Can Zoledronic Acid be Beneficial for Promoting Tumor Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy?
Abstract
:1. Introduction
2. Bisphosphonates
3. Hypotheses for BP Anti-Tumor Mechanism
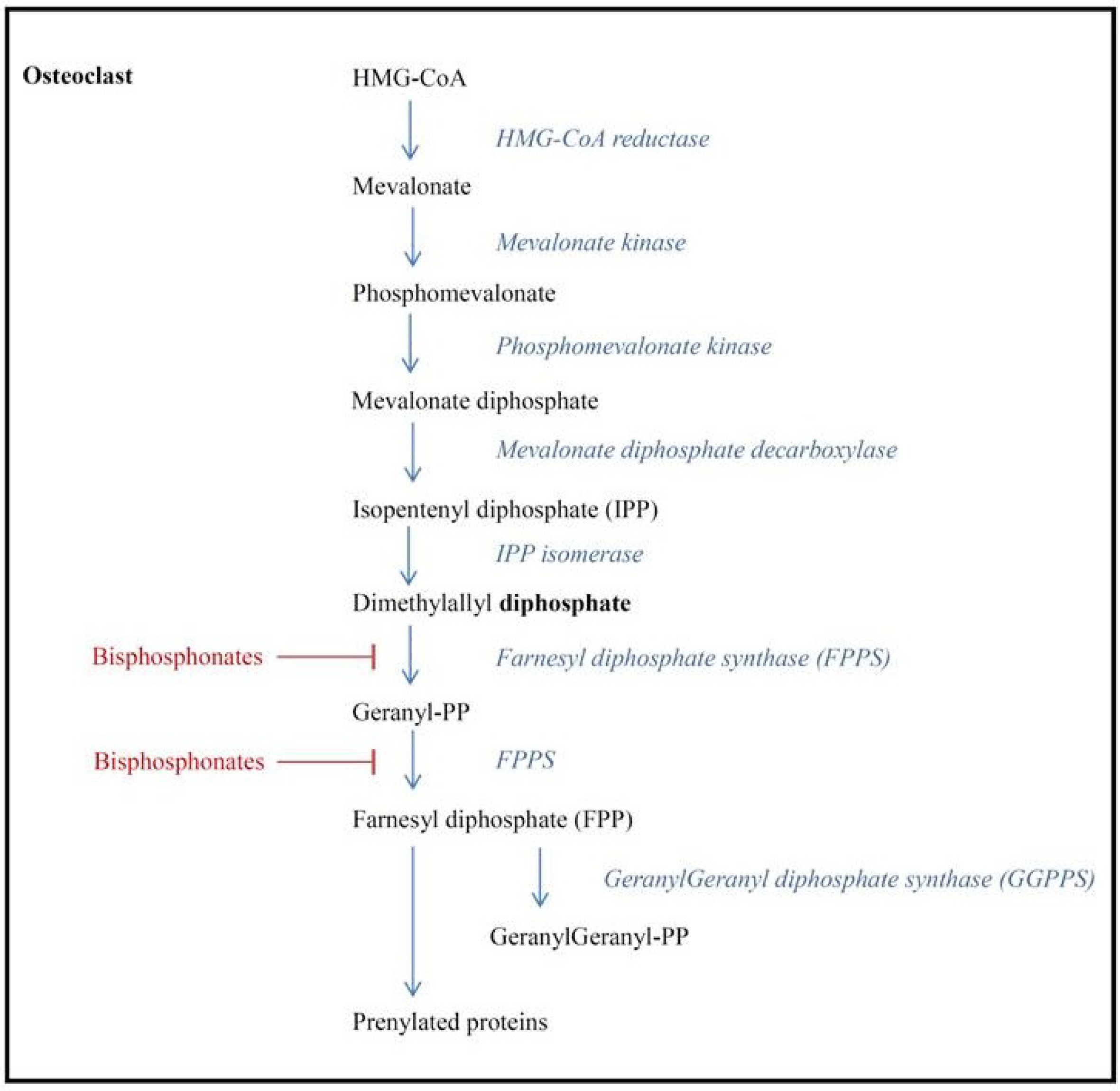

4. Preclinical Treatment Efficacy Data
5. Clinical Evidence
6. The Future of Bisphosphonates and Neoadjuvant Therapy
| Study | Intervention | Inclusion criteria | Primary endpoint | Secondary endpoints | (Estimated) enrollment | Estimated completed enrollment |
|---|---|---|---|---|---|---|
| NEOZOL | 8 cycles CT only (first 4 cycles doxorubicin + cyclophosphamide, last 4 cycles docetaxel) | Breast cancer (TNM IIB, IIIa) 3 cm and largers in maximal diameter | Decrease in serum VEGF concentration treatment |
| 76 | November 2013 |
| 8 cycles CT with zoledronic acid (4 mg i.v.) | ||||||
| ZoNantax | Cyclophosphamide, adriamycin (every 21 days for 4 cycles) Trastuzumab 8 mg/kg loading dose, then 3 times every 21 days for 3 cycles plus docetaxel 8 cycles with zoledronic acid (4 mg i.v.) | Stage IIA to IIIB HER-2 positive breast cancer | Residual cancer burden |
| 56 | November 2014 |
| Studies with completed accrual | ||||||
| Aftet al. | Neoadjuvant/adjuvant CT only (with four cycles of neoadjuvant epirubicin plus docetaxel and two cycles of adjuvant epirubicin and docetaxel) | Clinical stage II–III breast cancer | Number of patients with detectable DTCs at 3 months |
| 120 | Completed |
| Neoadjuvant/adjuvant CT in combination with zoledronic acid (4 mg i.v.) 3-weekly for 1 year | ||||||
| NEOZOTAC | 6 cycles CT only (docetaxel, adryamycin, cyclophosphamide) | T2 (≥2 cm and positive lymph nodes), T2 (≥3 cm), ≥ T3, T4, any N, M0 breast cancer | Pathologic complete response |
| 250 | Completed |
| 6 cycles CT only with zoledronic acid (4 mg i.v.) | ||||||
| JONIE-1 | Stage IIA, IIB, HER2-negative breast cancer | Pathologic complete response |
| 188 | Completed | |
| ANZAC | 6 cycles CT only | T2 breast tumor or above | Increase in apoptotic index between diagnostic core biopsy and repeat core biopsy |
| 40 | Completed |
| 6 cycles CT with zoledronic acid (4 mg i.v.) after first cycle chemotherapy only | ||||||
7. Conclusions
Conflicts of Interest
References
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P. Neoadjuvant vs. adjuvant systemic treatment in breast cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 188–194. [Google Scholar] [CrossRef]
- Mieog, J.S.; van der Hage, J.A.; van de Velde, C.J. Neoadjuvant chemotherapy for operable breast cancer. Br. J. Surg. 2007, 94, 1189–1200. [Google Scholar] [CrossRef]
- Ottewell, P.D.; Monkkonen, H.; Jones, M.; Lefley, D.V.; Coleman, R.E.; Holen, I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J. Natl. Cancer Inst. 2008, 100, 1167–1178. [Google Scholar] [CrossRef]
- Valachis, A.; Polyzos, N.P.; Coleman, R.E.; Gnant, M.; Eidtmann, H.; Brufsky, A.M.; Aft, R.; Tevaarwerk, A.J.; Swenson, K.; Lind, P.; et al. Adjuvant therapy with zoledronic acid in patients with breast cancer: A systematic review and meta-analysis. Oncologist 2013, 18, 353–361. [Google Scholar] [CrossRef]
