Age-Related Macular Degeneration: Advances in Management and Diagnosis
Abstract
:1. Introduction
2. Risk Factors for AMD
3. Clinical Features, Histopathology, and Imaging
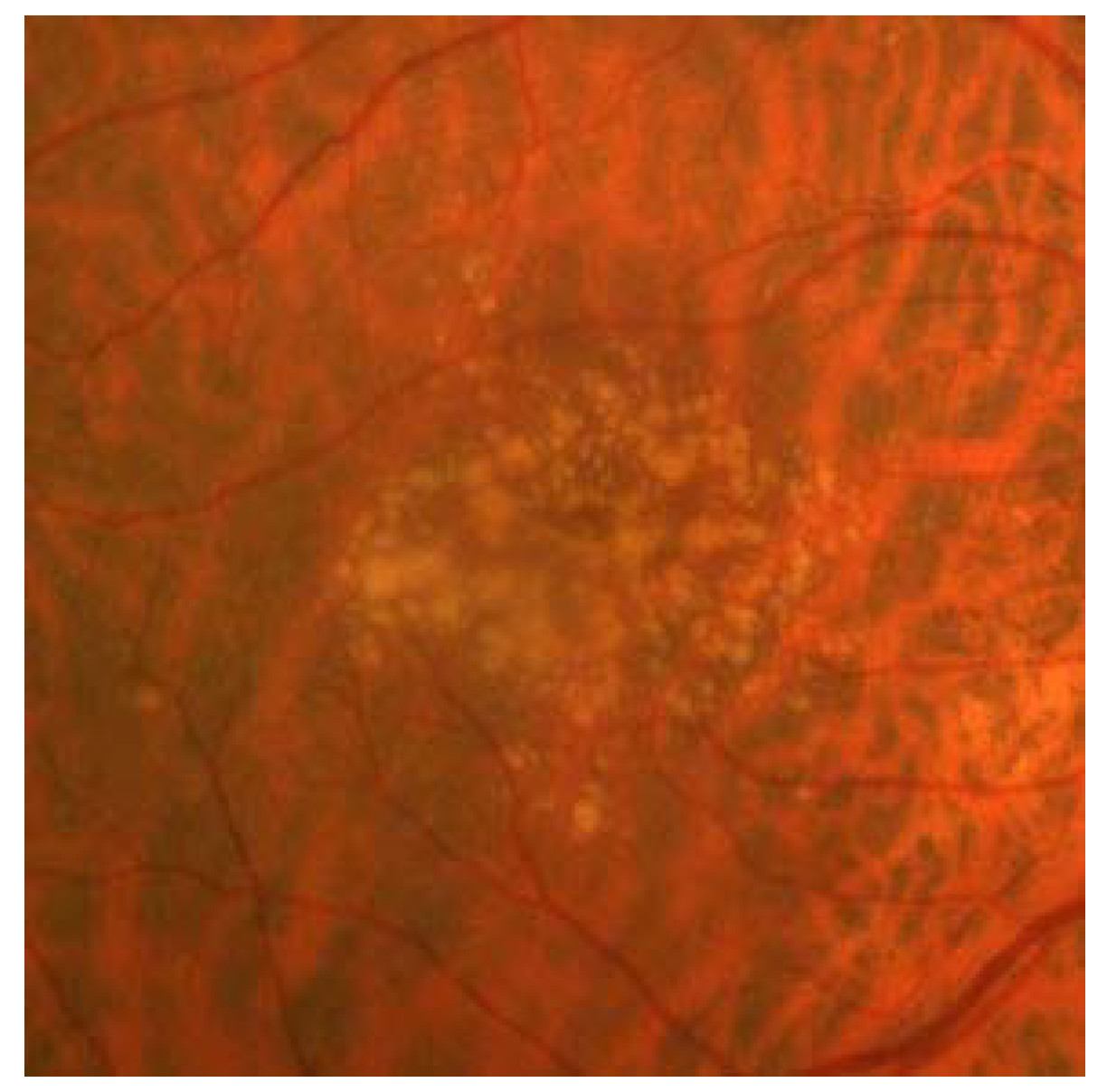
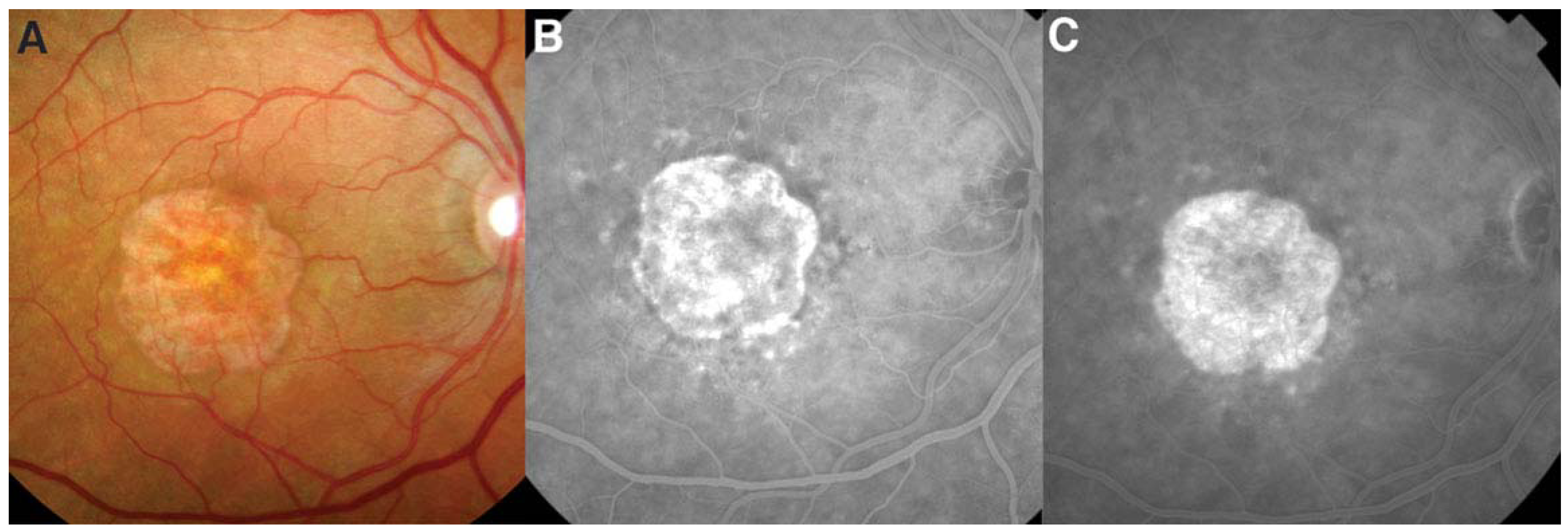
4. Geographic Atrophy
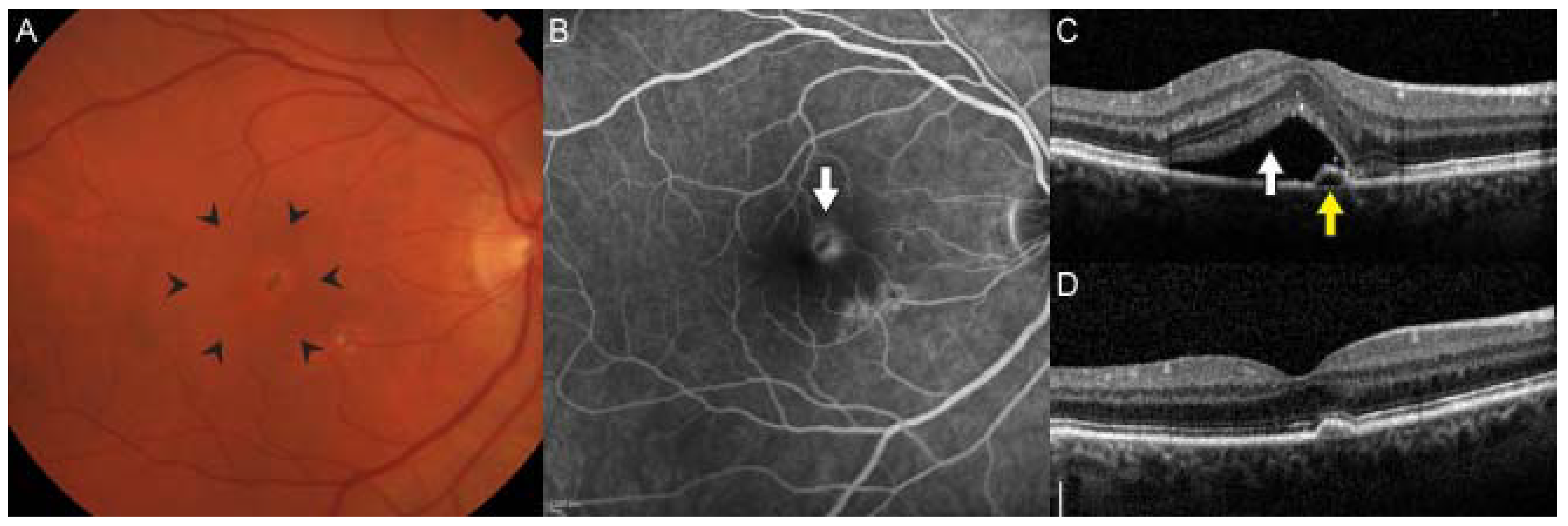
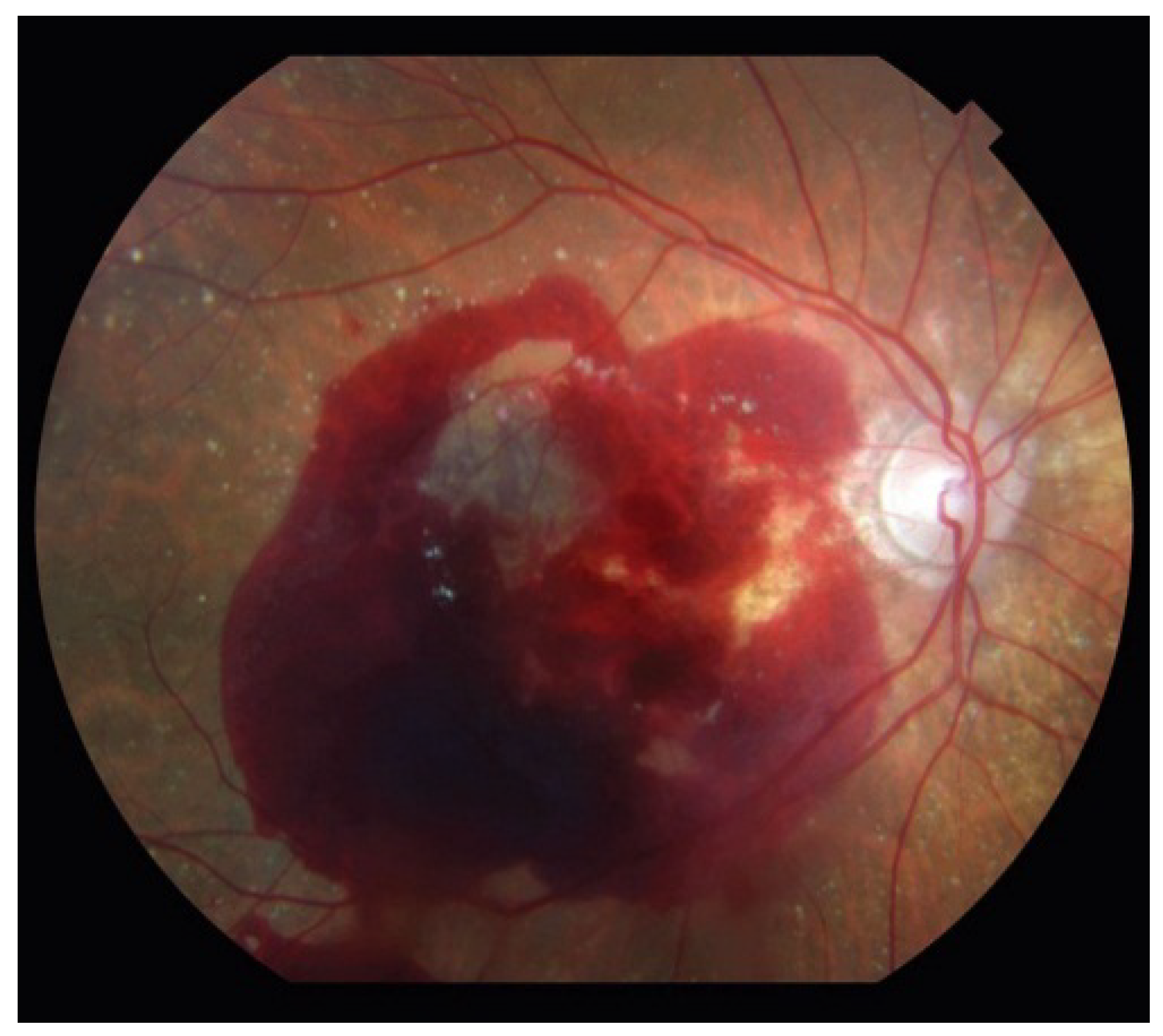
5. Neovascular AMD
6. Treatment of Non-Neovascular AMD
7. Treatment of Neovascular AMD
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Miller, J.W. Age-related macular degeneration revisited—Piecing the puzzle: The LXIX edward jackson memorial lecture. Am. J. Ophthalmol. 2013, 155. [Google Scholar] [CrossRef]
- Friedman, D.S.; O’Colmain, B.J.; Munoz, B.; Tomany, S.C.; McCarty, C.; de Jong, P.T.; Nemesure, B.; Mitchell, P.; Kempen, J. Prevalence of age-related macular degeneration in the united states. Arch. Ophthalmol. 2004, 122, 564–572. [Google Scholar] [CrossRef] [PubMed]
- WHO. Prevention of Blindness and Visual Impairment; Priority Eye Diseases. Available online: http://www.who.int/blindness/causes/priority/en/index8.html (accessed on 30 June 2014).
