e228. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Bertrand, M.E.; Moses, J.W.; Ohman, E.M.; Lincoff, A.M.; Ware, J.H.; Pocock, S.J.; McLaurin, B.T.; Cox, D.A.; Jafar, M.Z.; et al. Routine upstream initiation vs deferred selective use of glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: The acuity timing trial. JAMA 2007, 297, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Giugliano, R.P.; White, J.A.; Bode, C.; Armstrong, P.W.; Montalescot, G.; Lewis, B.S.; van’t Hof, A.; Berdan, L.G.; Lee, K.L.; Strony, J.T.; et al. Early versus delayed, provisional eptifibatide in acute coronary syndromes. N. Engl. J. Med. 2009, 360, 2176–2190. [Google Scholar] [CrossRef] [PubMed]
- Lotrionte, M.; Biondi-Zoccai, G.G.; Agostoni, P.; Abbate, A.; Angiolillo, D.J.; Valgimigli, M.; Moretti, C.; Meliga, E.; Cuisset, T.; Alessi, M.C.; et al. Meta-analysis appraising high clopidogrel loading in patients undergoing percutaneous coronary intervention. Am. J. Cardiol. 2007, 100, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Bellemain-Appaix, A.; O’Connor, S.A.; Silvain, J.; Cucherat, M.; Beygui, F.; Barthelemy, O.; Collet, J.P.; Jacq, L.; Bernasconi, F.; Montalescot, G.; et al. Association of clopidogrel pretreatment with mortality, cardiovascular events, and major bleeding among patients undergoing percutaneous coronary intervention: A systematic review and meta-analysis. JAMA 2012, 308, 2507–2516. [Google Scholar] [CrossRef] [PubMed]
- Cuisset, T.; Frere, C.; Quilici, J.; Morange, P.E.; Nait-Saidi, L.; Carvajal, J.; Lehmann, A.; Lambert, M.; Bonnet, J.L.; Alessi, M.C. Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-st-segment elevation acute coronary syndrome undergoing coronary stenting. J. Am. Coll. Cardiol. 2006, 48, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.; Colonna, G.; Pasceri, V.; Pepe, L.L.; Montinaro, A.; Di Sciascio, G. Randomized trial of high loading dose of clopidogrel for reduction of periprocedural myocardial infarction in patients undergoing coronary intervention: Results from the armyda-2 (antiplatelet therapy for reduction of myocardial damage during angioplasty) study. Circulation 2005, 111, 2099–2106. [Google Scholar] [PubMed]
- Bellemain-Appaix, A.; Kerneis, M.; O’Connor, S.A.; Silvain, J.; Cucherat, M.; Beygui, F.; Barthelemy, O.; Collet, J.P.; Jacq, L.; Bernasconi, F.; et al. Reappraisal of thienopyridine pretreatment in patients with non-st elevation acute coronary syndrome: A systematic review and meta-analysis. BMJ 2014, 349, g6269. [Google Scholar] [CrossRef] [PubMed]
- Rade, J.J. Routine thienopyridine pretreatment for acute coronary syndrome without st elevation. BMJ 2014, 349, g6282. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; Anderson, F.A., Jr.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (grace). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef] [PubMed]

| Base Case | Value (Confidence Interval) | Reference |
|---|---|---|
| Baseline ischemic risk | 7% | [13,14] |
| Baseline bleeding risk | 2.5% | [13,14] |
| Delay to angiography | 0–96 h | |
| CABG rate | 7%–15% | |
| Clopidogrel effect on death/myocardial infarction rates | 0.80 (0.72–0.90) | [15] |
| Clopidogrel effect on bleeding rates | 1.38 (1.13–1.67) | [15] |
| Prasugrel vs. Clopidogrel on death/myocardial infarction rates | 0.98 (0.78–1.23) | [11] |
| 0.81 (0.73-0.91) | [3] | |
| Prasugrel vs. Clopidogrel on bleeding rates | 2.86 (1.44–5.68) | [11] |
| 1.32 (1.03–1.68) | [3] | |
| Ticagrelor vs. Clopidogrel on death/myocardial infarction rates | 0.83 (0.74–0.93) | [4] |
| Ticagrelor vs. Clopidogrel on bleeding rates | 1.03 (0.93–1.15) | [4] |
| No Pre-Treatment (at 30 Days) | P2Y12 Inhibitor | Pre-Treatment Risk (at 30 Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Death or MI | Major Bleeding | Death or MI | Major Bleeding | ARR (Death or MI) | ARI (Major Bleeding) | NCB | NNH | |
| 6.3% | 3.5% | Clopidogrel | 5.1% | 4.9% | 1.2% | 1.4% | −0.2% | 500 |
| Prasugrel | 5.1% | 9.2% | 1.2% | 5.7% | −4.5% | 22 | ||
| Ticagrelor | 4.2% | 6.8% | 2.1% | 3.3% | −1.2% | 83 | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gunton, J.; Hartshorne, T.; Langrish, J.; Chuang, A.; Chew, D. P2Y12 Inhibitor Pre-Treatment in Non-ST-Elevation Acute Coronary Syndrome: A Decision-Analytic Model. J. Clin. Med. 2016, 5, 72. https://doi.org/10.3390/jcm5080072
Gunton J, Hartshorne T, Langrish J, Chuang A, Chew D. P2Y12 Inhibitor Pre-Treatment in Non-ST-Elevation Acute Coronary Syndrome: A Decision-Analytic Model. Journal of Clinical Medicine. 2016; 5(8):72. https://doi.org/10.3390/jcm5080072
Chicago/Turabian StyleGunton, James, Trent Hartshorne, Jeremy Langrish, Anthony Chuang, and Derek Chew. 2016. "P2Y12 Inhibitor Pre-Treatment in Non-ST-Elevation Acute Coronary Syndrome: A Decision-Analytic Model" Journal of Clinical Medicine 5, no. 8: 72. https://doi.org/10.3390/jcm5080072






