Current Approaches in the Treatment of Relapsed and Refractory Acute Myeloid Leukemia
Abstract
:1. Introduction
2. Cytotoxic Chemotherapy
| Regimen | Agents | CR | TRM or 30 Day Mortality | Reference |
|---|---|---|---|---|
| HIDAC | Cytarabine 3 g/m2 every 12 h days 1–6 | 32%–47% | 12%–15% | [12,13] |
| FLAG FLAG-IDA | Fludarabine 30 mg/m2 days 1–5 | 48%–55% | 10%–11% | [14,15] |
| Cytarabine 2 g/m2 days 1–5 | ||||
| G-CSF 5 mcg/kg day 0 until ANC recovery | ||||
| Fludarabine 30 mg/m2 days 1–5 | 63% | 17% | [16] | |
| Cytarabine 2 g/m2 days 1–5 | ||||
| G-CSF 300 mcg day 0 until ANC recovery | ||||
| Idarubicin 8 mg/m2 days 1–3 | ||||
| FLA | Fludarabine 30 mg/m2 days 1–5 | 61% | 7% | [17] |
| Cytarabine 2 g/m2 days 1–5 | ||||
| CLAG CLAG-M | Cladribine 5 mg/m2 days 2–6 | 38%–50% | 0%–17% | [18,19,20] |
| Cytarabine 2 g/m2 days 2–6 | ||||
| G-CSF 300 mcg days 1–6 | ||||
| Cladribine 5 mg/m2 days 1–5 | 50%–58% (53% after first course) | 0%–7% | [18,21] | |
| Cytarabine 2 g/m2 days 1–5 | ||||
| G-CSF 300 mcg days 0–5 | ||||
| Mitoxantrone 10 mg/m2 days 1–3 | ||||
| MEC | Mitoxantrone 6 mg/m2 days 1–6 | 59%–66% | 3%–6% | [22,23] |
| Etoposide 80 mg/m2 days 1–6 | ||||
| Cytarabine 1 g/m2 days 1–6 | ||||
| Mitoxantrone 8 mg/m2 days 1–5 | 18%–24% | 7%–11% | [20,24,25] | |
| Etoposide 100 mg/m2 days 1–5 | ||||
| Cytarabine 1 mg/m2 days 1–5 | ||||
| MEC/Decitabine | Decitabine 20 mg/m2 days 1–10 | 30% (CR + CRp + CRi = 50%) | 20% | [26] |
| Mitoxantrone 8 mg/m2 days 16–20 | ||||
| Etoposide 100 mg/m2 days 16–20 | ||||
| Cytarabine 1 mg/m2 days 16–20 | ||||
| EMA-86 | Mitoxantrone 12 mg/m2 days 1–3 | 60% | 11% | [27] |
| Cytarabine 500 mg/m2 CI days 1–3 & 8–10 | ||||
| Etoposide 200 mg/m2 CI days 8–10 | ||||
| MAV | Mitoxantrone 10 mg/m2 days 4–8 | 58% | 11% | [28] |
| Cytarabine 100 mg/m2 CI days 1–8 | ||||
| Etoposide 100–120 mg/m2 days 4–8 | ||||
| FLAD | Fludarabine 30 mg/m2 days 1–3 | 53% | 7.5% | [29] |
| Cytarabine 2 g/m2 days 1–3 | ||||
| Liposomal daunorubicin 100 mg/m2 days 1–3 | ||||
| FLAM | Flavopiridol 50 mg/m2 days 1–3 | 28%–43% | 5%–28% | [30,31] |
| Cytarabine 2 g/m2/72 h starting day 6 | ||||
| Mitoxantrone 40 mg/m2 day 9 | ||||
| Hybrid FLAM | Flavopiridol 30mg/m2 bolus, 60 mg/m2 over 4 h days 1–3 | 40% | 9% | [32] |
| Cytarabine 2 g/m2/72 h starting day 6 | ||||
| Mitoxantrone 40 mg/m2 day 9 | ||||
| Clofarabine Cytarabine | Clofarabine 40 mg/m2 days 2–6 | 28%–51% | 6.2%–13% | [33] |
| Cytarabine 1 g/m2 days 1–5 | ||||
| Clofarabine 40 mg/m2 days 1–5; Cytarabine 1 g/m2 days 1–5 | [34,35] | |||
| Clofarabine 22.5 mg/m2 days 1–5; Cytarabine 1 g/m2 days 1–5 | ||||
| GCLAC | Clofarabine 25 mg/m2 days 1–5; Cytarabine 2 g/m2 days 1–5; G-CSF 5 mcg/kg day 0 until ANC recovery | 46%, (CR + CRp 61%) | 13% | [36,37] |
| HAA | Homoharringtonine 4 mg/m2 days 1–3 | 76%–80% | 0% | [38] |
| Cytarabine 150 mg/m2 days 1–7 | ||||
| Aclarubicin 12 mg/m2 days 1–7 | ||||
| CPX 351 | CPX 351 101 units/m2 days 1, 3, and 5 | 23%–37% (CR + CRi = 49%) | 7%–13% | [39] |
| CPX 351 100 units/m2 days 1, 3, 5 (first induction) and days 1 and 3 (second induction and consolidation) | [40] |
| Agent | Mechanism of Action | Ongoing Clinical Trial | Reference |
|---|---|---|---|
| Ruxolitinib | JAK1 and JAK2 inhibitor | NCT02257138, NCT00674479, NCT01251965 | [41,42] |
| Rapamycin | mTOR inhibitor | NCT01184898, NCT01869114, NCT00634244, NCT02109744 | [43,44] |
| Everolimus | mTOR inhibitor | NCT00819546 | [45] |
| Tosedostat | Aminopeptidase activity inhibitor | NCT01636609 | [46,47] |
| Vorinostat | Histone deacetylase inhibitor | NCT01130506, NCT01534260, NCT01550224, NCT01617226, NCT02083250 | [48,49,50] |
| AG-120 | IDH1 inhibitor | NCT02074839 | NCT02074839 |
| AG-221 | IDH2 inhibitor | NCT01915498 | [51] |
| Elacytarabine | Elaidic acid ester of cytarabine | No active studies found | [52] |
| Vosaroxin | Anticancer quinolone derivative | NCT01191801 | [53,54] |
| Pravastatin | HMG-CoA reductase inhibitor | NCT00840177 | [55,56] |