- Ferris, F.L., III; Fine, S.L.; Hyman, L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Rowland, M.L.; Harris, M.I. Racial/ethnic differences in age-related maculopathy. Third national health and nutrition examination survey. Ophthalmology 1995, 102, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Chou, C.F.; Klein, B.E.; Zhang, X.; Meuer, S.M.; Saaddine, J.B. Prevalence of age-related macular degeneration in the us population. Arch. Ophthalmol. 2011, 129, 75–80. [Google Scholar] [CrossRef] [PubMed]
- AREDS-Study-Group. Risk factors associated with age-related macular degeneration. A case-control study in the age-related eye disease study: Age-related eye disease study report number 3. Ophthalmology 2000, 107, 2224–2232. [Google Scholar]
- Klein, R.; Klein, B.E.; Jensen, S.C.; Meuer, S.M. The five-year incidence and progression of age-related maculopathy: The beaver dam eye study. Ophthalmology 1997, 104, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Smith, W.; Attebo, K.; Wang, J.J. Prevalence of age-related maculopathy in australia. The blue mountains eye study. Ophthalmology 1995, 102, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Linton, K.L. Prevalence of age-related maculopathy. The beaver dam eye study. Ophthalmology 1992, 99, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, H.M.; Krueger, D.E.; Maunder, L.R.; Milton, R.C.; Kini, M.M.; Kahn, H.A.; Nickerson, R.J.; Pool, J.; Colton, T.L.; Ganley, J.P.; et al. The framingham eye study monograph: An ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv. Ophthalmol. 1980, 24, 335–610. [Google Scholar] [CrossRef] [PubMed]
- Vingerling, J.R.; Dielemans, I.; Hofman, A.; Grobbee, D.E.; Hijmering, M.; Kramer, C.F.; de Jong, P.T. The prevalence of age-related maculopathy in the rotterdam study. Ophthalmology 1995, 102, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Fraser-Bell, S.; Donofrio, J.; Wu, J.; Klein, R.; Azen, S.P.; Varma, R. Sociodemographic factors and age-related macular degeneration in latinos: The los angeles latino eye study. Am. J. Ophthalmol. 2005, 139, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.; Tielsch, J.M.; Katz, J.; Quigley, H.A.; Gottsch, J.D.; Javitt, J.C.; Martone, J.F.; Royall, R.M.; Witt, K.A.; Ezrine, S. Racial differences in the cause-specific prevalence of blindness in east baltimore. N. Engl. J. Med. 1991, 325, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Christen, W.G.; Glynn, R.J.; Manson, J.E.; Ajani, U.A.; Buring, J.E. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA 1996, 276, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; Willett, W.C.; Speizer, F.E.; Hankinson, S.E. A prospective study of cigarette smoking and age-related macular degeneration in women. JAMA 1996, 276, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, R.D.; Hiller, R. Systemic hypertension and age-related maculopathy in the Framingham Study. Arch. Ophthalmol. 1986, 104, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, J.; Flowerdew, G.; Smith, E.; Brody, J.A.; Tso, M.O. Factors associated with age-related macular degeneration. An analysis of data from the first National Health and Nutrition Examination Survey. Am. J. Epidemiol. 1988, 128, 700–710. [Google Scholar] [PubMed]
- Hyman, L.; Schachat, A.P.; He, Q.; Leske, M.C. Hypertension, cardiovascular disease, and age-related macular degeneration. Age-Related Macular Degeneration Risk Factors Study Group. Arch. Ophthalmol. 2000, 188, 351–358. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Tomany, S.C.; Cruickshanks, K.J. The association of cardiovascular disease with the long-term incidence of age-related maculopathy: The Beaver Dam Eye Study. Ophthalmology 2003, 110, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, R.; Ikram, M.K.; Vingerling, J.R.; Witteman, J.C.; Hofman, A.; de Jong, P.T. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3771–3777. [Google Scholar] [CrossRef]
- Klein, R.; Klein, B.E.; Marino, E.K.; Kuller, L.H.; Furberg, C.; Burke, G.L.; Hubbard, L.D. Early age-related maculopathy in the cardiovascular health study. Ophthalmology 2003, 110, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Mitchell, P.; Smith, W.; Wang, J.J. Cardiovascular risk factors and the long-term incidence of age-related macular degeneration: The Blue Mountains Eye study. Ophthalmology 2007, 114, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Eye-Disease-Case-Control-Study-Group. Risk factors for neovascular age-related macular degeneration. Arch. Ophthalmol. 1992, 110, 1701–1708. [Google Scholar]
- Klein, R.; Klein, B.E.; Franke, T. The relationship of cardiovascular disease and its risk factors to age-related maculopathy. The beaver dam eye study. Ophthalmology 1993, 100, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Vinding, T. Age-related macular degeneration. An epidemiological study of 1000 elderly individuals. With reference to prevalence, funduscopic findings, visual impairment and risk factors. Acta Ophthalmol. Scand. Suppl. 1995, 217, 1–32. [Google Scholar] [PubMed]
