Status Epilepticus: Epidemiology and Public Health Needs
Abstract
:1. Introduction
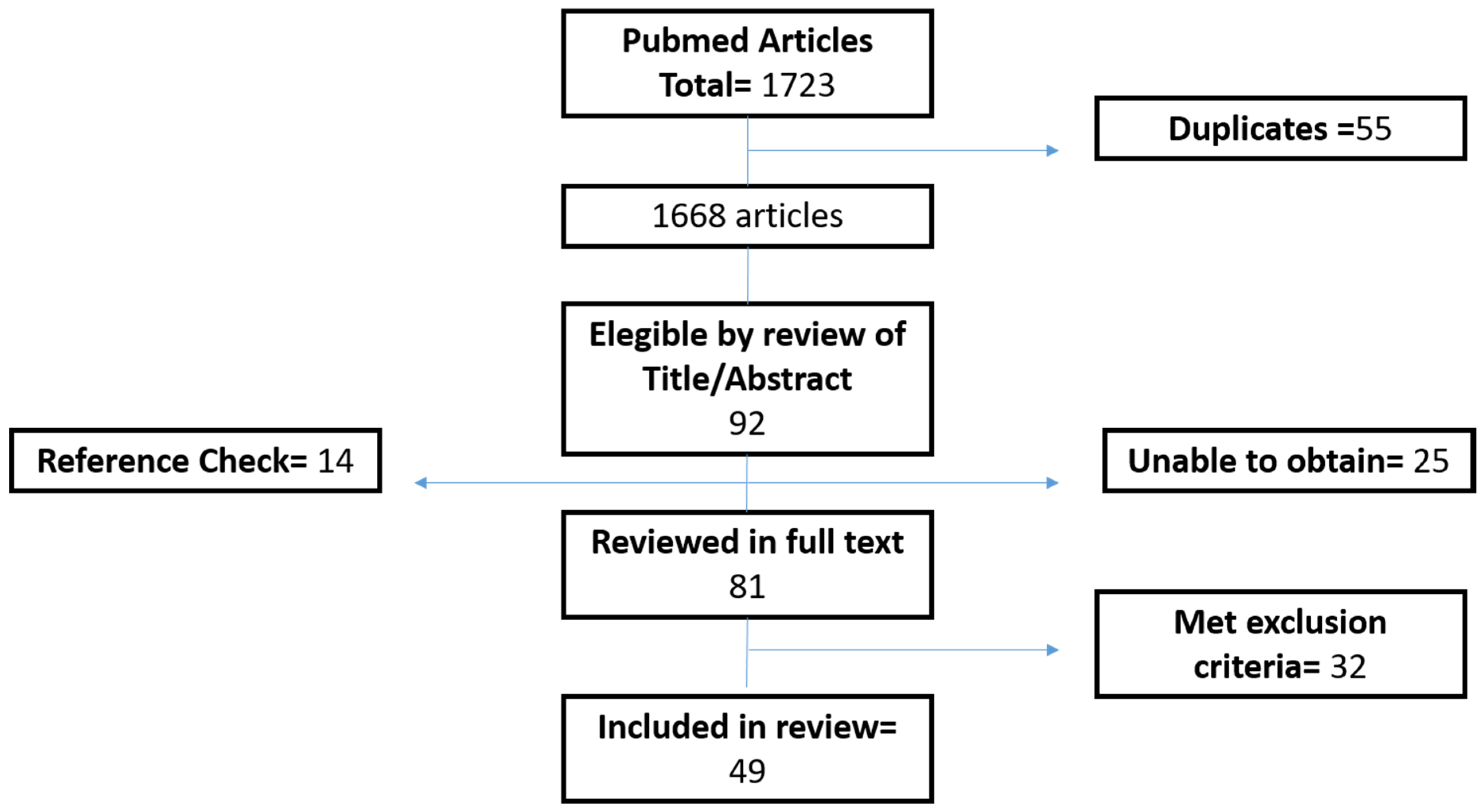
2. Methods
- status epilepticus, filtered by observational studies, reviews and systematic reviews.
- status epilepticus and public health.
- status epilepticus and epidemiology.
- status epilepticus and incidence.
- status epilepticus and prevalence.