| Bortezomib | Proteasome inhibitor | NCT01174888, NCT01127009, NCT01736943, NCT01861314, NCT01534260, NCT01075425, NCT00410423 | [57] |
| Lenalidomide | Immunomodulatory agent | NCT01681537, NCT01904643, NCT01629082, NCT01132586, NCT01246622, NCT01743859, NCT01016600, NCT00466895, NCT01615042 | [58] |
| CPI-613 | Lipoate derivative | NCT01768897 | [59] |
| ABT-199 | BCL-2 inhibitor | NCT01994837 | [60] |
| Erismodegib | Hedgehog inhibitor | NCT02129101 | NCT02129101 |
| PF-04449913 | Hedgehog inhibitor | NCT02038777 | NCT02038777 |
| Agent | Mechanism of Action | Ongoing Clinical Trial | Reference |
|---|---|---|---|
| Gemtuzumab ozogamicin | Conjugated Antibody targeting CD33 | NCT01869803, NCT00766116, NCT02221310 | [61,62,63] |
| SGN-CD33A | Conjugated Antibody targeting CD33 | NCT01902329 | [64,65] |
| Lintuzumab | Unconjugated Antibody targeting CD33 | No active studies found | [66] |
| CSL362 | Unconjugated Antibody targeting CD123 | No active studies found | [67,68] |
| AMG330 | Bispecific T-cell Engaging Antibody targeting CD33 and CD3 | No active studies found | [69,70] |
| MGD006 | Dual Affinity Re-Targeting Antibody targeting CD123 and CD3 | NCT02152956 | [71] |
| CD16x33 BiKE | Bispecific Killer Cell Engager Antibody against CD16 and CD33 | No active studies found | [72] |
| CART33 | Chimeric Antigen Receptor-Transduced T Cells targeting CD33 | NCT01864902 | [73] |
| CART123 | Chimeric Antigen Receptor-Transduced T Cells targeting CD123 | NCT02159495 | [74,75] |
| WT1 peptide vaccine | Vaccine targeting WT1 | NCT00965224 | [76,77] |
| WT1-specific CD8(+) T-cell infusion | Adoptive Cell Transfer | NCT01640301 | [78,79,80] |
| Haploidentical NK cell infusion | Adoptive Cell Transfer | NCT01947322, NCT01385423, NCT01370213, NCT00303667, NCT01621477, NCT00526292, NCT00789776, NCT02259348, NCT01795378, NCT01898793, NCT01386619 | [81] |
| AlloHSCT | Adoptive Cell Transfer | More than 40 active clinical trials identified | [82,83,84,85] |
| Donor lymphocyte infusion (post alloHCT) | Adoptive Cell Transfer | NCT01758367, NCT01390311, NCT00068718, NCT01523223, NCT01760655, NCT00534118, NCT00005799, NCT00448357 | [86,87,88,89] |
3. Targeted Agents
4. Immunotherapy
5. Low-Intensity Therapy
6. Discussion
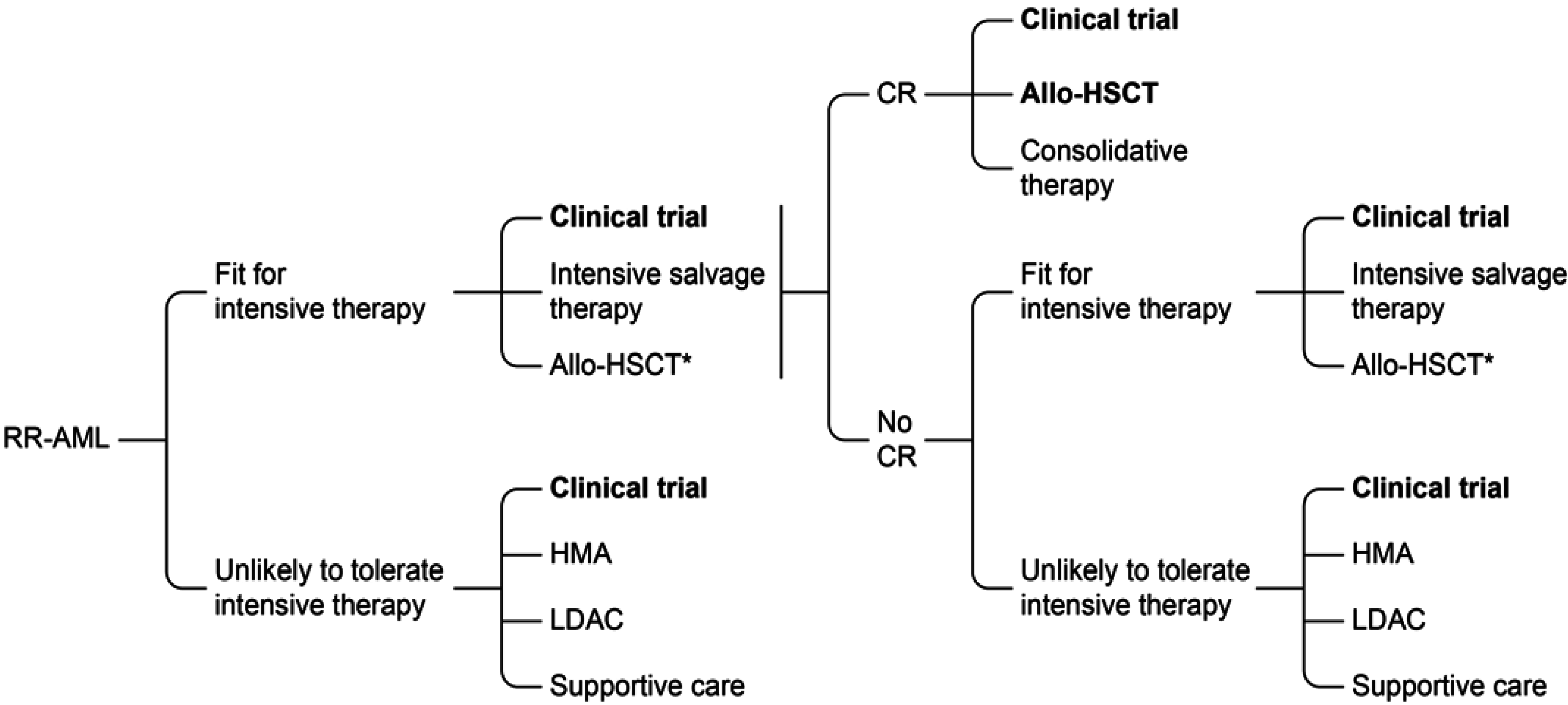
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2015; American Cancer Society: Atlanta, GA, USA, 2015; p. 4. [Google Scholar]
- Walter, R.B.; Othus, M.; Burnett, A.K.; Lowenberg, B.; Kantarjian, H.M.; Ossenkoppele, G.J.; Hills, R.K.; Ravandi, F.; Pabst, T.; Evans, A.; et al. Resistance prediction in aml: Analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOC and MD Anderson Cancer Center. Leukemia 2014, 29, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Lowenberg, B.; Downing, J.R.; Burnett, A. Acute myeloid leukemia. N. Engl. J. Med. 1999, 341, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Bergua, J.M.; Montesinos, P.; Martinez-Cuadrón, D.; Fernández-Abellán, P.; Serrano, J.; Sayas, M.J.; Prieto-Fernandez, J.; Garcia, R.; Garcia-Huerta, A.J.; Barrios, M.; et al. A prognostic index for patients with refractory or in first relapsed acute myeloid leukemia treated with FLAG-ida or FLAGO-ida. Blood 2014, 124, 1049. [Google Scholar]
- Breems, D.A.; Van Putten, W.L.; Huijgens, P.C.; Ossenkoppele, G.J.; Verhoef, G.E.; Verdonck, L.F.; Vellenga, E.; De Greef, G.E.; Jacky, E.; Van der Lelie, J.; et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J. Clin. Oncol. 2005, 23, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S.; Yamaguchi, T.; Miyawaki, S.; Uchida, N.; Sakura, T.; Kanamori, H.; Usuki, K.; Yamashita, T.; Okoshi, Y.; Shibayama, H.; et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica 2010, 95, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Kantarjian, H.; Garcia-Manero, G.; Pierce, S.; Cardenas-Turanzas, M.; Cortes, J.; Ravandi, F. Improving outcomes for patients with acute myeloid leukemia in first relapse: A single center experience. Am. J. Hematol. 2015, 90, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074.