- Yan, T.; Yin, W.; Zhou, Q.; Jiang, Y.; Du, Y.; Shao, Z.; Lu, J. The efficacy of zoledronic acid in breast cancer adjuvant therapy: A meta-analysis of randomised controlled trials. Eur. J. Cancer 2012, 48, 187–195. [Google Scholar]
- Winter, M.C.; Holen, I.; Coleman, R.E. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat. Rev. 2008, 34, 453–475. [Google Scholar] [CrossRef]
- Aapro, M.; Abrahamsson, P.A.; Body, J.J.; Coleman, R.E.; Colomer, R.; Costa, L.; Crino, L.; Dirix, L.; Gnant, M.; Gralow, J.; et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann. Oncol. 2008, 19, 420–432. [Google Scholar]
- Pavlakis, N.; Schmidt, R.; Stockler, M. Bisphosphonates for breast cancer. Cochrane Database Syst. Rev. 2005, 3. [Google Scholar] [CrossRef]
- Van Beek, E.R.; Lowik, C.W.; Ebetino, F.H.; Papapoulos, S.E. Binding and antiresorptive properties of heterocycle-containing bisphosphonate analogs: Structure-activity relationships. Bone 1998, 23, 437–442. [Google Scholar] [CrossRef]
- Russell, R.G.; Muhlbauer, R.C.; Bisaz, S.; Williams, D.A.; Fleisch, H. The influence of pyrophosphate, condensed phosphates, phosphonates and other phosphate compounds on the dissolution of hydroxyapatite in vitro and on bone resorption induced by parathyroid hormone in tissue culture and in thyroparathyroidectomised rats. Calcif. Tissue Res. 1970, 6, 183–196. [Google Scholar]
- Dunford, J.E.; Thompson, K.; Coxon, F.P.; Luckman, S.P.; Hahn, F.M.; Poulter, C.D.; Ebetino, F.H.; Rogers, M.J. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001, 296, 235–242. [Google Scholar]
- Kavanagh, K.L.; Guo, K.; Dunford, J.E.; Wu, X.; Knapp, S.; Ebetino, F.H.; Rogers, M.J.; Russel, R.G.; Opperman, U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. USA 2006, 103, 7829–7834. [Google Scholar] [CrossRef]
- Luckman, S.P.; Hughes, D.E.; Coxon, F.P.; Graham, R.; Russel, G.; Rogers, M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J. Bone Miner Res. 1998, 13, 581–589. [Google Scholar] [CrossRef]
- Green, J.R. Bisphosphonates: Preclinical review. Oncologist 2004, 9, 3–13. [Google Scholar] [CrossRef]
- Plotkin, L.I.; Weinstein, R.S.; Parfitt, A.M.; Roberson, P.K.; Manolagas, S.C.; Bellido, T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 1999, 104, 1363–1374. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to bone: Causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Roodman, G.D. Biology of osteoclast activation in cancer. J. Clin. Oncol. 2001, 19, 3562–3571. [Google Scholar]
- Coleman, R.E. Adjuvant bisphosphonates in breast cancer: Are we witnessing the emergence of a new therapeutic strategy? Eur. J. Cancer 2009, 45, 1909–1915. [Google Scholar] [CrossRef]
- Fournier, P.; Boissier, S.; Filleur, S.; Guglielmi, J.; Cabon, F.; Colombel, M.; Clezardin, P. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res. 2002, 62, 6538–6544. [Google Scholar]