- status epilepticus and etiology.
- status epilepticus and population.
- status epilepticus and mortality.
- status epilepticus and prognosis.
- status epilepticus and costs.
3. Results
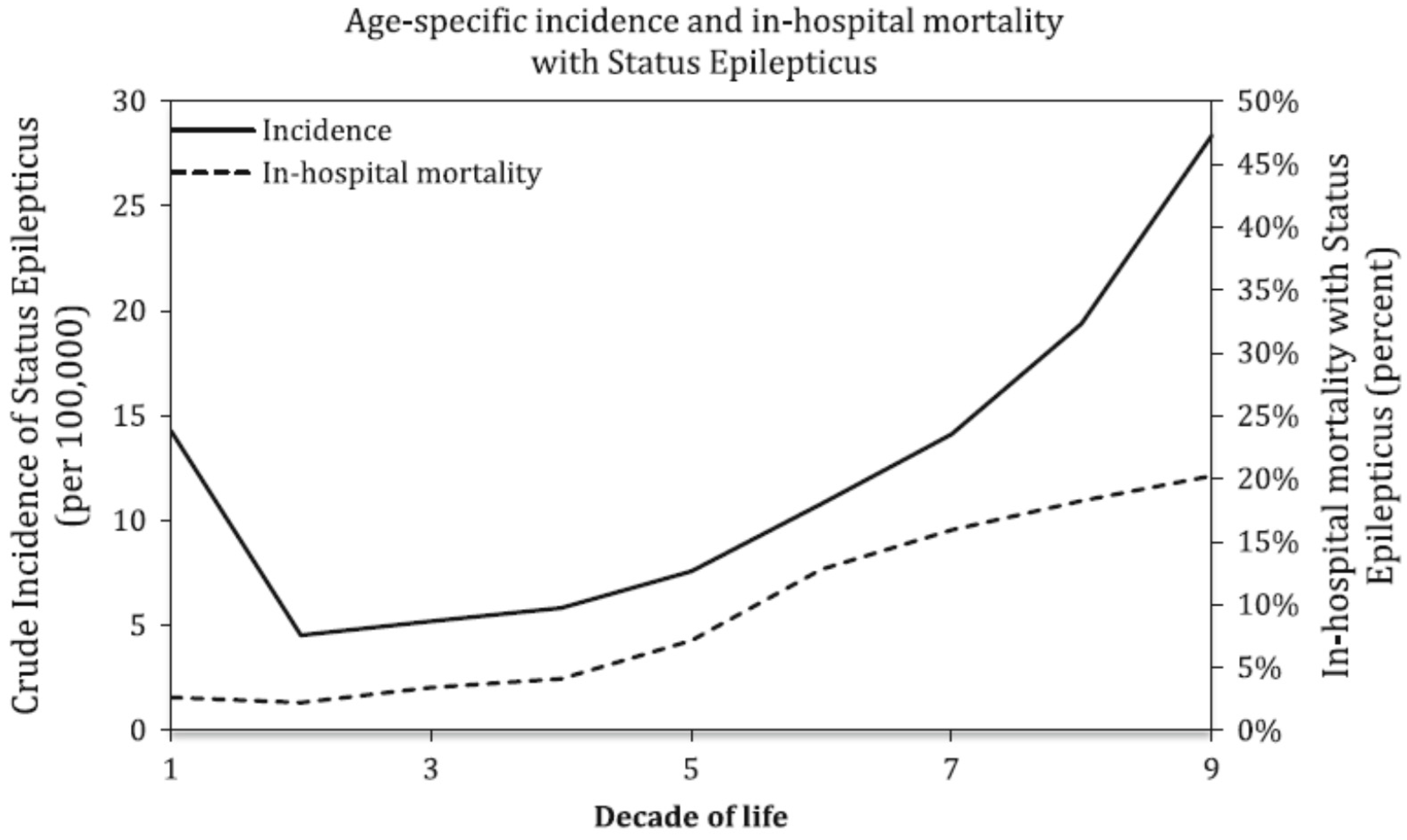
3.1. Demographics
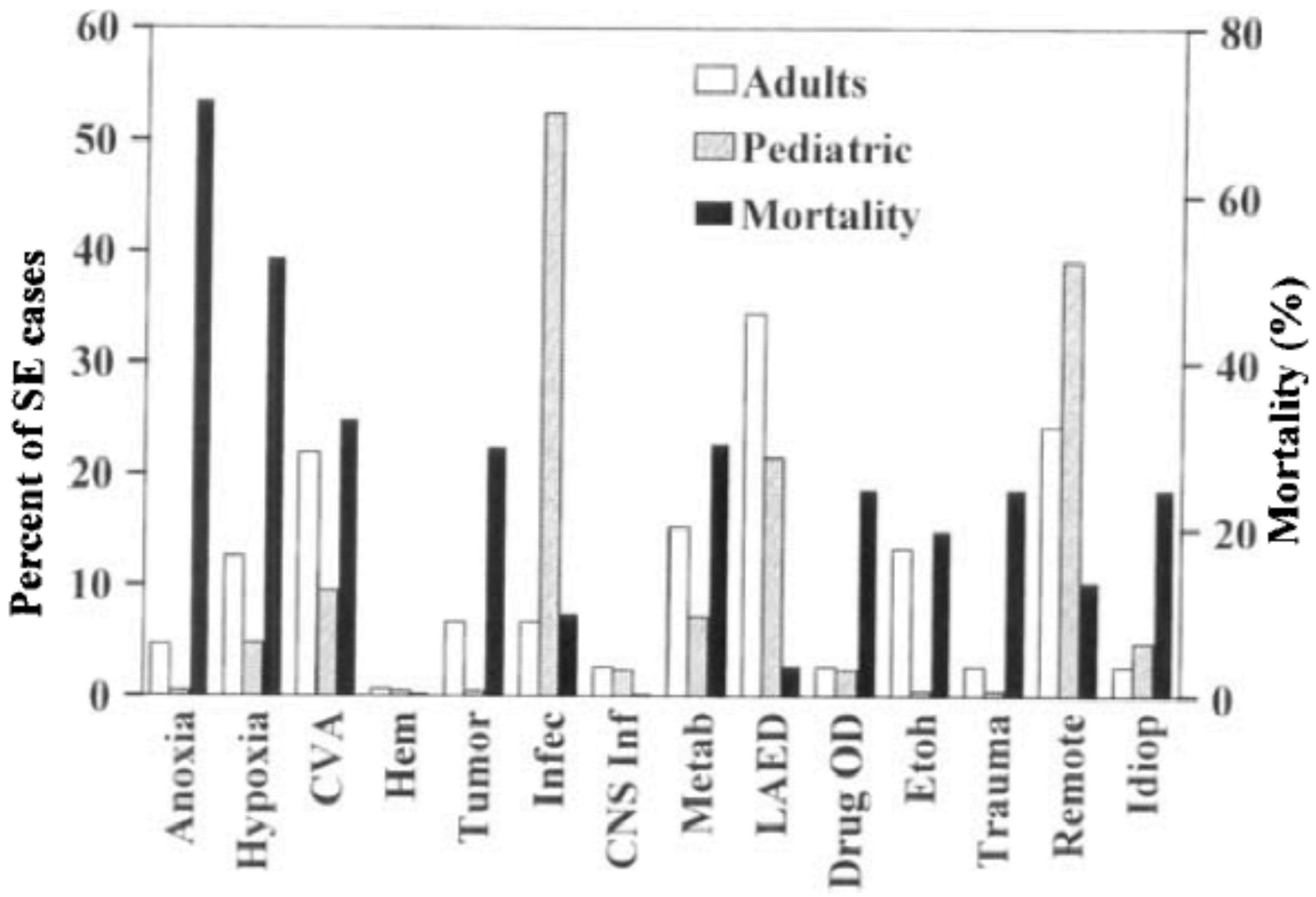
3.2. Risk Factors, Etiology and Comorbidities
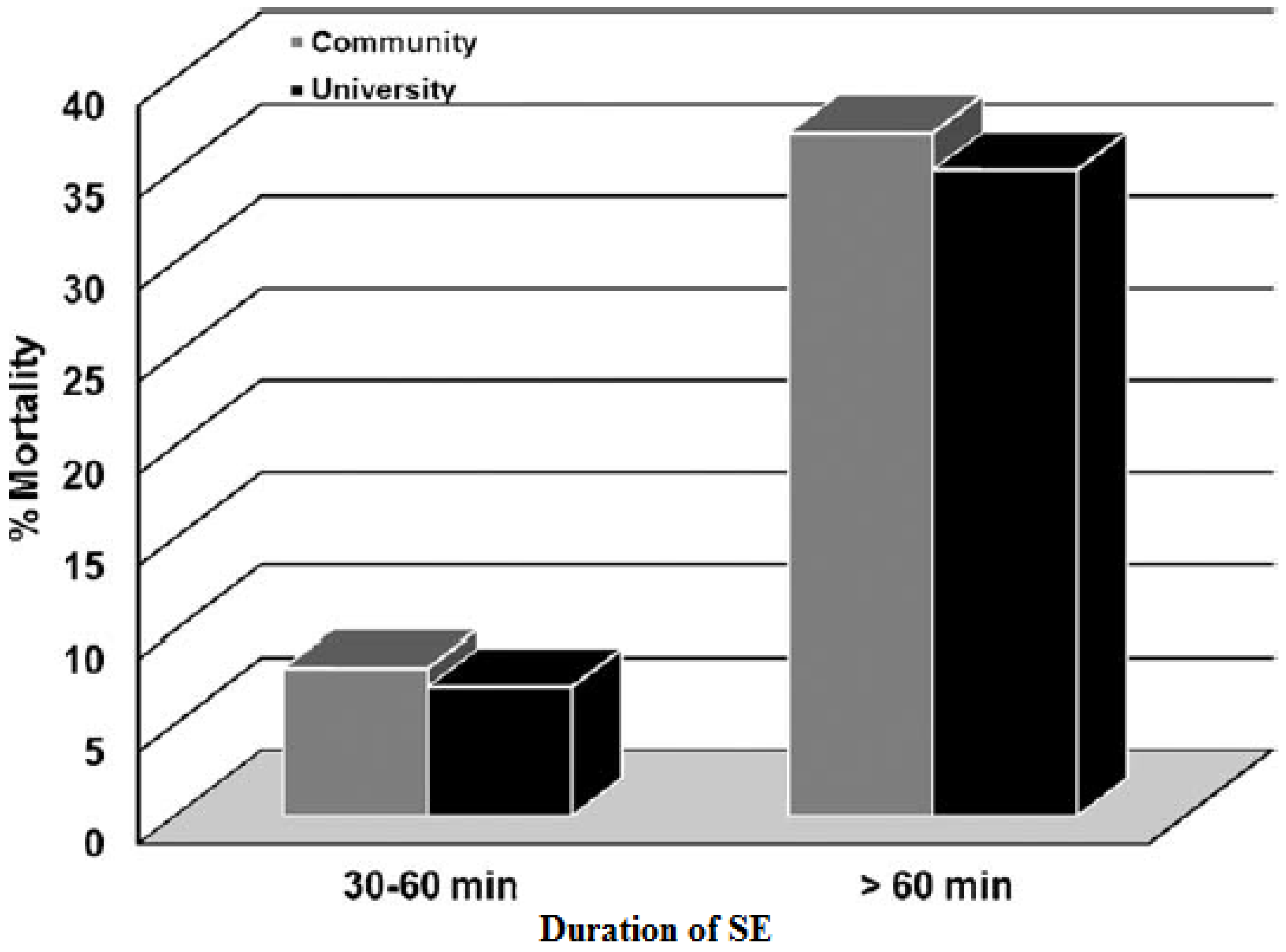
3.3. Acute Complications and Mortality
3.4. Costs
4. Discussion
Acknowledgments
Conflicts of Interest
References
- Meldrum, B.S.; Horton, R.W. Physiology of status epilepticus in primates. Arch. Neurol. 1973, 28, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lowestein, D.H. Status epilepticus: An overview of the clinical problem. Epilepsia 1999, 40 (Suppl. 1), S3–S8. [Google Scholar] [CrossRef]
- Lowestein, D.H.; Alldredge, B.K. Status Epilepticus. N. Engl. J. Med. 1998, 338, 970–976. [Google Scholar] [CrossRef] [PubMed]
- Lowestein, D.H.; Bleck, T.; Macdonald, R.L. It’s time to revise the definition of Status Epilepticus. Epilepsia 1999, 40, 120–122. [Google Scholar] [CrossRef]
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.O.; Scheffer, I.E.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Blume, W.T.; Lüders, H.O.; Mizrahi, E.; Tassinari, C.; van Emde Boas, W.; Engel, J., Jr. Glossary of descriptive terminology for ictal semiology: Report of the ILAE Task Force on Clasiffication and terminology. Epilepsia 2001, 42, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Hamer, H.; Knake, S. The epidemiology of convulsive and nonconvulsive status epilepticus. Epilepsia 2007, 48 (Suppl. 8), 82–84. [Google Scholar] [CrossRef] [PubMed]
- Skinner, H.J.; Dubon-Murcia, S.A.; Thompson, A.R.; Medina, M.T.; Edwards, J.C.; Nicholas, J.S.; Holden, K.R. Adult convulsive status epilepticus in the developing country of Honduras. Seizure 2010, 19, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Kortland, L.M.; Knake, S.; Rosenow, F.; Strzelczyk, A. Cost of status epilepticus: A systematic review. Seizure 2015, 24, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Dham, B.S.; Hunter, K.; Rincon, F. The Epidemiology of status Epilepticus in the United States. Neurocrit Care 2014, 20, 476–483. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, R.J.; Hauser, W.A.; Towne, A.R.; Boggs, J.G.; Pellock, J.M.; Penberthy, L.; Garnett, L.; Fortner, C.A.; Ko, D. A prospective, population-based epidemiologic study of Status epilepticus in Richmond, Virginia. Neurology 1996, 46, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Hesdorffer, D.C.; Logroscino, G.; Cascino, G.; Annegers, J.F.; Hauser, W.A. Incidence of Status Epilepticus in Rochester, Minnesota, 1965–1984. Neurology 1998, 50, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, E.J.; DeLorenzo, R.J. Status epilepticus in the elderly patients: Epidemiology and treatment options. Drugs Aging 2001, 18, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.; Joynt, G.; Huan, L.I.; Wong, K.S. Status epilepticus in Hong Kong Chinese: Aetiology, outcome and predictors of death and morbidity. Seizure 2003, 12, 478–482. [Google Scholar] [CrossRef]
- Li, J.M.; Chen, L.; Zhou, B.; Zhu, Y.; Zhou, D. Convulsive status epilepticus in adults and adolescents of southwest China: Mortality, etiology and predictors of death. Epilepsy Behav. 2009, 14, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, I.; Ohtsuka, Y.; Tsuda, T.; Kobayashi, K.; Inoue, H.; Narahara, K.; Shiraga, H.; Kimura, T.; Ogawa, M.; Terasaki, T.; et al. An epidemilogical study of children with status epilepticus in Okoyama, Japan: Incidence, etiologies, and outcomes. Epilepsy Res. 2011, 96, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Vignatelli, L.; Rinaldi, R.; Galeotti, M.; de Carolis, P.; D’Alessandro, R. Epidemiology of Status Epilepticus in a rural area of northern Italy: A 2-year population based study. Eur. J. Neurol. 