- Ding, L.; Ley, T.J.; Larson, D.E.; Miller, C.A.; Koboldt, D.C.; Welch, J.S.; Ritchey, J.K.; Young, M.A.; Lamprecht, T.; McLellan, M.D.; et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 2012, 481, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Meggendorfer, M.; Alpermann, T.; Perglerová, K.; Kern, W.; Schnittger, S.; Haferlach, C.; Haferlach, T. Genetic patterns of relapsed aml differ significantly from first manifestation and are dependent on cytogenetic risk groups at diagnosis: Results in 175 patients with paired samples. Blood 2014, 124, 1029. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Karp, J.E. New considerations in the design of clinical trials for the treatment of acute leukemia. Clin. Investig. 2011, 1, 509–517. [Google Scholar] [CrossRef]
- Karanes, C.; Kopecky, K.J.; Head, D.R.; Grever, M.R.; Hynes, H.E.; Kraut, E.H.; Vial, R.H.; Lichtin, A.; Nand, S.; Samlowski, W.E.; et al. A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk. Res. 1999, 23, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Herzig, R.H.; Lazarus, H.M.; Wolff, S.N.; Phillips, G.L.; Herzig, G.P. High-dose cytosine arabinoside therapy with and without anthracycline antibiotics for remission reinduction of acute nonlymphoblastic leukemia. J. Clin. Oncol. 1985, 3, 992–997. [Google Scholar] [PubMed]
- Lee, S.R.; Yang, D.H.; Ahn, J.S.; Kim, Y.K.; Lee, J.J.; Choi, Y.J.; Shin, H.J.; Chung, J.S.; Cho, Y.Y.; Chae, Y.S.; et al. The clinical outcome of flag chemotherapy without idarubicin in patients with relapsed or refractory acute myeloid leukemia. J. Korean Med. Sci. 2009, 24, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Montillo, M.; Mirto, S.; Petti, M.C.; Latagliata, R.; Magrin, S.; Pinto, A.; Zagonel, V.; Mele, G.; Tedeschi, A.; Ferrara, F. Fludarabine, cytarabine, and G-CSF (FLAG) for the treatment of poor risk acute myeloid leukemia. Am. J. Hematol. 1998, 58, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Virchis, A.; Koh, M.; Rankin, P.; Mehta, A.; Potter, M.; Hoffbrand, A.V.; Prentice, H.G. Fludarabine, cytosine arabinoside, granulocyte-colony stimulating factor with or without idarubicin in the treatment of high risk acute leukaemia or myelodysplastic syndromes. Br. J. Haematol. 2004, 124, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Milligan, D.W.; Wheatley, K.; Littlewood, T.; Craig, J.I.; Burnett, A.K. Fludarabine and cytosine are less effective than standard ade chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: Results of the MRC AML-HR randomized trial. Blood 2006, 107, 4614–4622. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.G.; Welch, J.S.; Augustin, K.; Hladnik, L.; DiPersio, J.F.; Abboud, C.N. Cladribine in the treatment of acute myeloid leukemia: A single-institution experience. Clin. Lymphoma Myeloma 2009, 9, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Wrzesien-Kus, A.; Robak, T.; Lech-Maranda, E.; Wierzbowska, A.; Dmoszynska, A.; Kowal, M.; Holowiecki, J.; Kyrcz-Krzemien, S.; Grosicki, S.; Maj, S.; et al. A multicenter, open, non-comparative, phase II study of the combination of cladribine (2-chlorodeoxyadenosine), cytarabine, and G-CSF as induction therapy in refractory acute myeloid leukemia—A report of the polish adult leukemia group (PALG). Eur. J. Haematol. 2003, 71, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Price, S.L.; Lancet, J.E.; George, T.J.; Wetzstein, G.A.; List, A.F.; Ho, V.Q.; Fernandez, H.F.; Pinilla-Ibarz, J.; Kharfan-Dabaja, M.A.; Komrokji, R.S. Salvage chemotherapy regimens for acute myeloid leukemia: Is one better? Efficacy comparison between CLAG and MEC regimens. Leuk. Res. 2011, 35, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Wierzbowska, A.; Robak, T.; Pluta, A.; Wawrzyniak, E.; Cebula, B.; Holowiecki, J.; Kyrcz-Krzemien, S.; Grosicki, S.; Giebel, S.; Skotnicki, A.B.; et al. Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: A final report of the polish adult leukemia group. Eur. J. Haematol. 2008, 80, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Amadori, S.; Arcese, W.; Isacchi, G.; Meloni, G.; Petti, M.C.; Monarca, B.; Testi, A.M.; Mandelli, F. Mitoxantrone, etoposide, and intermediate-dose cytarabine: An effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J. Clin. Oncol. 1991, 9, 1210–1214. [Google Scholar] [PubMed]
- Trifilio, S.M.; Rademaker, A.W.; Newman, D.; Coyle, K.; Carlson-Leuer, K.; Mehta, J.; Altman, J.; Frankfurt, O.; Tallman, M.S. Mitoxantrone and etoposide with or without intermediate dose cytarabine for the treatment of primary induction failure or relapsed acute myeloid leukemia. Leuk. Res. 2012, 36, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.L.; Lee, S.J.; Advani, R.; Tallman, M.S.; Sikic, B.I.; Letendre, L.; Dugan, K.; Lum, B.; Chin, D.L.; Dewald, G.; et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: A phase III trial (E2995). J. Clin. Oncol. 2004, 22, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, H.E.; Patel, S.; Ho, M.; Owen, T.; Pollyea, D.A.; Majeti, R.; Gotlib, J.; Coutre, S.; Liedtke, M.; Berube, C.; et al. Second-line mitoxantrone, etoposide, and cytarabine for acute myeloid leukemia: A single-center experience. Am. J. Hematol. 2010, 85, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Halpern, A.B.; Estey, E.H.; Othus, M.; Orlowski, K.F.; Powell, M.A.; Chen, T.L.; Becker, P.S.; Scott, B.L.; Hendrie, P.C.; Ostronoff, F.; et al. Mitoxantrone, etoposide, and cytarabine (MEC) following epigenetic priming with decitabine in adults with relapsed/refractory acute myeloid leukemia (AML) or high-risk myelodysplastic syndrome (MDS): A phase 1 study. Blood 2014, 124, 3730. [Google Scholar] [CrossRef] [PubMed]
- Archimbaud, E.; Thomas, X.; Leblond, V.; Michallet, M.; Fenaux, P.; Cordonnier, C.; Dreyfus, F.; Troussard, X.; Jaubert, J.; Travade, P.; et al. Timed sequential chemotherapy for previously treated patients with acute myeloid leukemia: Long-term follow-up of the etoposide, mitoxantrone, and cytarabine-86 trial. J. Clin. Oncol. 1995, 13, 11–18. [Google Scholar] [PubMed]
- Link, H.; Freund, M.; Diedrich, H.; Wilke, H.; Austein, J.; Henke, M.; Wandt, H.; Fackler-Schwalbe, E.; Schlimok, G.; Hoffmann, R.; et al. Mitoxantrone, cytosine arabinoside, and vp-16 in 36 patients with relapsed and refractory acute myeloid leukemia. Haematol. Blood Transfus. 1990, 33, 322–325. [Google Scholar] [PubMed]
- De Astis, E.; Clavio, M.; Raiola, A.M.; Ghiso, A.; Guolo, F.; Minetto, P.; Galaverna, F.; Miglino, M.; Di Grazia, C.; Ballerini, F.; et al. Liposomal daunorubicin, fludarabine, and cytarabine (FLAD) as bridge therapy to stem cell transplant in relapsed and refractory acute leukemia. Ann. Hematol. 2014, 93, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Litzow, M.R.; Wang, X.V.; Carroll, M.P.; Karp, J.E.; Ketterling, R.; Kaufmann, S.H.; Lazarus, H.M.; Luger, S.M.; Paietta, E.M.; Rowe, J.M.; et al. A randomized phase II trial of three novel regimens for relapsed/ refractory acute myeloid leukemia (AML) demonstrates encouraging results with a flavopiridol-based regimen: Results of eastern cooperative oncology group (ECOG) trial E1906. Blood 2014, 124, 3742. [Google Scholar]