- Klein, R.; Cruickshanks, K.J.; Myers, C.E.; Sivakumaran, T.A.; Iyengar, S.K.; Meuer, S.M.; Schubert, C.R.; Gangnon, R.E.; Klein, B.E. The relationship of atherosclerosis to the 10-year cumulative incidence of age-related macular degeneration: The beaver dam studies. Ophthalmology 2013, 120, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Knudtson, M.D.; Klein, B.E. Statin use and the five-year incidence and progression of age-related macular degeneration. Am. J. Ophthalmol. 2007, 144, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.E.; Woddside, J.V.; Gilchrist, S.E.; Graydon, R.; Fletcher, A.E.; Chan, W.; Knox, A.; Cartmill, B.; Chakravarthy, U. Cardiovascular disease and hypertension are strong risk factors for choroidal neovascularization. Ophthalmology 2008, 115, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Deng, Y.; Klein, B.E.; Hyman, L.; Seddon, J.; Frank, R.N.; Wallace, R.B.; Hendrix, S.L.; Kuppermann, B.D.; Langer, R.D.; et al. Cardiovascular disease, its risk factors and treatment, and age-related macular degeneration: Women’s Health Initiative Sight Exam ancillary study. Am. J. Ophthalmol. 2007, 143, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Berman, E.R.; Rothman, H. Lipofuscin of human retinal pigment epithelium. Am. J. Ophthalmol. 1980, 90, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal pigment epithelial cell count, distribution, and correlations in normal human eyes. Am. J. Ophthalmol. 1996, 121, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Sarks, S.H. Ageing and degeneration in the macular region: A clinico-pathological study. Br. J. Ophthalmol. 1976, 60, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Sarks, S.; Cherepanoff, S.; Killingsworth, M.; Sarks, J. Relationship of basal laminar deposit and membranous debris to the clinical presentation of early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 968–977. [Google Scholar] [CrossRef]
- Klein, M.L.; Ferris, F.L., III; Armstrong, J.; Hwang, T.S.; Chew, E.Y.; Bressler, S.B.; Chandra, S.R. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology 2008, 115, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Valckenberg, S.; Bindewald-Wittich, A.; Dolar-Szczasny, J.; Dreyhaupt, J.; Wolf, S.; Scholl, H.P.; Holz, F.G. Correlation between the area of increased autofluorescence surrounding geographic atrophy and disease progression in patients with amd. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2648–2654. [Google Scholar] [CrossRef]
- Holz, F.G.; Bellman, C.; Staudt, S.; Schutt, F.; Volcker, H.E. Fundus autofluorescence and development of geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1051–1056. [Google Scholar]
- AREDS-Study-Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: Areds report no. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar]
- Newsome, D.A.; Swartz, M.; Leone, N.C.; Elston, R.C.; Miller, E. Oral zinc in macular degeneration. Arch. Ophthalmol. 1988, 106, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Stampfer, M.J.; Seddon, J.M.; Hung, S.; Spiegelman, D.; Rimm, E.B.; Willett, W.C.; Hankinson, S.E. Prospective study of zinc intake and the risk of age-related macular degeneration. Ann. Epidemiol. 2001, 11, 328–336. [Google Scholar] [CrossRef]
- Smith, W.; Mitchell, P.; Rochester, C. Serum beta carotene, alpha tocopherol, and age-related maculopathy: The blue mountains eye study. Am. J. Ophthalmol. 1997, 124, 838–840. [Google Scholar] [CrossRef] [PubMed]
- VandenLangenberg, G.M.; Mares-Perlman, J.A.; Klein, R.; Klein, B.E.; Brady, W.E.; Palta, M. Associations between antioxidant and zinc intake and the 5-year incidence of early age-related maculopathy in the beaver dam eye study. Am. J. Epidemiol. 1998, 148, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Mares-Perlman, J.A.; Fisher, A.I.; Klein, R.; Palta, M.; Block, G.; Millen, A.E.; Wright, J.D. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third national health and nutrition examination survey. Am. J. Epidemiol. 2001, 153, 424–432. [Google Scholar] [PubMed]
- Taylor, H.R.; Tikellis, G.; Robman, L.D.; McCarty, C.A.; McNeil, J.J. Vitamin E supplementation and macular degeneration: Randomised controlled trial. BMJ 2002, 325. [Google Scholar] [CrossRef]
- AREDS2-Study-Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The age-related eye disease study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Regillo, C.D. Lampalizumab (anti-factor D) in patients with geography atrophy: The mahalo phase 2 results. In Proceedings of the 2013 Annual Meeting of the American Academy of Ophthalmology, New Orleans, LA, USA, 16–19 November 2013.
