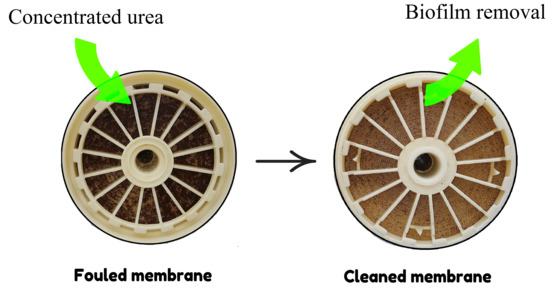
Pilot-Scale Assessment of Urea as a Chemical Cleaning Agent for Biofouling Control in Spiral-Wound Reverse Osmosis Membrane Elements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Key Performance Indicators
2.3. Membrane Autopsy
2.4. Biomass Quantification
2.4.1. Adenosine Triphosphate (ATP)
2.4.2. Total Organic Carbon (TOC)
2.5. Biofilm Composition
2.5.1. SEM–EDX Analysis
2.5.2. ATR–FTIR Analysis
3. Results
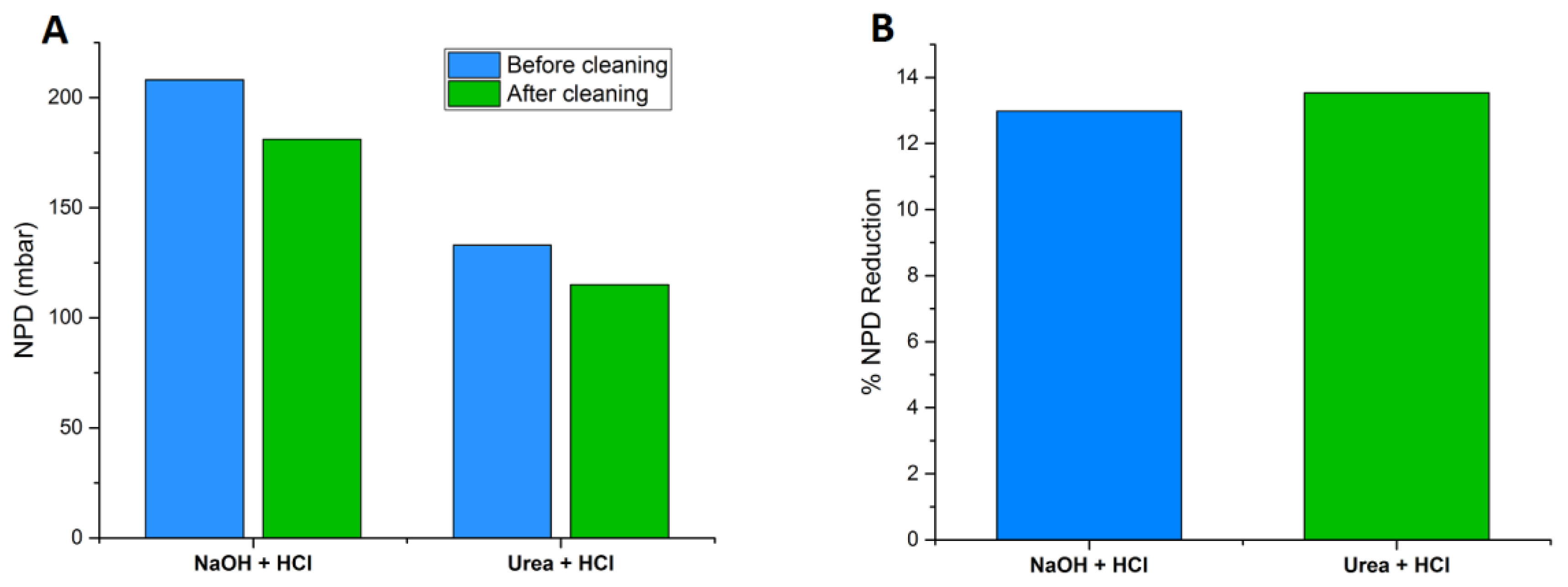
3.1. Membrane Performance
3.2. Visual Analysis
3.3. Biomass Parameters
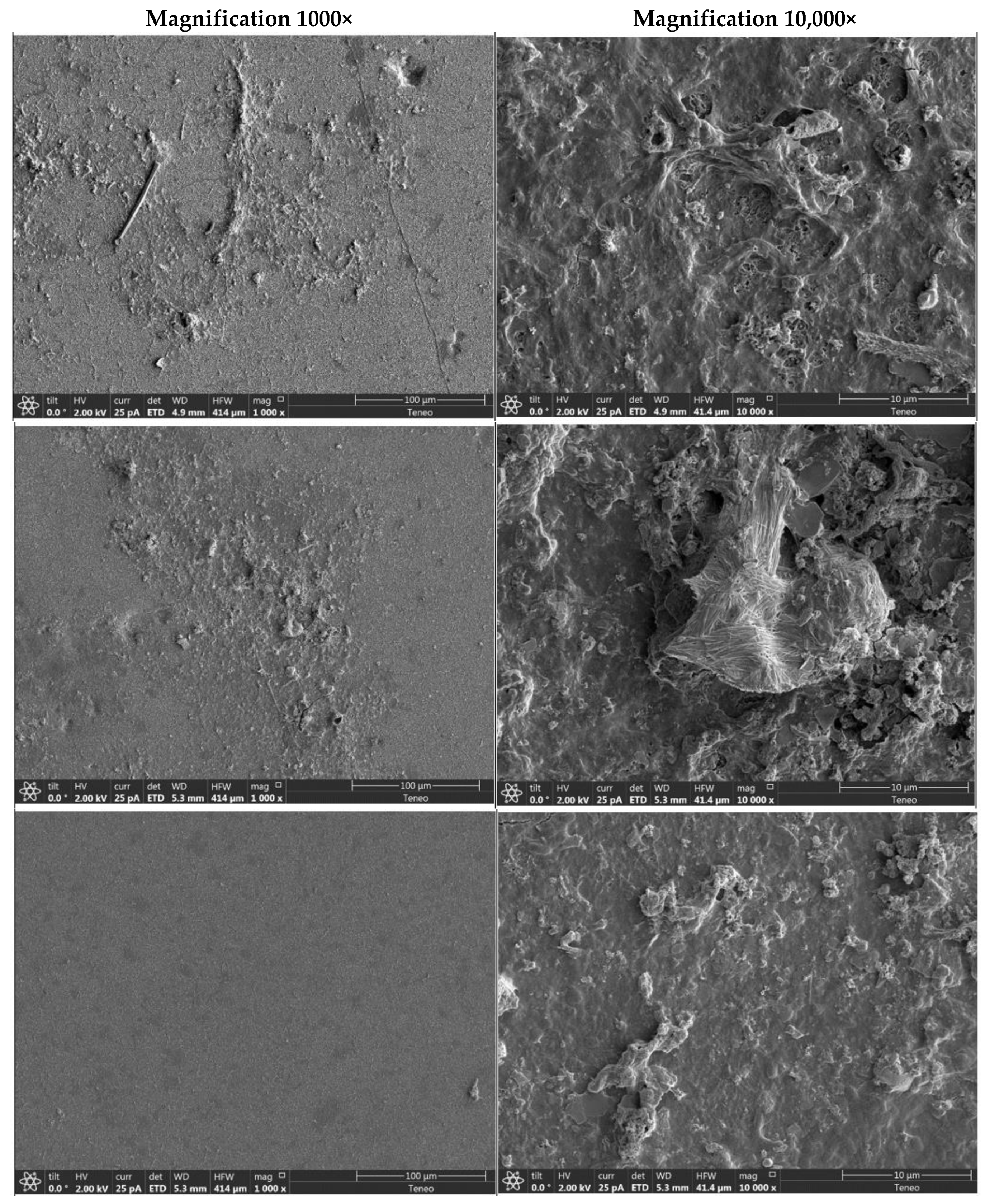
3.4. Elemental Composition and Surface Morphology
3.5. Molecular Composition
4. Discussion
4.1. Membrane Regeneration
4.2. Biofilm Solubilization and Removal
4.3. Relevance of Urea Use
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fritzmann, C.; Löwenberg, J.; Wintgens, T.; Melin, T. State-of-the-art of reverse osmosis desalination. Desalination 2007, 216, 1–76. [Google Scholar] [CrossRef]
- Tang, F.; Hu, H.-Y.; Sun, L.-J.; Wu, Q.-Y.; Jiang, Y.-M.; Guan, Y.-T.; Huang, J.-J. Fouling of reverse osmosis membrane for municipal wastewater reclamation: Autopsy results from a full-scale plant. Desalination 2014, 349, 73–79. [Google Scholar] [CrossRef]
- Polasek, V.; Talo, S.; Sharif, T. Conversion from hollow fiber to spiral technology in large seawater RO systems—Process design and economics. Desalination 2003, 156, 239–247. [Google Scholar] [CrossRef]
- Komlenic, R. Rethinking the causes of membrane biofouling. Filtr. Sep. 2010, 47, 26–28. [Google Scholar] [CrossRef]
- Hydranautics. Troubleshooting Your RO—Hydranautics. 2001. Available online: http://www.membranes.com/docs/trc/TROUBLES.PDF (accessed on 13 February 2019).