- Karp, J.E.; Smith, B.D.; Levis, M.J.; Gore, S.D.; Greer, J.; Hattenburg, C.; Briel, J.; Jones, R.J.; Wright, J.J.; Colevas, A.D. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: A phase II trial in adults with poor-risk acute myelogenous leukemia. Clin. Cancer Res. 2007, 13, 4467–4473. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.E.; Smith, B.D.; Resar, L.S.; Greer, J.M.; Blackford, A.; Zhao, M.; Moton-Nelson, D.; Alino, K.; Levis, M.J.; Gore, S.D.; et al. Phase 1 and pharmacokinetic study of bolus-infusion flavopiridol followed by cytosine arabinoside and mitoxantrone for acute leukemias. Blood 2011, 117, 3302–3310. [Google Scholar] [CrossRef] [PubMed]
- Faderl, S.; Gandhi, V.; O'Brien, S.; Bonate, P.; Cortes, J.; Estey, E.; Beran, M.; Wierda, W.; Garcia-Manero, G.; Ferrajoli, A.; et al. Results of a phase 1-2 study of clofarabine in combination with cytarabine (ARA-C) in relapsed and refractory acute leukemias. Blood 2005, 105, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Scappini, B.; Gianfaldoni, G.; Caracciolo, F.; Mannelli, F.; Biagiotti, C.; Romani, C.; Pogliani, E.M.; Simonetti, F.; Borin, L.; Fanci, R.; et al. Cytarabine and clofarabine after high-dose cytarabine in relapsed or refractory AML patients. Am. J. Hematol. 2012, 87, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Faderl, S.; Wetzler, M.; Rizzieri, D.; Schiller, G.; Jagasia, M.; Stuart, R.; Ganguly, S.; Avigan, D.; Craig, M.; Collins, R.; et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: Results from the classic I trial. J. Clin. Oncol. 2012, 30, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.S.; Kantarjian, H.M.; Appelbaum, F.R.; Petersdorf, S.H.; Storer, B.; Pierce, S.; Shan, J.; Hendrie, P.C.; Pagel, J.M.; Shustov, A.R.; et al. Clofarabine with high dose cytarabine and granulocyte colony-stimulating factor (G-CSF) priming for relapsed and refractory acute myeloid leukaemia. Br. J. Haematol. 2011, 155, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.S.; Kantarjian, H.M.; Appelbaum, F.R.; Storer, B.; Pierce, S.; Shan, J.; Faderl, S.; Estey, E.H. Retrospective comparison of clofarabine versus fludarabine in combination with high-dose cytarabine with or without granulocyte colony-stimulating factor as salvage therapies for acute myeloid leukemia. Haematologica 2013, 98, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Mao, L.; Qian, J.; Qian, W.; Meng, H.; Mai, W.; Tong, H.; Tong, Y.; Jin, J. Homoharringtonine in combination with cytarabine and aclarubicin in the treatment of refractory/relapsed acute myeloid leukemia: A single-center experience. Ann. Hematol. 2013, 92, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.J.; Lancet, J.E.; Kolitz, J.E.; Ritchie, E.K.; Roboz, G.J.; List, A.F.; Allen, S.L.; Asatiani, E.; Mayer, L.D.; Swenson, C.; et al. First-in-man study of CPX-351: A liposomal carrier containing cytarabine and daunorubicin in a fixed 5:1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J. Clin. Oncol. 2011, 29, 979–985. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Goldberg, S.L.; Feldman, E.J.; Rizzeri, D.A.; Hogge, D.E.; Larson, M.; Pigneux, A.; Recher, C.; Schiller, G.; Warzocha, K.; et al. Phase II, multicenter, randomized trial of CPX-351 (cytarabine:Daunorubicin) liposome injection versus intensive salvage therapy in adults with first relapse AML. Cancer 2015, 121, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Pemmaraju, N.; Kantarjian, H.; Kadia, T.; Cortes, J.; Borthakur, G.; Newberry, K.; Garcia-Manero, G.; Ravandi, F.; Jabbour, E.; Dellasala, S.; et al. A phase I/II study of the janus kinase (JAK) 1 and 2 inhibitor ruxolitinib in patients with relapsed or refractory acute myeloid leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Eghtedar, A.; Verstovsek, S.; Estrov, Z.; Burger, J.; Cortes, J.; Bivins, C.; Faderl, S.; Ferrajoli, A.; Borthakur, G.; George, S.; et al. Phase 2 study of the JAK kinase inhibitor ruxolitinib in patients with refractory leukemias, including postmyeloproliferative neoplasm acute myeloid leukemia. Blood 2012, 119, 4614–4618. [Google Scholar] [CrossRef] [PubMed]
- Recher, C.; Beyne-Rauzy, O.; Demur, C.; Chicanne, G.; Dos Santos, C.; Mas, V.M.; Benzaquen, D.; Laurent, G.; Huguet, F.; Payrastre, B. Antileukemic activity of rapamycin in acute myeloid leukemia. Blood 2005, 105, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Liesveld, J.L.; O'Dwyer, K.; Walker, A.; Becker, M.W.; Ifthikharuddin, J.J.; Mulford, D.; Chen, R.; Bechelli, J.; Rosell, K.; Minhajuddin, M.; et al. A phase I study of decitabine and rapamycin in relapsed/refractory AML. Leuk. Res. 2013, 37, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chapuis, N.; Saint Marcoux, F.; Recher, C.; Prebet, T.; Chevallier, P.; Cahn, J.Y.; Leguay, T.; Bories, P.; Witz, F.; et al. A phase Ib GOELAMS study of the mTOR inhibitor RAD001 in association with chemotherapy for aml patients in first relapse. Leukemia 2013, 27, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Lowenberg, B.; Morgan, G.; Ossenkoppele, G.J.; Burnett, A.K.; Zachee, P.; Duhrsen, U.; Dierickx, D.; Muller-Tidow, C.; Sonneveld, P.; Krug, U.; et al. Phase I/II clinical study of tosedostat, an inhibitor of aminopeptidases, in patients with acute myeloid leukemia and myelodysplasia. J. Clin. Oncol. 2010, 28, 4333–4338. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.; Feldman, E.; Yee, K.; Rizzieri, D.; Advani, A.S.; Charman, A.; Spruyt, R.; Toal, M.; Kantarjian, H. Two dosing regimens of tosedostat in elderly patients with relapsed or refractory acute myeloid leukaemia (OPAL): A randomised open-label phase 2 study. Lancet Oncol. 2013, 14, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.W.; Loaiza-Bonilla, A.; Juckett, M.; DiPers, J.F.; Roy, V.; Slack, J.; Wu, W.T.; Laumann, K.; Espinoza-Delgado, I.; Gore, S.D.; et al. A phase 2 study of vorinostat in acute myeloid leukemia. Haematol. Hematol. J. 2009, 94, 1375–1382. [Google Scholar] [CrossRef]
- Walter, R.B.; Medeiros, B.C.; Gardner, K.M.; Orlowski, K.F.; Gallegos, L.; Scott, B.L.; Hendrie, P.C.; Estey, E.H. Gemtuzumab ozogamicin in combination with vorinostat and azacitidine in older patients with relapsed or refractory acute myeloid leukemia: A phase I/II study. Haematologica 2014, 99, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Gojo, I.; Tan, M.; Fang, H.B.; Sadowska, M.; Lapidus, R.; Baer, M.R.; Carrier, F.; Beumer, J.H.; Anyang, B.N.; Srivastava, R.K.; et al. Translational phase I trial of vorinostat (suberoylanilide hydroxamic acid) combined with cytarabine and etoposide in patients with relapsed, refractory, or high-risk acute myeloid leukemia. Clin. Cancer Res. 2013, 19, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.; Tallman, M.; Pollyea, D.A.; Flinn, I.W.; Fathi, A.T.; Stone, R.M.; Levine, R.L.; Agresta, S.; Schenkein, D.; Yang, H.; et al. Clinical safety and activity in a phase I trial of AG-221, a first in class, potent inhibitor of the IDH2-mutant protein, in patients with IDH2 mutant positive advanced hematologic malignancies. In Proceedings of the 105th Annual Meeting of the American Association for Cancer Research, San Diego, CA, USA, 6–8 April 2014.