2005, 12, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Vignatelli, L.; Tonon, C.; D’Alessandro, R. Incidence and short-term prognosis of status epilepticus in adults in Bologna, Italy. Epilepsia 2003, 44, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Canouï-Poitrine, F.; Bastuji-Garin, S.; Alonso, E.; Darcel, G.; Verstichel, P.; Caillet, P.; Paillaud, E. Risk and prognostic factors of status epilepticus in the elderly: A case-control study. Epilepsia 2011, 52, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Coeytaux, A.; Jallon, P.; Galobardes, B.; Morabia, A. Incidence of status epilepticus in french-speaking Switzerland (EPISTAR). Neurology 2000, 55, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.F.; Neville, B.G.; Peckham, C.; Bedford, H.; Wade, A.; Scott, R.C. Incidence, cause, and short-term outcome of convulsive status epilepticus in chilhood: Prospective population based study. Lancet 2006, 368, 222–229. [Google Scholar] [CrossRef]
- Garzon, E.; Fernandes, R.M.; Sakamoto, A.C. Analysis of clinical characteristics and risk factors for mortality in human status epilepticus. Seizure 2003, 12, 337–345. [Google Scholar] [CrossRef]
- Maldonado, A.; Ramos, W.; Pérez, J.; Huamán, L.A.; Gutiérrez, E.L. Convulsive status epilepticus: Clinico-epidemiologic characteristics and risk factors in Peru. Neurologia 2010, 25, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Amare, A.; Zenebe, G.; Hammack, J.; Davey, G. Status epilepticus: Clinical presentation, cause, outcome and predictors of death in 119 Ethiopian patients. Epilepsia 2008, 49, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Ozdilek, B.; Midi, I.; Agan, K.; Bingol, C.A. Episodes of status epilepticus in young adults: Etiologic factors, subtypes, and outcomes. Epilepsy Behav. 2013, 27, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Urtecho, J.; Seifi, A.; Maltenfort, M.; Vibbert, M.; McBride, W.; Moussouttas, M.; Jallo, J.; Bell, R.; Rincon, F. Incidence, risk factors, and impact of status epilepticus in sepsis in the United States. Crit. Care 2011, 15, 328. [Google Scholar] [CrossRef]
- Sadarangani, M.; Seaton, C.; Scott, J.A.G.; Ogutu, B.; Edwards, T.; Prins, A.; Gatakaa, H.; Idro, R.; Berkley, J.A.; Peshu, N.; et al. Incidence and outcome of status epilepticus in Kenyan children: A cohort study. Lancet Neurol. 2008, 7, 145–150. [Google Scholar] [CrossRef]