- SUEZ. Utilization of Chemical Treatments to Maintain and Restore Membrane Performance—SUEZ Water Technologies & Solutions. 2017. Available online: https://www.suezwatertechnologies.com/kcpguest/document-library.do (accessed on 13 February 2019).
- DOW. DOW FILMTEC™ Membranes—Cleaning Procedures for DOW FILMTEC FT30 Elements. Available online: http://msdssearch.dow.com/PublishedLiteratureDOWCOM/dh_060a/0901b8038060a66f.pdf?filepath=liquids (accessed on 13 February 2019).
- Bucs, S.S.; Farhat, N.; Kruithof, J.C.; Picioreanu, C.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Review on strategies for biofouling mitigation in spiral wound membrane systems. Desalination 2018, 434, 189–197. [Google Scholar] [CrossRef]
- Farhat, N.M.; Loubineaud, E.; Prest, E.I.E.C.; El-Chakhtoura, J.; Salles, C.; Bucs, S.S.; Trampé, J.; van den Broek, W.B.P.; van Agtmaal, J.M.C.; van Loosdrecht, M.C.M.; et al. Application of monochloramine for wastewater reuse: Effect on biostability during transport and biofouling in RO membranes. J. Membr. Sci. 2018, 551, 243–253. [Google Scholar] [CrossRef]
- Siddiqui, A.; Pinel, I.; Prest, E.; Bucs, S.; van Loosdrecht, M.; Kruithof, J.; Vrouwenvelder, J.S. Application of DBNPA dosage for biofouling control in spiral wound membrane systems. Desalin. Water Treat. 2017, 68, 12–22. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Hinrichs, C.; van der Meer, W.G.; van Loosdrecht, M.C.; Kruithof, J.C. Pressure drop increase by biofilm accumulation in spiral wound RO and NF membrane systems: Role of substrate concentration, flow velocity, substrate load and flow direction. Biofouling 2009, 25, 543–555. [Google Scholar] [CrossRef]
- Chinu, K.J.; Johir, A.H.; Vigneswaran, S.; Shon, H.K.; Kandasamy, J. Biofilter as pretreatment to membrane based desalination: Evaluation in terms of fouling index. Desalination 2009, 247, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, A.; Lehmann, S.; Bucs, S.S.; Fresquet, M.; Fel, L.; Prest, E.I.E.C.; Ogier, J.; Schellenberg, C.; van Loosdrecht, M.C.M.; Kruithof, J.C.; et al. Predicting the impact of feed spacer modification on biofouling by hydraulic characterization and biofouling studies in membrane fouling simulators. Water Res. 2017, 110, 281–287. [Google Scholar] [CrossRef]
- Karkhanechi, H.; Takagi, R.; Matsuyama, H. Biofouling resistance of reverse osmosis membrane modified with polydopamine. Desalination 2014, 336, 87–96. [Google Scholar] [CrossRef]