- Roboz, G.J.; Rosenblat, T.; Arellano, M.; Gobbi, M.; Altman, J.K.; Montesinos, P.; O’Connell, C.; Solomon, S.R.; Pigneux, A.; Vey, N.; et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J. Clin. Oncol. 2014, 32, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Lancet, J.E.; Ravandi, F.; Ricklis, R.M.; Cripe, L.D.; Kantarjian, H.M.; Giles, F.J.; List, A.F.; Chen, T.; Allen, R.S.; Fox, J.A.; et al. A phase Ib study of vosaroxin, an anticancer quinolone derivative, in patients with relapsed or refractory acute leukemia. Leukemia 2011, 25, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Ritchie, E.; Sayar, H.; Lancet, J.; Craig, M.D.; Vey, N.; Strickland, S.A.; Schiller, G.; Jabbour, E.; Erba, H.P.; et al. Improved survival in patients with first relapsed or refractory acute myeloid leukemia (AML) treated with vosaroxin plus cytarabine versus placebo plus cytarabine: Results of a phase 3 double-blind randomized controlled multinational study (VALOR). Blood 2014, 124, LBA 6. [Google Scholar]
- Kornblau, S.M.; Banker, D.E.; Stirewalt, D.; Shen, D.; Lemker, E.; Verstovsek, S.; Estrov, Z.; Faderl, S.; Cortes, J.; Beran, M.; et al. Blockade of adaptive defensive changes in cholesterol uptake and synthesis in AML by the addition of pravastatin to idarubicin + high-dose ARA-C: A phase 1 study. Blood 2007, 109, 2999–3006. [Google Scholar] [PubMed]
- Advani, A.S.; McDonough, S.; Copelan, E.; Willman, C.; Mulford, D.A.; List, A.F.; Sekeres, M.A.; Othus, M.; Appelbaum, F.R. SWOG0919: A phase 2 study of idarubicin and cytarabine in combination with pravastatin for relapsed acute myeloid leukaemia. Br. J. Haematol. 2014, 167, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Advani, A.S.; Elson, P.; Kalaycio, M.E.; Mukherjee, S.; Gerds, A.T.; Hamilton, B.K.; Hobson, S.; Smith, A.; Rush, M.L.; Bogati, S.; et al. Bortezomib + MEC (mitoxantrone, etoposide, cytarabine) for relapsed/refractory acute myeloid leukemia: Final results of an expanded phase 1 trial. Blood 2014, 124, 978. [Google Scholar]
- Chen, Y.; Kantarjian, H.; Estrov, Z.; Faderl, S.; Ravandi, F.; Rey, K.; Cortes, J.; Borthakur, G. A phase II study of lenalidomide alone in relapsed/refractory acute myeloid leukemia or high-risk myelodysplastic syndromes with chromosome 5 abnormalities. Clin. Lymphoma Myeloma Leuk. 2012, 12, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Pardee, T.S.; Stadelman, K.; Isom, S.; Ellis, L.R.; Berenzon, D.; Hurd, D.D.; Harrelson, R.; Manuel, M.; Dralle, S.; Lyerly, S.; et al. The mitochondrial metabolism inhibitor CPI-613 is highly active in combination with high dose ARA-C (HIDAC) and mitoxantrone in a phase I study for relapsed or refractory acute myeloid leukemia (AML). Blood 2014, 124, 3744. [Google Scholar]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.J.; Busman, T.; McKeegan, E.; Salem, A.; Zhu, M.; Ricker, J.L.; Blum, W.; et al. A phase 2 study of ABT-199 (GDC-0199) in patients with acute myelogenous leukemia (AML). Blood 2014, 124, 118. [Google Scholar]
- Thol, F.; Schlenk, R.F. Gemtuzumab ozogamicin in acute myeloid leukemia revisited. Expert. Opin. Biol. Ther. 2014, 14, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Petersdorf, S.; Kopecky, K.; Stuart, R.K.; Larson, R.A.; Nevill, T.J.; Stenke, L.; Slovak, M.L.; Tallman, M.S.; Willman, C.L.; Erba, H.; et al. Preliminary results of Southwest Oncology Group Sstudy S0106: An international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood 2009, 114, 326–327. [Google Scholar]
- Pilorge, S.; Rigaudeau, S.; Rabian, F.; Sarkozy, C.; Taksin, A.L.; Farhat, H.; Merabet, F.; Ghez, S.; Raggueneau, V.; Terre, C.; et al. Fractionated gemtuzumab ozogamicin and standard dose cytarabine produced prolonged second remissions in patients over the age of 55 years with acute myeloid leukemia in late first relapse. Am. J. Hematol. 2014, 89, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.S.K.; Walter, R.B.; Jeffrey, S.C.; Burke, P.J.; Yu, C.P.; Kostner, H.; Stone, I.; Ryan, M.C.; Sussman, D.; Lyon, R.P.; et al. SGN-CD33A: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood 2013, 122, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Stein, E.M.; Stein, A.; Walter, R.B.; Fathi, A.T.; Lancet, J.E.; Kovacsovics, T.J.; Advani, A.S.; DeAngelo, D.J.; O'Meara, M.M.; Zhao, B.; et al. Interim analysis of a phase 1 trial of SGN-CD33A in patients with CD33-positive acute myeloid leukemia (AML). Blood 2014, 124, 623. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.J.; Brandwein, J.; Stone, R.; Kalaycio, M.; Moore, J.; O’Connor, J.; Wedel, N.; Roboz, G.J.; Miller, C.; Chopra, R.; et al. Phase III randomized multicenter study of a humanized anti-CD33 monoclonal antibody, lintuzumab, in combination with chemotherapy, versus chemotherapy alone in patients with refractory or first-relapsed acute myeloid leukemia. J. Clin. Oncol. 2005, 23, 4110–4116. [Google Scholar] [CrossRef] [PubMed]
- Busfield, S.J.; Biondo, M.; Wong, M.; Ramshaw, H.S.; Lee, E.M.; Ghosh, S.; Braley, H.; Panousis, C.; Roberts, A.W.; He, S.Z.; et al. Targeting of acute myeloid leukemia in vitro and in vivo with an anti-CD123 mAB engineered for optimal ADCC. Leukemia 2014, 28, 2213–2221. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Roboz, G.J.; Walter, R.B.; Altman, J.K.; Ferguson, A.; Curcio, T.J.; Orlowski, K.F.; Garrett, L.; Busfield, S.J.; Barnden, M.; et al. First-in man, phase 1 study of CSL362 (anti-IL3Rα/anti-CD123 monoclonal antibody) in patients with CD123+ acute myeloid leukemia (AML) in CR at high risk for early relapse. Blood 2014, 124, 120. [Google Scholar]
- Laszlo, G.S.; Gudgeon, C.J.; Harrington, K.H.; Dell’Aringa, J.; Newhall, K.J.; Means, G.D.; Sinclair, A.M.; Kischel, R.; Frankel, S.R.; Walter, R.B. Cellular determinants for preclinical activity of a novel CD33/CD3 bispecific T-cell engager (BiTE) antibody, AMG 330, against human AML. Blood 2014, 123, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Henn, A.; Raum, T.; Bajtus, M.; Matthes, K.; Hendrich, L.; Wahl, J.; Hoffmann, P.; Kischel, R.; Kvesic, M.; et al. Preclinical characterization of AMG 330, a CD3/CD33-bispecific T-cell-engaging antibody with potential for treatment of acute myelogenous leukemia. Mol. Cancer Ther. 2014, 13, 1549–1557. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.; Stewart, S.; Baughman, J.; Rettig, M.; Chichili, G.; Bonvini, E.; Wigginton, J.; Lechleider, R.; DiPersio, J. A phase I trial of MGD006 in patients with relapsed acute myeloid leukemia (AML). J. Immun. Ther. Cancer 2014, 2, 87. [Google Scholar] [CrossRef]
- Wiernik, A.; Foley, B.; Zhang, B.; Verneris, M.R.; Warlick, E.; Gleason, M.K.; Ross, J.A.; Luo, X.; Weisdorf, D.J.; Walcheck, B.; et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 × 33 bispecific killer cell engager and ADAM17 inhibition. Clin. Cancer Res. 2013, 19, 3844–3855. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Wang, Y.; Lv, H.Y.; Han, Q.W.; Fan, H.; Guo, B.; Wang, L.L.; Han, W.D. Treatment of CD33-directed chimeric antigen receptor-modified T cells in one patient with relapsed and refractory acute myeloid leukemia. Mol. Ther. 2015, 23, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Tasian, S.K.; Ruella, M.; Shestova, O.; Li, Y.; Porter, D.L.; Carroll, M.; Danet-Desnoyers, G.; Scholler, J.; Grupp, S.A.; et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood 2014, 123, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Pizzitola, I.; Anjos-Afonso, F.; Rouault-Pierre, K.; Lassailly, F.; Tettamanti, S.; Spinelli, O.; Biondi, A.; Biagi, E.; Bonnet, D. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia 2014, 28, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Berneman, Z.N.; Van de Velde, A.L.; Willemen, Y.; Anguille, S.; Saevels, K.; Germonpré, P.; Huizing, M.T.; Peeters, M.; Snoeckx, A.; Parizel, P.; et al. Vaccination with WT1 mRNA-electroporated dendritic cells: Report of clinical outcome in 66 cancer patients. Blood 2014, 124, 310. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, T.L.; Frattini, M.G.; Chanel, S.M.; Dao, T.; Bernal, Y.; Jurcic, J.G.; Zhang, R.; Simancek, P.; Tallman, M.S.; Scheinberg, D.A.; et al. Phase II trial of WT1 analog peptide vaccine in patients with acute myeloid leukemia (AML) in complete remission (CR). Blood 2012, 120, 3624. [Google Scholar]