- Tarallo, V.H.Y.; Gelfand, B.D.; Dridi, S.; Kerur, N.; Kim, Y.; Cho, W.G.; Kaneko, H.; Fowler, B.J.; Bogdanovich, S.; Albuquerque, R.J.; et al. Dicer1 loss and alu rna induce age-related macular degeneration via the NLRP3 inflammasome and MYD88. Cell 2012, 149, 847–859. [Google Scholar] [CrossRef] [PubMed]
- Kubota, R.; Boman, N.L.; David, R.; Mallikaarjun, S.; Patil, S.; Birch, D. Safety and effect on rod function of ACU-4429, a novel small-molecule visual cycle modulator. Retina 2012, 32, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Mata, N.L.; Lichter, J.B.; Vogel, R.; Han, Y.; Bui, T.V.; Singerman, L.J. Investigation of oral fenretinide for treatment of geographic atrophy in age-related macular degeneration. Retina 2013, 33, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Trichonas, G.; Murakami, Y.; Thanos, A.; Morizane, Y.; Kayama, M.; Debouck, C.M.; Hisatomi, T.; Miller, J.W.; Vavvas, D.G. Receptor interacting protein kinases mediate retinal detachment-induced photoreceptor necrosis and compensate for inhibition of apoptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 21695–21700. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C. Canadian journal of ophthalmology lecture: Translational research advances in glaucoma neuroprotection. Can. J. Ophthalmol. 2013, 48, 141–145. [Google Scholar] [CrossRef]
- Wong, W.T.; Kam, W.; Cunningham, D.; Harrington, M.; Hammel, K.; Meyerle, C.B.; Cukras, C.; Chew, E.Y.; Sadda, S.R.; Ferris, F.L. Treatment of geographic atrophy by the topical administration of OT-551: Results of a phase II clinical trial. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6131–6139. [Google Scholar] [CrossRef]
- Zhang, K.; Hopkins, J.J.; Heier, J.S.; Birch, D.G.; Halperin, L.S.; Albini, T.A.; Brown, D.M.; Jaffe, G.J.; Tao, W.; Williams, G.A. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 6241–6245. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Hubschman, J.P.; Heilwell, G.; Franco-Cardenas, V.; Pan, C.K.; Ostrick, R.M.; Mickunas, E.; Gay, R.; Klimanskaya, I.; Lanza, R. Embryonic stem cell trials for macular degeneration: A preliminary report. Lancet 2012, 379, 713–720. [Google Scholar] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Elliot, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- MPS-Study-Group. Argon laser photocoagulation for senile macular degeneration. Results of a randomized clinical trial. Arch. Ophthalmol. 1982, 100, 912–918. [Google Scholar]
- MPS-Study-Group. Argon laser photocoagulation for neovascular maculopathy. Three-year results from randomized clinical trials. Arch. Ophthalmol. 1986, 104, 694–701. [Google Scholar]
- MPS-Study-Group. Laser photocoagulation for juxtafoveal choroidal neovascularization. Five-year results from randomized clinical trials. Arch. Ophthalmol. 1994, 112, 500–509. [Google Scholar]
- MPS-Study-Group. The influence of treatment extent on the visual acuity of eyes treated with krypton laser for juxtafoveal choroidal neovascularization. Arch. Ophthalmol. 1995, 113, 190–194. [Google Scholar]
- MPS-Study-Group. Persistent and recurrent neovascularization after laser photocoagulation for subfoveal choroidal neovascularization of age-related macular degeneration. Arch. Ophthalmol. 1994, 112, 489–499. [Google Scholar]
- MPS-Study-Group. Laser photocoagulation of subfoveal neovascular lesions of age-related macular degeneration. Updated findings from two clinical trials. Arch. Ophthalmol. 1993, 111, 1200–1209. [Google Scholar]
- TAP-Study-Group. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: One-year results of 2 randomized clinical trials—Tap report. Arch. Ophthalmol. 1999, 117, 1329–1345. [Google Scholar]
- Bressler, N.M. Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: Two-year results of 2 randomized clinical trials-tap report 2. Arch. Ophthalmol. 2001, 119, 198–207. [Google Scholar] [PubMed]
- Blumenkranz, M.S.; Bressler, N.M.; Bressler, S.B.; Donati, G.; Fish, G.E.; Haynes, L.A.; Lewis, H.; Miller, J.W.; Mones, J.M.; Potter, M.J.; et al. Verteporfin therapy for subfoveal choroidal neovascularization in age-related macular degeneration: Three-year results of an open-label extension of 2 randomized clinical trials—Tap report no. 5. Arch. Ophthalmol. 2002, 120, 1307–1314. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T., Jr.; Feinsod, M.; Guyer, D.R. Pegaptanib for neovascular age-related macular degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [PubMed]