- Santini, D.; Vincenzi, B.; Galluzzo, S.; Battistoni, F.; Rocci, L.; Venditti, O.; Schiavon, G.; Angeletti, S.; Uzzali, F.; Caraglia, M.; et al. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin. Cancer Res. 2007, 13, 4482–4486. [Google Scholar] [CrossRef]
- Gober, H.J.; Kistowska, M.; Angman, L.; Jeno, P.; Mori, L.; de Libero, G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef]
- Benzaid, I.; Monkkonen, H.; Stresing, V.; Bonnelye, E.; Green, J.; Monkkonen, J.; Touraine, J.L.; Clezardin, P. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vgamma9Vdelta2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011, 71, 4562–4572. [Google Scholar] [CrossRef]
- Benzaid, I.; Monkkonen, H.; Bonnelye, E.; Monkkonen, J.; Clezardin, P. In vivo phosphoantigen levels in bisphosphonate-treated human breast tumors trigger Vgamma9Vdelta2 T-cell antitumor cytotoxicity through ICAM-1 engagement. Clin. Cancer Res. 2012, 18, 6249–6259. [Google Scholar] [CrossRef]
- Rogers, T.L.; Holen, I. Tumour macrophages as potential targets of bisphosphonates. J. Transl. Med. 2011, 9, 177. [Google Scholar] [CrossRef]
- Neville-Webbe, H.L.; Rostami-Hodjegan, A.; Evans, C.A.; Coleman, R.E.; Holen, I. Sequence- and schedule-dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int. J. Cancer 2005, 113, 364–371. [Google Scholar] [CrossRef]
- Ottewell, P.D.; Woodward, J.K.; Lefley, D.V.; Evans, C.E.; Coleman, R.E.; Holen, I. Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Mol. Cancer Ther. 2009, 8, 2821–2832. [Google Scholar] [CrossRef]
- Ottewell, P.D.; Deux, B.; Monkkonen, H.; Cross, S.; Coleman, R.E.; Clezardin, P.; Holen, I. Differential effect of doxorubicin and zoledronic acid on intraosseous vs. extraosseous breast tumor growth in vivo. Clin. Cancer Res. 2008, 14, 4658–4666. [Google Scholar] [CrossRef]
- Holen, I.; Wang, N.; Reeves, K.J.; Fowles, A.M.; Croucher, P.I.; Eaton, C.L.; Ottewell, P.D. Zoledronic acid specifically inhibits development of bone metastases in the postmenopausal setting—Evidence from an in vivo breast cancer model. Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Diel, I.J.; Solomayer, E.F.; Costa, S.D.; Gollan, C.; Goerner, R.; Wallwiener, R.; Kaufmann, M.; Bastert, G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N. Engl. J. Med. 1998, 339, 357–363. [Google Scholar] [CrossRef]
- Diel, I.J.; Jaschke, A.; Solomayer, E.F.; Gollan, C.; Bastert, C.; Sohn, C.; Schuetz, F. Adjuvant oral clodronate improves the overall survival of primary breast cancer patients with micrometastases to the bone marrow: A long-term follow-up. Ann. Oncol. 2008, 19, 2007–2011. [Google Scholar] [CrossRef]
- Powles, T.; Paterson, A.; McCloskey, E.; Schein, P.; Scheffler, B.; Tidy, A.; Ashley, S.; Smith, I.; Ottestad, L.; Kanis, J. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer (ISRCTN83688026). Breast Cancer Res. 2006, 8, 13. [Google Scholar]