- Goswami, M.; Hensel, N.; Smith, B.D.; Prince, G.T.; Qin, L.; Levitsky, H.I.; Strickland, S.A.; Jagasia, M.; Savani, B.N.; Fraser, J.W.; et al. Expression of putative targets of immunotherapy in acute myeloid leukemia and healthy tissues. Leukemia 2014, 28, 1167–1170. [Google Scholar] [CrossRef] [PubMed]
- Cilloni, D.; Renneville, A.; Hermitte, F.; Hills, R.K.; Daly, S.; Jovanovic, J.V.; Gottardi, E.; Fava, M.; Schnittger, S.; Weiss, T.; et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: A European LeukemiaNet study. J. Clin. Oncol. 2009, 27, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Chapuis, A.G.; Ragnarsson, G.B.; Nguyen, H.N.; Chaney, C.N.; Pufnock, J.S.; Schmitt, T.M.; Duerkopp, N.; Roberts, I.M.; Pogosov, G.L.; Ho, W.Y.; et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci. Transl. Med. 2013, 5. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; Defor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef] [PubMed]
- Armistead, P.M.; de Lima, M.; Pierce, S.; Qiao, W.; Wang, X.; Thall, P.F.; Giralt, S.; Ravandi, F.; Kantarjian, H.; Champlin, R.; et al. Quantifying the survival benefit for allogeneic hematopoietic stem cell transplantation in relapsed acute myelogenous leukemia. Biol. Blood Marrow Transplant. 2009, 15, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, A.; Hardan, I.; Shem-Tov, N.; Yerushalmi, R.; Nagler, A. Allogeneic hematopoietic stem-cell transplantation in AML and MDS using myeloablative versus reduced-intensity conditioning: Long-term follow-up. Leukemia 2010, 24, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Sebert, M.; Porcher, R.; Robin, M.; Ades, L.; Boissel, N.; Raffoux, E.; Xhaard, A.; Dhedin, N.; Larghero, J.; Himberlin, C.; et al. Equivalent outcomes using reduced intensity or conventional myeloablative conditioning transplantation for patients aged 35 years and over with AML. Bone Marrow Transplant. 2015, 50, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Bejanyan, N.; Oran, B.; Shanley, R.; Warlick, E.; Ustun, C.; Vercellotti, G.; Verneris, M.; Wagner, J.E.; Weisdorf, D.; Brunstein, C. Clinical outcomes of aml patients relapsing after matched-related donor and umbilical cord blood transplantation. Bone Marrow Transplant. 2014, 49, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Itonaga, H.; Tsushima, H.; Taguchi, J.; Fukushima, T.; Taniguchi, H.; Sato, S.; Ando, K.; Sawayama, Y.; Matsuo, E.; Yamasaki, R.; et al. Treatment of relapsed adult T-cell leukemia/lymphoma after allogeneic hematopoietic stem cell transplantation: The Nagasaki Transplant Group experience. Blood 2013, 121, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Takami, A.; Yano, S.; Yokoyama, H.; Kuwatsuka, Y.; Yamaguchi, T.; Kanda, Y.; Morishima, Y.; Fukuda, T.; Miyazaki, Y.; Nakamae, H.; et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: A retrospective analysis by the adult acute myeloid leukemia working group of the Japan Society for Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1785–1790. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Czibere, A.; Platzbecker, U.; Bug, G.; Uharek, L.; Luft, T.; Giagounidis, A.; Zohren, F.; Bruns, I.; Wolschke, C.; et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia 2013, 27, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, A.; Fiala, M.A.; Abboud, C.N.; Cashen, A.F.; Eissenberg, L.; Graubert, T.; Jacoby, M.A.; Pusic, I.; Schroeder, M.A.; Stockerl-Goldstein, K.E.; et al. A phase I study of azacitidine after donor lymphocyte infusion for relapsed acute myeloid leukemia post allogeneic stem cell transplantation. Blood 2013, 122, 3320. [Google Scholar]
- O’Donnell, M.R.; Tallman, M.S.; Abboud, C.N.; Altman, J.K.; Appelbaum, F.R.; Arber, D.A.; Attar, E.; Borate, U.; Coutre, S.E.; Damon, L.E.; et al. Acute myeloid leukemia, version 2.2013. J. Nat. Compr. Cancer Netw. 2013, 11, 1047–1055. [Google Scholar]
- Gandhi, V.; Estey, E.; Keating, M.J.; Plunkett, W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J. Clin. Oncol. 1993, 11, 116–124. [Google Scholar] [PubMed]
- Chow, K.U.; Boehrer, S.; Napieralski, S.; Nowak, D.; Knau, A.; Hoelzer, D.; Mitrou, P.S.; Weidmann, E. In AML cell lines ARA-C combined with purine analogues is able to exert synergistic as well as antagonistic effects on proliferation, apoptosis and disruption of mitochondrial membrane potential. Leuk. Lymphoma 2003, 44, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Thomas, X.; Fenaux, P.; Dombret, H.; Delair, S.; Dreyfus, F.; Tilly, H.; Vekhoff, A.; Cony-Makhoul, P.; Leblond, V.; Troussard, X.; et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF) to increase efficacy of intensive sequential chemotherapy with etoposide, mitoxantrone and cytarabine (EMA) in previously treated acute myeloid leukemia: A multicenter randomized placebo-controlled trial (EMA91 trial). Leukemia 1999, 13, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.; Ayres, M.; Nowak, B.; Gandhi, V. Biochemical modulation of cytarabine triphosphate by clofarabine. Cancer Chemother. Pharmacol. 2005, 55, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.S.; Medeiros, B.C.; Stein, A.S.; Othus, M.; Appelbaum, F.R.; Forman, S.J.; Scott, B.L.; Hendrie, P.C.; Gardner, K.M.; Pagel, J.M.; et al. G-CSF priming, clofarabine, and high dose cytarabine (GCLAC) for upfront treatment of acute myeloid leukemia, advanced myelodysplastic syndrome or advanced myeloproliferative neoplasm. Am. J. Hematol. 2014. [CrossRef]
- Verdonck, L.F.; Lokhorst, H.M.; Roovers, D.J.; van Heugten, H.G. Multidrug-resistant acute leukemia cells are responsive to prolonged exposure of daunorubicin: Implications for liposome-encapsulated daunorubicin. Leuk. Res. 1998, 22, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ermacora, A.; Michieli, M.; Pea, F.; Visani, G.; Bucalossi, A.; Russo, D. Liposome encapsulated daunorubicin (daunoxome) for acute leukemia. Haematologica 2000, 85, 324–325. [Google Scholar]
- Lancet, J.E.; Roboz, G.J.; Cripe, L.D.; Michelson, G.C.; Fox, J.A.; Leavitt, R.D.; Chen, T.; Hawtin, R.; Craig, A.R.; Ravandi, F.; et al. A phase 1b/2 study of combination vosaroxin and cytarabine in patients with relapsed or refractory acute myeloid leukemia. Haematologica 2014, 100, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Marcucci, G.; Maharry, K.; Wu, Y.Z.; Radmacher, M.D.; Mrozek, K.; Margeson, D.; Holland, K.B.; Whitman, S.P.; Becker, H.; Schwind, S.; et al. IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. J. Clin. Oncol. 2010, 28, 2348–2355. [Google Scholar] [CrossRef] [PubMed]
- Stuart, S.D.; Schauble, A.; Gupta, S.; Kennedy, A.D.; Keppler, B.R.; Bingham, P.M.; Zachar, Z. A strategically designed small molecule attacks alpha-ketoglutarate dehydrogenase in tumor cells through a redox process. Cancer Metab. 2014, 2. [Google Scholar] [CrossRef]