- Corey, L.A.; Pellock, J.M.; DeLorenzo, R.J. Status epilepticus in a population-based Virginia twin sample. Epilepsia 2004, 45, 159–165. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, R.J.; Pellock, J.M.; Towne, A.R.; Boggs, J.G. Epidemiology of Status Epilepticus. J. Clin. Neurophysiol. 1995, 12, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Maytal, J.; Shinnar, S.; Moshe, S.L.; Alvarez, L.A. Low morbidity and mortality of Status epilepticus in children. Pediatrics 1989, 83, 323–331. [Google Scholar] [PubMed]
- Fountain, N.B. Status epilepticus: Risk factors and complications. Epilepsia 2000, 41 (Suppl. 2), S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Koubeissi, M.; Alshekhlee, A. In-hospital mortality of generalized convulsive status epilepticus. A large US sample. Neurology 2007, 69, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Chuang, Y.C.; Chang, H.W.; Chang, W.N.; Lai, S.L.; Huang, C.R.; Tsai, N.W.; Wang, H.C.; Lin, Y.J.; Lu, C.H. Factors predictive of outcome in patients with de novo status epilepticus. QJM 2009, 102, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Neligan, A.; Shorvon, S.D. Prognostic factors, morbidity and mortality in tonic-clonic status epilepticus: A review. Epilepsy Res. 2011, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Raspall-Chaure, M.; Chin, R.F.; Neville, B.G.; Scott, R.C. Outcome of paediatric convulsive status epilepticus: A systematic review. Lancet Neurol. 2006, 5, 769–779. [Google Scholar] [CrossRef]
- Waterhouse, E.J.; Vaughan, J.K.; Barnes, T.Y.; Boggs, J.G.; Towne, A.R.; Kopec-Garnett, L.; DeLorenzo, R.J. Synergistic effect of status epilepticus and brain ischemic brain injury on mortality. Epilepsy Res. 1998, 29, 175–183. [Google Scholar] [CrossRef]
- Shneker, B.F.; Fountain, N.B. Assessement of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology 2003, 61, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Neligan, A.; Shorvon, S.D. Frequency and prognosis of convulsive status epilepticus of different causes: A systematic review. Arch. Neurol. 2010, 67, 931–940. [Google Scholar] [CrossRef] [PubMed]