- Saarto, T.; Blomqvist, C.; Virkkunen, P.; Elomaa, I. Adjuvant clodronate treatment does not reduce the frequency of skeletal metastases in node-positive breast cancer patients: 5-Year results of a randomized controlled trial. J. Clin. Oncol. 2001, 19, 10–17. [Google Scholar]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, H.; Heck, D.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Pristauz, G.; et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-Month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011, 12, 631–641. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Schippinger, W.; Luschin-Ebengreuth, H.; Postlberger, S.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Bjelic-Radisic, V.; et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N. Engl. J. Med. 2009, 360, 679–691. [Google Scholar] [CrossRef]
- Gnant, M.; Mlineritsch, B.; Luschin-Ebengreuth, G.; Stoeger, H.; Dubsky, P.; Jakesz, R.; Singer, C.; Eidtmann, H.; Fesl, C.; Eiermann, W.; et al. Long-term follow-up in ABCSG-12: Significantly improved overall survival with adjuvant zoledronic acid in premenopausal patients with endocrine-receptor-positive early breast cancer. Cancer Res. 2011, 71. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Harker, W.G.; Beck, J.T.; Bosserman, L.; Vogel, C.; Seidler, C.; Jin, L.; Warsi, G.; Argonza-Aviles, E.; Hohneker, J.; et al. Final 5-year results of Z-FAST trial: Adjuvant zoledronic acid maintains bone mass in postmenopausal breast cancer patients receiving letrozole. Cancer 2012, 118, 1192–1201. [Google Scholar] [CrossRef]
- Coleman, R.; de Boer, R.; Eidtmann, H.; Llombart, A.; Davidson, N.; Neven, P.; von Minckwitz, G.; Sleeboom, H.P.; Forbes, J.; Barrios, C.; et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): Final 60-month results. Ann. Oncol. 2013, 24, 398–405. [Google Scholar] [CrossRef]
- Llombart, A.; Frassoldati, A.; Paija, O.; Sleeboom, H.P.; Jerusalem, G.; Mebis, J.; Deleu, I.; Miller, J.; Schenk, N.; Neven, P. Immediate administration of zoledronic acid reduces aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer: 12-Month analysis of the E-ZO-FAST trial. Clin. Breast Cancer 2012, 12, 40–48. [Google Scholar] [CrossRef]
- Coleman, R.E.; Marshall, H.; Cameron, D.; Dodwell, D.; Burkinshaw, R.; Keane, M.; Gil, M.; Houston, S.J.; Grieve, R.J.; Barrett-Lee, P.J.; et al. Breast-cancer adjuvant therapy with zoledronic acid. N. Engl. J. Med. 2011, 365, 1396–1405. [Google Scholar] [CrossRef]
- Coleman, R.E.; Winter, M.C.; Cameron, D.; Bell, R.; Dodwell, D.; Keane, M.M.; Gil, M.; Ritchie, D.; Passos-Coelho, J.L.; Wheatley, D.; et al. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: Exploratory evidence for direct anti-tumour activity in breast cancer. Br. J. Cancer 2010, 102, 1099–1105. [Google Scholar] [CrossRef]