- Ben-Batalla, I.; Schultze, A.; Wroblewski, M.; Erdmann, R.; Heuser, M.; Waizenegger, J.S.; Riecken, K.; Binder, M.; Schewe, D.; Sawall, S.; et al. Axl, a prognostic and therapeutic target in acute myeloid leukemia mediates paracrine crosstalk of leukemia cells with bone marrow stroma. Blood 2013, 122, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- Uy, G.L.; Rettig, M.P.; Motabi, I.H.; McFarland, K.; Trinkaus, K.M.; Hladnik, L.M.; Kulkarni, S.; Abboud, C.N.; Cashen, A.F.; Stockerl-Goldstein, K.E.; et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 2012, 119, 3917–3924. [Google Scholar] [CrossRef] [PubMed]
- Konig, H.; Levis, M. Targeting FLT3 to treat leukemia. Expert Opin. Ther. Targets 2015, 19, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Pratz, K.W.; Sato, T.; Murphy, K.M.; Stine, A.; Rajkhowa, T.; Levis, M. FLT3-mutant allelic burden and clinical status are predictive of response to FLT3 inhibitors in AML. Blood 2010, 115, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Kantarjian, H.; Foran, J.M.; Ghirdaladze, D.; Zodelava, M.; Borthakur, G.; Gammon, G.; Trone, D.; Armstrong, R.C.; James, J.; et al. Phase I study of quizartinib administered daily to patients with relapsed or refractory acute myeloid leukemia irrespective of FMS-like tyrosine kinase 3-internal tandem duplication status. J. Clin. Oncol. 2013, 31, 3681–3687. [Google Scholar] [CrossRef] [PubMed]
- Wander, S.A.; Levis, M.J.; Fathi, A.T. The evolving role of FLT3 inhibitors in acute myeloid leukemia: Quizartinib and beyond. Ther. Adv. Hematol. 2014, 5, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J.E.; Tallman, M.S.; Schiller, G.; Trone, D.; Gammon, G.; Goldberg, S.; Perl, A.E.; Marie, J.P.; Martinelli, G.; Levis, M. Results of a phase 2 randomized, open-label, study of lower doses of quizartinib (AC220; ASP2689) in subjects with FLT3-ITD positive relapsed or refractory acute myeloid leukemia (AML). Blood 2013, 122, 494. [Google Scholar]
- Levis, M.J.; Perl, A.E.; Dombret, H.; Dohner, H.; Steffen, B.; Rousselot, P.; Martinelli, G.; Estey, E.H.; Burnett, A.K.; Gammon, G.; et al. Final results of a phase 2 open-label, monotherapy efficacy and safety study of quizartinib (AC220) in patients with FLT3-ITD positive or negative relapsed/refractory acute myeloid leukemia after second-line chemotherapy or hematopoietic stem cell transplantation. Blood 2012, 120, 673. [Google Scholar]
- Röllig, C.; Müller-Tidow, C.; Hüttmann, A.; Noppeney, R.; Kunzmann, V.; Baldus, C.D.; Brandts, C.H.; Krämer, A.; Schäfer-Eckart, K.; Neubauer, A.; et al. Sorafenib versus placebo in addition to standard therapy in younger patients with newly diagnosed acute myeloid leukemia: Results from 267 patients treated in the randomized placebo-controlled SAL-Soraml trial. 2014, 124, 6. [Google Scholar]
- Hourigan, C.S.; McCarthy, P.; de Lima, M. Back to the future! The evolving role of maintenance therapy after hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2014, 20, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Li, S.; Lane, A.A.; Connolly, C.; Del Rio, C.; Valles, B.; Curtis, M.; Ballen, K.; Cutler, C.; Dey, B.R.; et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for FMS-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol. Blood Marrow Transplant. 2014, 20, 2042–2048. [Google Scholar] [CrossRef] [PubMed]
- Sammons, S.L.; Pratz, K.W.; Smith, B.D.; Karp, J.E.; Emadi, A. Sorafenib is tolerable and improves clinical outcomes in patients with FLT3-ITD acute myeloid leukemia prior to stem cell transplant and after relapse post-transplant. Am. J. Hematol. 2014, 89, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, M.R.; Levis, M.J. FLT3 inhibitors for acute myeloid leukemia: A review of their efficacy and mechanisms of resistance. Int. J. Hematol. 2013, 97, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, E.I.; Turner, D.C.; Buaboonnam, J.; Hu, S.; Orwick, S.; Roberts, M.S.; Janke, L.J.; Ramachandran, A.; Stewart, C.F.; Inaba, H.; et al. Crenolanib is active against models of drug-resistant FLT3-ITD-positive acute myeloid leukemia. Blood 2013, 122, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Kim, H.W.; Lee, I.Y.; Lee, J.; Lee, J.; Jung, D.S.; Lee, S.Y.; Park, S.H.; Hwang, H.; Choi, J.S.; et al. G-749, a novel FLT3 kinase inhibitor, can overcome drug resistance for the treatment of acute myeloid leukemia. Blood 2014, 123, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Keegan, K.; Li, C.; Li, Z.H.; Ma, J.; Ragains, M.; Coberly, S.; Hollenback, D.; Eksterowicz, J.; Liang, L.M.; Weidner, M.; et al. Preclinical evaluation of AMG 925, a FLT3/CDK4 dual kinase inhibitor for treating acute myeloid leukemia. Mol. Cancer Ther. 2014, 13, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Zhao, Y.; Mitaksov, V.; Fremont, D.H.; Kasai, Y.; Molitoris, A.; Ries, R.E.; Miner, T.L.; McLellan, M.D.; DiPersio, J.F.; et al. Identification of somatic JAK1 mutations in patients with acute myeloid leukemia. Blood 2008, 111, 4809–4812. [Google Scholar] [CrossRef] [PubMed]
- Shadman, M.; Mawad, R.; Dean, C.; Shannon-Dorcy, K.; Sandhu, V.; Hendrie, P.C.; Scott, B.L.; Walter, R.B.; Becker, P.S.; Pagel, J.; et al. Idarubicin, cytarabine and pravastatin as induction therapy for untreated acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 2014, 124, 3732. [Google Scholar]
- Kobune, M.; Takimoto, R.; Murase, K.; Iyama, S.; Sato, T.; Kikuchi, S.; Kawano, Y.; Miyanishi, K.; Sato, Y.; Niitsu, Y.; et al. Drug resistance is dramatically restored by hedgehog inhibitors in CD34+ leukemic cells. Cancer Sci. 2009, 100, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Krupka, C.; Kufer, P.; Kischel, R.; Zugmaier, G.; Bögeholz, J.; Köhnke, T.; Lichtenegger, F.S.; Schneider, S.; Metzeler, K.H.; Fiegl, M.; et al. CD33 target validation and sustained depletion of AML blasts in long-term cultures by the bispecific T-cell-engaging antibody AMG 330. Blood 2014, 123, 356. [Google Scholar]
- Mardiros, A.; Dos Santos, C.; McDonald, T.; Brown, C.E.; Wang, X.; Budde, L.E.; Hoffman, L.; Aguilar, B.; Chang, W.-C.; Bretzlaff, W.; et al. T cells expressing CD123-specific chimeric antigen receptors exhibit specific cytolytic effector functions and antitumor effects against human acute myeloid leukemia. Blood 2013, 122, 3138–3148. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Levitsky, H.I. Evaluation of current cancer immunotherapy: Hemato-oncology. Cancer J. (Sudbury, Mass.) 2011, 17, 309–324. [Google Scholar] [CrossRef]
- Jabbour, E.; Daver, N.; Champlin, R.; Mathisen, M.; Oran, B.; Ciurea, S.; Khouri, I.; Cornelison, A.M.; Ghanem, H.; Cardenas-Turanzas, M.; et al. Allogeneic stem cell transplantation as initial salvage for patients with acute myeloid leukemia refractory to high-dose cytarabine-based induction chemotherapy. Am. J. Hematol. 2014, 89, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.B.; Buckley, S.A.; Pagel, J.M.; Wood, B.L.; Storer, B.E.; Sandmaier, B.M.; Fang, M.; Gyurkocza, B.; Delaney, C.; Radich, J.P.; et al. Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood 2013, 122, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Duval, M.; Klein, J.P.; He, W.; Cahn, J.Y.; Cairo, M.; Camitta, B.M.; Kamble, R.; Copelan, E.; de Lima, M.; Gupta, V.; et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J. Clin. Oncol. 2010, 28, 3730–3738. [Google Scholar] [CrossRef] [PubMed]