- Brown, D.M.; Michels, M.; Kaiser, P.K.; Heier, J.S.; Sy, J.P.; Ianchulev, T. Ranibizumab versus verteporfin photodynamic therapy for neovascular age-related macular degeneration: Two-year results of the ANCHOR study. Ophthalmology 2009, 116. [Google Scholar] [CrossRef]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.F.; Maguire, M.G.; Fine, S.L.; Ying, G.S.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Redford, M.; Ferris, F.L., III. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: Two-year results. Ophthalmology 2012, 119, 1388–1398. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Browning, D.J.; Kaiser, P.K.; Rosenfeld, P.J.; Stewart, M.W. Aflibercept for age-related macular degeneration: A game-changer or quiet addition? Am. J. Ophthalmol. 2012, 154, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.W. Aflibercept (VEGF trap-eye): The newest anti-VEGF drug. Br. J. Ophthalmol. 2012, 96, 1157–1158. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.; Martin, J.; Ruan, Q.; Rafique, A.; Rosconi, M.P.; Shi, E.; Pyles, E.A.; Yancopoulos, G.D.; Stahl, N.; Wiegand, S.J. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF trap, ranibizumab and bevacizumab. Angiogenesis 2012, 15, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Heir, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.-F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U. A phase 2b study of e10030,a platelet derived growth factor (PDGF) inhibitor in combination with a vascular endothelial growth factor (VEGF) inhibitor for neovascular age-related macular degeneration (AMD). In Proceedings of the 36th Annual Meeting of the Macula Society, Dana Point, CA, USA; 2013. [Google Scholar]
- Yonekawa, Y.; Andreoli, C.; Miller, J.B.; Loewenstein, J.I.; Sobrin, L.; Eliott, D.; Vavvas, D.G.; Miller, J.W.; Kim, I.K. Conversion to aflibercept for chronic refractory or recurrent neovascular age-related macular degeneration. Am. J. Ophthalmol. 2013, 156. [Google Scholar] [CrossRef]
- Bakall, B.; Folk, J.C.; Boldt, H.C.; Sohn, E.H.; Stone, E.M.; Russell, S.R.; Mahajan, V.B. Aflibercept therapy for exudative age-related macular degeneration resistant to bevacizumab and ranibizumab. Am. J. Ophthalmol. 2013, 156. [Google Scholar] [CrossRef]
- Ho, V.Y.; Yeh, S.; Olsen, T.W.; Bergstrom, C.S.; Yan, J.; Cribbs, B.E.; Hubbard, G.B., III. Short-term outcomes of aflibercept for neovascular age-related macular degeneration in eyes previously treated with other vascular endothelial growth factor inhibitors. Am. J. Ophthalmol. 2013, 156. [Google Scholar] [CrossRef]
- Cho, H.; Shah, C.P.; Weber, M.; Heier, J.S. Aflibercept for exudative amd with persistent fluid on ranibizumab and/or bevacizumab. Br. J. Ophthalmol. 2013, 97, 1032–1035. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Boyer, D.S.; Cruess, A.F.; Slakter, J.S.; Pilz, S.; Weisberger, A. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: Twelve-month results of the denali study. Ophthalmology 2012, 119, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.; Schmidt-Erfurth, U.; Lanzetta, P.; Wolf, S.; Simader, C.; Tokaji, E.; Pilz, S.; Weisberger, A. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: Twelve-month mont blanc study results. Ophthalmology 2012, 119, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.L.; Chakravarthy, U.; Kaiser, P.K.; Slakter, J.S.; Jan, E.; Bandello, F.; O’Shaughnessy, D.; Gertner, M.E.; Danielson, L.; Moshfeghi, D.M. Stereotactic radiotherapy for neovascular age-related macular degeneration: 52-week safety and efficacy results of the intrepid study. Ophthalmology 2013, 120, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghi, A.A.; Morales-Canton, V.; Quiroz-Mercado, H.; Velez-Montoya, R.; Zavala-Ayala, A.; Shusterman, E.M.; Kaiser, P.K.; Sanislo, S.R.; Gertner, M.; Moshfeghi, D.M. 16 GY low-voltage x-ray irradiation followed by as needed ranibizumab therapy for age-related macular degeneration: 12 month outcomes of a “radiation-first” strategy. Br. J. Ophthalmol. 2012, 96, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Naranjo, J.L.; Quiroz-Mercados, H.; Sanchez-Bermudez, G.; Schoonewolff, F.; Salinas-Longoria, S.; Romero-Vera, R.; Tao, W.; Beckman, R.; Morales-Canton, V. Safety of implantation of the NT-503 device in patients with choroidal neovascularization secondary to age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54. ARVO E-abstract 3298. [Google Scholar]
- Clinicaltrials.Gov. Phase I Dose Escalation Safety Study of Retinostat in Advanced Age-Related Macular Degeneration (AMD) (GEM). Clinicaltrials.Gov Website. Avaibale online: http://clinicaltrials.gov/show/nct01301443 (accessed on 19 May 2014).