- Araújo, P.A.; Miller, D.J.; Correia, P.B.; van Loosdrecht, M.C.M.; Kruithof, J.C.; Freeman, B.D.; Paul, D.R.; Vrouwenvelder, J.S. Impact of feed spacer and membrane modification by hydrophilic, bactericidal and biocidal coating on biofouling control. Desalination 2012, 295, 1–10. [Google Scholar] [CrossRef]
- Beyer, F.; Rietman, B.M.; Zwijnenburg, A.; van den Brink, P.; Vrouwenvelder, J.S.; Jarzembowska, M.; Laurinonyte, J.; Stams, A.J.M.; Plugge, C.M. Long-term performance and fouling analysis of full-scale direct nanofiltration (NF) installations treating anoxic groundwater. J. Membr. Sci. 2014, 468, 339–348. [Google Scholar] [CrossRef]
- Huiting, H.; Kappelhof, J.W.N.M.; Bosklopper, T.G.J. Operation of NF/RO plants: From reactive to proactive. Desalination 2001, 139, 183–189. [Google Scholar] [CrossRef]
- Vrouwenvelder, H.S.; van Paassen, J.A.M.; Folmer, H.C.; Hofman, J.A.M.H.; Nederlof, M.M.; van der Kooij, D. Biofouling of membranes for drinking water production. Desalination 1998, 118, 157–166. [Google Scholar] [CrossRef]
- Beyer, F.; Laurinonyte, J.; Zwijnenburg, A.; Stams, A.J.M.; Plugge, C.M. Membrane Fouling and Chemical Cleaning in Three Full-Scale Reverse Osmosis Plants Producing Demineralized Water. J. Eng. 2017, 2017. [Google Scholar] [CrossRef]
- Whittaker, C.; Ridgway, H.; Olson, B.H. Evaluation of Cleaning Strategies for Removal of Biofilms from Reverse-Osmosis Membranes. Appl. Environ. Microbiol. 1984, 48, 395–403. [Google Scholar] [Green Version]
- Chen, X.; Stewart, P.S. Biofilm removal caused by chemical treatments. Water Res. 2000, 34, 4229–4233. [Google Scholar] [CrossRef]
- Rasmussen, K.; Reilly, C.; Li, Y.; Jones, R.S. Real-time imaging of anti-biofilm effects using CP-OCT. Biotechnol. Bioeng. 2016, 113, 198–205. [Google Scholar] [CrossRef]
- Sanawar, H.; Pinel, I.; Farhat, N.M.; Bucs, S.S.; Zlopasa, J.; Kruithof, J.C.; Witkamp, G.J.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Enhanced biofilm solubilization by urea in reverse osmosis membrane systems. Water Res. X 2018, 1, 100004. [Google Scholar] [CrossRef]
- Xu, P.; Bellona, C.; Drewes, J.E. Fouling of nanofiltration and reverse osmosis membranes during municipal wastewater reclamation: Membrane autopsy results from pilot-scale investigations. J. Membr. Sci. 2010, 353, 111–121. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Samieirad, S. Chemical cleaning of reverse osmosis membrane fouled by wastewater. Desalination 2010, 257, 80–86. [Google Scholar] [CrossRef]
- Hydranautics. Foulants and Cleaning Procedures for Composite Polyamide RO Membrane Elements (ESPA, ESNA, CPA, LFC, NANO and SWC). Available online: http://www.membranes.com/docs/tsb/TSB107.pdf (accessed on 13 February 2019).