- Luznik, L.; O’Donnell, P.V.; Symons, H.J.; Chen, A.R.; Leffell, M.S.; Zahurak, M.; Gooley, T.A.; Piantadosi, S.; Kaup, M.; Ambinder, R.F.; et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol. Blood Marrow Transplant. 2008, 14, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Raiola, A.M.; Dominietto, A.; di Grazia, C.; Lamparelli, T.; Gualandi, F.; Ibatici, A.; Bregante, S.; Van Lint, M.T.; Varaldo, R.; Ghiso, A.; et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol. Blood Marrow Transplant. 2014, 20, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Craddock, C.; Labopin, M.; Houhou, M.; Robin, M.; Finke, J.; Chevallier, P.; Yakoub-Agha, I.; Bourhis, J.-H.; Sengelov, H.; Blaise, D.; et al. Activity and tolerability of azacitidine in patients who relapse after allogeneic stem cell transplantation (allo-SCT) for acute myeloid leukemia (AML) and myelodysplasia (MDS): A survey from the european society for blood and marrow transplantation (EBMT). Blood 2014, 124, 2506–2506. [Google Scholar]
- Sarkozy, C.; Gardin, C.; Gachard, N.; Merabet, F.; Turlure, P.; Malfuson, J.V.; Pautas, C.; Micol, J.B.; Thomas, X.; Quesnel, B.; et al. Outcome of older patients with acute myeloid leukemia in first relapse. Am. J. Hematol. 2013, 88, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, B.; Sliesoraitis, S.; Lyerly, S.; Klepin, H.D.; Lawrence, J.; Isom, S.; Ellis, L.R.; Manuel, M.; Dralle, S.; Berenzon, D.; et al. Efficacy of the hypomethylating agents as frontline, salvage, or consolidation therapy in adults with acute myeloid leukemia (AML). Ann. Hematol. 2014, 93, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Ivanoff, S.; Gruson, B.; Chantepie, S.P.; Lemasle, E.; Merlusca, L.; Harrivel, V.; Charbonnier, A.; Votte, P.; Royer, B.; Marolleau, J.P. 5-azacytidine treatment for relapsed or refractory acute myeloid leukemia after intensive chemotherapy. Am. J. Hematol. 2013, 88, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, E.K.; Feldman, E.J.; Christos, P.J.; Rohan, S.D.; Lagassa, C.B.; Ippoliti, C.; Scandura, J.M.; Carlson, K.; Roboz, G.J. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk. Lymphoma 2013, 54, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
- Bejar, R.; Lord, A.; Stevenson, K.; Bar-Natan, M.; Perez-Ladaga, A.; Zaneveld, J.; Wang, H.; Caughey, B.; Stojanov, P.; Getz, G.; et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood 2014, 124, 2705–2712. [Google Scholar] [CrossRef] [PubMed]
- Itzykson, R.; Kosmider, O.; Cluzeau, T.; Mansat-De Mas, V.; Dreyfus, F.; Beyne-Rauzy, O.; Quesnel, B.; Vey, N.; Gelsi-Boyer, V.; Raynaud, S.; et al. Impact of TET2 mutations on response rate to azacitidine in myelodysplastic syndromes and low blast count acute myeloid leukemias. Leukemia 2011, 25, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Estey, E.H. Primacy of resistance rather than toxicity in determining outcome of therapy for AML. Clin. Lymphoma Myeloma Leuk. 2014, 14, S56–S58. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Karp, J.E. Minimal residual disease in acute myeloid leukaemia. Nat. Rev. Clin. Oncol. 2013, 10, 460–471. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.; Yu, Y.; Kaye, J.A.; Davis, K.L. Medicare fee-for-service enrollees with primary acute myeloid leukemia: An analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl. Health Econ. Health Policy 2013, 11, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Karp, J.E. Development of therapeutic agents for older patients with acute myelogenous leukemia. Curr. Opin. Investig. Drugs 2010, 11, 669–677. [Google Scholar] [PubMed]
- Pemovska, T.; Kontro, M.; Yadav, B.; Edgren, H.; Eldfors, S.; Szwajda, A.; Almusa, H.; Bespalov, M.M.; Ellonen, P.; Elonen, E.; et al. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discov. 2013, 3, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.S.; Oehler, V.; Estey, E.H.; Martins, T.; Perdue, A.; David, J.; Chien, S.; Hendrie, P.C.; Ostronoff, F.; Blau, C.A. Feasibility trial of individualized therapy for relapsed or refractory acute myeloid leukemia based on a high throughput in vitro drug sensitivity assay. Blood 2014, 124, 3748. [Google Scholar] [CrossRef] [PubMed]
- Hourigan, C.S.; Karp, J.E. Personalized therapy for acute myeloid leukemia. Cancer Discov. 2013, 3, 1336–1338. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, S.; Rassidakis, G.Z.; Estey, E.; Kantarjian, H.; Liakou, C.I.; Huang, X.; Xiao, L.; Andreeff, M.; Konopleva, M.; Medeiros, L.J. Overexpression of CXCR4 predicts adverse overall and event-free survival in patients with unmutated FLT3 acute myeloid leukemia with normal karyotype. Cancer 2007, 109, 1152–1156. [Google Scholar] [CrossRef] [PubMed]
- Freeman, S.D.; Virgo, P.; Couzens, S.; Grimwade, D.; Russell, N.; Hills, R.K.; Burnett, A.K. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J. Clin. Oncol. 2013, 31, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Inaba, H.; Coustan-Smith, E.; Cao, X.; Pounds, S.B.; Shurtleff, S.A.; Wang, K.Y.; Raimondi, S.C.; Onciu, M.; Jacobsen, J.; Ribeiro, R.C.; et al. Comparative analysis of different approaches to measure treatment response in acute myeloid leukemia. J. Clin. Oncol. 2012, 30, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, D.; Freeman, S.D. Defining minimal residual disease in acute myeloid leukemia: Which platforms are ready for “prime time"? Blood 2014, 124, 3345–3355. [Google Scholar] [CrossRef] [PubMed]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.F.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; Wall, K.; et al. Molecular detection of minimal residual disease provides the most powerful independent prognostic factor irrespective of clonal architecture in nucleophosmin (NPML) mutant acute myeloid leukemia. 2014, 124, 70. [Google Scholar]
- Steinbach, D.; Bader, P.; Willasch, A.; Bartholomae, S.; Debatin, K.M.; Zimmermann, M.; Creutzig, U.; Reinhardt, D.; Gruhn, B. Prospective validation of a new method of monitoring minimal residual disease in childhood acute myeloid leukemia. Clin. Cancer Res. 2015, 15, 1353–1359. [Google Scholar] [CrossRef]
- Goswami, M.; McGowan, K.S.; Lu, K.; Jain, N.; Candia, J.; Hensel, N.F.; Tang, J.; Calvo, K.R.; Battiwalla, M.; Barrett, A.J.; et al. A multigene array for measurable residual disease detection in AML patients undergoing SCT. Bone Marrow Transplant. 2015. [CrossRef]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.M.; Knoth, H.; Rollig, C.; Schetelig, J.; et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the relaza trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- De Lima, M.; Giralt, S.; Thall, P.F.; de Padua Silva, L.; Jones, R.B.; Komanduri, K.; Braun, T.M.; Nguyen, H.Q.; Champlin, R.; Garcia-Manero, G. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: A dose and schedule finding study. Cancer 2010, 116, 5420–5431. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, N.R.; Mo, C.C.; Karp, J.E.; Hourigan, C.S. Current Approaches in the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. J. Clin. Med. 2015, 4, 665-695. https://doi.org/10.3390/jcm4040665
Ramos NR, Mo CC, Karp JE, Hourigan CS. Current Approaches in the Treatment of Relapsed and Refractory Acute Myeloid Leukemia. Journal of Clinical Medicine. 2015; 4(4):665-695. https://doi.org/10.3390/jcm4040665
Chicago/Turabian StyleRamos, Nestor R., Clifton C. Mo, Judith E. Karp, and Christopher S. Hourigan. 2015. "Current Approaches in the Treatment of Relapsed and Refractory Acute Myeloid Leukemia" Journal of Clinical Medicine 4, no. 4: 665-695. https://doi.org/10.3390/jcm4040665