- DeLorenzo, R.; Kirmani, B.; Deshpande, L.S.; Jakkampudi, V.; Towne, A.R.; Waterhouse, E.; Garnett, L.; Ramakrishnan, V. Comparisons of mortality and clinical presentations of status epilepticus in private practice community and university hospital setting in Richmond, Virginia. Seizure 2009, 2009, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, E.J.; Garnett, L.K.; Towne, A.R.; Morton, L.D.; Barnes, T.; Ko, D.; DeLorenzo, R.J. Prospective population-based study of intermittent and continuous convulsive status epilepticus in Richmond, Virginia. Epilepsia 1999, 40, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Pujar, S.S.; Neville, B.G.; Scott, R.C.; Chin, R.F. Death within 8 years after chilhood convulsive status epilepticus: A population-based study. Brain 2011, 134, 2819–2827. [Google Scholar] [CrossRef] [PubMed]
- Hesdorffer, D.C.; Logroscino, G.; Cascino, G.; Annegers, J.F.; Hauser, W.A. Riks of unprovoked seizure after acute symptomatic seizure: Effect ot status epilepticus. Ann. Neurol. 1998, 44, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.A.; Chin, R.F.; Camfield, C.S.; Wiebe, S.; Levin, S.D.; Speechley, K.N. Convulsive status epilepticus and health-related quality of life in children with epilepsy. Neurology 2014, 83, 752–757. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.; Lokin, J.K.; Fitzsimmons, B.-F.M.; Mendelsohn, F.A.; Mayer, S.A. Predictor of functional disability and mortality after status epilepticus. Neurology 2002, 58, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Penberthy, L.T.; Towne, A.; Garnett, L.K.; Perlin, J.B.; DeLorenzo, R.J. Estimating the economic burden of status epilepticus to the health care system. Seizure 2005, 14, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Strzelczyk, A.; Knake, S.; Oertel, W.H.; Rosenow, F.; Hamer, H.M. Inpatient treatment costs of status epilepticus in adults in Germany. Seizure 2013, 22, 882–885. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Report on Ageing and Health. In Proceedings of the 2014 IEEE Geoscience and Remote Sensing Symposium IGARSS, Quebec City, QC, Canada, 13–18 July 2014; pp. 1–5.