- Van de Ven, S.; Liefers, G.J.; Putter, H.; van Warmerdam, L.J.; Kessels, L.W.; Dercksen, W.; Pepels, M.J.; Maartense, E.; van Laarhoven, H.W.M.; Vriens, B.; et al. Toxicity data of a phase III randomized trial with NEOadjuvant chemotherapy (TAC) with or without zoledronic acid (ZA) for patients with HER2-negative large resectable or locally advanced breast cancer (BC). Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Brown, E.; Lei, X.; Litton, J.; Meric-Bernstram, F.; Mettendorf, E.; Hernandez, L.; Valero, V.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Bisphosphonates and pathologic complete response to taxane- and anthracycline-based neoadjuvant chemotherapy in patients with breast cancer. Cancer 2012, 118, 326–332. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Kohno, N.; Horiguchi, J.; Miura, D.; Ishikawa, T.; Hayashi, M.; Takao, S.; Kim, S.J.; Tanino, H.; Miyashita, M.; et al. A randomized controlled trial comparing zoledronic acid plus chemotherapy with chemotherapy alone as a neoadjuvant treatment in patients with HER2-negative primary breast cancer. Cancer Res. 2012, 72. [Google Scholar] [CrossRef]
- Horiguchi, J.; Hasegawa, Y.; Miura, D.; Ishikawa, T.; Hayashi, M.; Takao, S.; Kim, S.J.; Tanino, H.; Miyashita, M.; Konishi, M.; et al. A randomized controlled trial comparing zoledronic acid plus chemothearpy with chemotherapy alone as a neoadjuvant treatment in patients with HER2-negative primary breast cancer. J. Clin. Oncol. 2013, 31, 1029. [Google Scholar] [CrossRef]
- Aft, R.; Naughton, M.; Trinkaus, K.; Watson, M.; Ylagan, L.; Chavez-MacGregor, M.; Zhai, J.; Kuo, S.; Shannon, W.; Diemer, K.; et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: An open label, randomised, phase 2 trial. Lancet Oncol. 2010, 11, 421–428. [Google Scholar] [CrossRef]
- Winter, M.C.; Wilson, C.; Syddall, S.P.; Cross, S.S.; Evans, A.; Ingram, C.E.; Jolley, I.J.; Hatton, M.Q.; Freeman, J.V.; Mori, S.; et al. Neoadjuvant chemotherapy with or without zoledronic acid in early breast cancer—A randomized biomarker pilot study. Clin. Cancer Res. 2013, 19, 2755–2765. [Google Scholar] [CrossRef]
- ClinicalTrials. Available online: http://www.clinicaltrials.gov (accessed on 3 September 2013).
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Charehbili, A.; Fontein, D.B.Y.; Kroep, J.R.; Liefers, G.-J.; Nortier, J.W.R.; Velde, C.J.H.v.d. Can Zoledronic Acid be Beneficial for Promoting Tumor Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy? J. Clin. Med. 2013, 2, 188-200. https://doi.org/10.3390/jcm2040188
Charehbili A, Fontein DBY, Kroep JR, Liefers G-J, Nortier JWR, Velde CJHvd. Can Zoledronic Acid be Beneficial for Promoting Tumor Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy? Journal of Clinical Medicine. 2013; 2(4):188-200. https://doi.org/10.3390/jcm2040188
Chicago/Turabian StyleCharehbili, Ayoub, Duveken B. Y. Fontein, Judith R. Kroep, Gerrit-Jan Liefers, Johannes W. R. Nortier, and Cornelis J. H. van de Velde. 2013. "Can Zoledronic Acid be Beneficial for Promoting Tumor Response in Breast Cancer Patients Treated with Neoadjuvant Chemotherapy?" Journal of Clinical Medicine 2, no. 4: 188-200. https://doi.org/10.3390/jcm2040188