- Campochiaro, P.A.; Channa, R.; Berger, B.B.; Heier, J.S.; Brown, D.M.; Fiedler, U.; Hepp, J.; Stumpp, M.T. Treatment of diabetic macular edema with a designed ankyrin repeat protein that binds vascular endothelial growth factor: A phase I/II study. Am. J. Ophthalmol. 2013, 155, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, P.K.; Symons, R.C.; Shah, S.M.; Quinlan, E.J.; Tabandeh, H.; Do, D.V.; Reisen, G.; Lockridge, J.A.; Short, B.; Guerciolini, R.; et al. Rnai-based treatment for neovascular age-related macular degeneration by sirna-027. Am. J. Ophthalmol. 2010, 150. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Yan, M.; Luo, D.; Zhu, W.; Kaiser, P.K.; Yu, D.C. A phase 1 study of kh902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology 2011, 118, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Lpath, Inc. Overview. Lpath Incorporated Website. Available online: http://www.lpath.com/about-lpath/overview (accessed on 19 May 2014).
- Kaiser, P.K.; Boyer, D.S.; Campochiaro, P.A.; Guerro-Nranjo, J.L.; Heier, J.; Kornfield, J.; Kuppermann, B.; Quiroz-Mercado, H.; Salinas-Longoria, S.; Schwartz, S. Integrin peptide therapy: The first wet amd experience. Investig. Ophthalmol. Vis. Sci. 2013, 54. ARVO E-abstract 2177. [Google Scholar]
- Allergan. Allergan and Molecular Partners Enter into Exclusive Alliance. Allergan Website. Available online: http://agn.Client.Shareholder.Com/releasedetail.Cfm?Releaseid=701294 (accessed on 19 May 2014).
- Ohr Pharmaceutics, Inc. Squalamine. Ohr Pharmaceuticals Website. Available online: http://www.Ohrpharmaceutical.Com/research/squalamine (accessed on 19 May 2014).
- Grassmann, F.; Schoenberger, P.G.; Brandl, C.; Schick, T.; Hasler, D.; Meister, G.; Fleckenstein, M.; Linder, M.; Helbig, H.; Fauser, S.; et al. A circulating microRNA profile is associated with late-stage neovascular age-related macular degeneration. PLoS One 2014, 9, e107461. [Google Scholar] [CrossRef] [PubMed]
- Askou, A.L. Development of gene therapy for treatment of age-related macular degeneration. Acta Ophthalmol. 2014, 92, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Yanai, R.; Mulki, L.; Hasegawa, E.; Takeuchi, K.; Sweigard, H.; Suzuki, J.; Gaissert, P.; Vavvas, D.G.; Sonoda, K.H.; Rothe, M.; et al. Cytochrome P450-generated metabolites derived from ω-3 fatty acids attenuate neovascularisation. Proc. Natl. Acad. Sci. USA 2014, 111, 9603–9608. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; Bressler, S.B.; Elman, M.J.; Danis, R.P.; Domalpally, A.; Heier, J.S.; Kim, J.E.; Garfinkel, R. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology 2014, 121, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.R.; Scott, I.U.; Kim, I.K.; Quillen, D.A.; Iannaccone, A.; Edwards, J.G. Diagnostic sensitivity and specificity of dark adaptometry for detection of age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1427–1431. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonekawa, Y.; Miller, J.W.; Kim, I.K. Age-Related Macular Degeneration: Advances in Management and Diagnosis. J. Clin. Med. 2015, 4, 343-359. https://doi.org/10.3390/jcm4020343
Yonekawa Y, Miller JW, Kim IK. Age-Related Macular Degeneration: Advances in Management and Diagnosis. Journal of Clinical Medicine. 2015; 4(2):343-359. https://doi.org/10.3390/jcm4020343
Chicago/Turabian StyleYonekawa, Yoshihiro, Joan W. Miller, and Ivana K. Kim. 2015. "Age-Related Macular Degeneration: Advances in Management and Diagnosis" Journal of Clinical Medicine 4, no. 2: 343-359. https://doi.org/10.3390/jcm4020343