- Morgenstern, L.B.; Smith, M.A.; Sánchez, B.N.; Brown, D.L.; Zahuranec, D.B.; Garcia, N.; Kerber, K.A.; Skolarus, L.E.; Meurer, W.J.; Burke, J.F.; et al. Persistent ischemic stroke disparities despite delclining incidence in Mexican americans. Ann. Neurol. 2013, 74, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Barry, E.; Hauser, W.A. Status epilepticus and antiepileptic medication levels. Neurology 1994, 44, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Chin, R.; Neville, B.; Scott, R.C. A systematic review of the epidemiology of status epilepticus. Eur. J. Neurol. 2004, 11, 800–810. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Malaria Report; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Greenwood, B.; Bojang, K. Malaria. Lancet 2005, 365, 1487–1498. [Google Scholar] [CrossRef]
| Cause | Incidence | Population | Mortality |
|---|---|---|---|
| Stroke | 20% | Elderly | 20%–40% |
| Alcohol abuse | 8.1%–25% | Adults | 0%–10% |
| Drug abuse | 2%–14% | Adults | 20% |
| AED reduction or withdrawal or low AED levels | 34% | Adults | |
| Severe acute anoxia/hypoxia | 8%–13% | Adults | 60%–80% |
| CNS infection 1 | 1%–12% | Children | 30% |
| Brain tumors | 2%–15% | Adults | 0%–20% |
| Trauma | 0%–10% | Adults | 11%–25% |
| Cryptogenic | 5% | Variable |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, S.; Rincon, F. Status Epilepticus: Epidemiology and Public Health Needs. J. Clin. Med. 2016, 5, 71. https://doi.org/10.3390/jcm5080071
Sánchez S, Rincon F. Status Epilepticus: Epidemiology and Public Health Needs. Journal of Clinical Medicine. 2016; 5(8):71. https://doi.org/10.3390/jcm5080071
Chicago/Turabian StyleSánchez, Sebastián, and Fred Rincon. 2016. "Status Epilepticus: Epidemiology and Public Health Needs" Journal of Clinical Medicine 5, no. 8: 71. https://doi.org/10.3390/jcm5080071