- Bisselink, R.; de Schepper, W.; Trampé, J.; van den Broek, W.; Pinel, I.; Krutko, A.; Groot, N. Mild desalination demo pilot: New normalization approach to effectively evaluate electrodialysis reversal technology. Water Res. Ind. 2016, 14, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Vrouwenvelder, J.S.; van Paassen, J.A.M.; van Agtmaal, J.M.C.; van Loosdrecht, M.C.M.; Kruithof, J.C. A critical flux to avoid biofouling of spiral wound nanofiltration and reverse osmosis membranes: Fact or fiction? J. Membr. Sci. 2009, 326, 36–44. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.S.; Manolarakis, S.A.; van der Hoek, J.P.; van Paassen, J.A.M.; van der Meer, W.G.J.; van Agtmaal, J.M.C.; Prummel, H.D.M.; Kruithof, J.C.; van Loosdrecht, M.C.M. Quantitative biofouling diagnosis in full scale nanofiltration and reverse osmosis installations. Water Res. 2008, 42, 4856–4868. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Cornelissen, E.R.; van der Kooij, D. Threshold concentrations of biomass and iron for pressure drop increase in spiral-wound membrane elements. Water Res. 2011, 45, 1607–1616. [Google Scholar] [CrossRef]
- Kwan, S.E.; Bar-Zeev, E.; Elimelech, M. Biofouling in forward osmosis and reverse osmosis: Measurements and mechanisms. J. Membr. Sci. 2015, 493, 703–708. [Google Scholar] [CrossRef]
- Kim, L.H.; Jung, Y.; Yu, H.W.; Chae, K.J.; Kim, I.S. Physicochemical interactions between rhamnolipids and Pseudomonas aeruginosa biofilm layers. Environ. Sci. Technol. 2015, 49, 3718–3726. [Google Scholar] [CrossRef]
- Quiles, F.; Humbert, F.; Delille, A. Analysis of changes in attenuated total reflection FTIR fingerprints of Pseudomonas fluorescens from planktonic state to nascent biofilm state. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2010, 75, 610–616. [Google Scholar] [CrossRef]
- Baker, J.; Stephenson, T.; Dard, S.; Côté, P. Characterisation of fouling of nanofiltration membranes used to treat surface waters. Environ. Technol. 1995, 16, 977–985. [Google Scholar] [CrossRef]
- Tran, T.; Bolto, B.; Gray, S.; Hoang, M.; Ostarcevic, E. An autopsy study of a fouled reverse osmosis membrane element used in a brackish water treatment plant. Water Res. 2007, 41, 3915–3923. [Google Scholar] [CrossRef] [Green Version]
- Vrouwenvelder, J.S.; Graf von der Schulenburg, D.A.; Kruithof, J.C.; Johns, M.L.; van Loosdrecht, M.C.M. Biofouling of spiral-wound nanofiltration and reverse osmosis membranes: A feed spacer problem. Water Res. 2009, 43, 583–594. [Google Scholar] [CrossRef]
- Siebdrath, N.; Farhat, N.; Ding, W.; Kruithof, J.; Vrouwenvelder, J.S. Impact of membrane biofouling in the sequential development of performance indicators: Feed channel pressure drop, permeability, and salt rejection. J. Membr. Sci. 2019, 585, 199–207. [Google Scholar] [CrossRef]
- American Water Works Association Research Foundation; Lyonnaise des Eaux; Water Research Commision of South Africa. Water Treatment Membrane Processes; Mallevialle, J., Odendaal, P.E., Wiesner, M.R., Eds.; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Bertheas, U.; Majamaa, K.; Arzu, A.; Pahnke, R. Use of DBNPA to control biofouling in RO systems. Desal. Water Treat. 2009, 3, 175–178. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Fu, X.; Wickramasinghe, R.; Chen, J. Chemical cleaning of PS ultrafilters fouled by the fermentation broth of glutamic acid. Sep. Purif. Technol. 2005, 42, 181–187. [Google Scholar] [CrossRef]
- Al-Amoudi, A.; Lovitt, R.W. Fouling strategies and the cleaning system of NF membranes and factors affecting cleaning efficiency. J. Membr. Sci. 2007, 303, 4–28. [Google Scholar] [CrossRef]





| Module | Code | Cleaning Protocol | Comment |
|---|---|---|---|
| 1 | Control | None | Control module. No cleaning |
| 2 | NaOH + HCl | (i) NaOH (pH 12, 35 °C) recirculated for 1 h (ii) HCl (pH 1, room temperature) (~18 °C) recirculated for 30 min | Reference module cleaned with conventional alkali/acid solutions as applied by DOW and Evides |
| 3 | Urea + HCl | (i) CO(NH2)2 (1340 g/L, 35 °C) recirculated for 1 h (ii) HCl (pH 1, room temperature) (~18 °C) recirculated for 30 min | NaOH replaced by saturated urea solution |
| Performance Parameters | Cleaning with NaOH + HCl | Cleaning with Urea + HCl | ||||
|---|---|---|---|---|---|---|
| Initial | After NaOH | After HCl | Initial | After Urea | After HCl | |
| Normalized Feed Channel Pressure Drop (mbar) | 208 | 202 | 181 | 133 | 173 | 115 |
| Normalized Flux (Lm−2 h−1) | 29.07 | 29.98 | 29.07 | 29.68 | 29.98 | 31.19 |
| Element | C | N | O | Mg | Al | Si | P | S | K | Ca | Ti | Mn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 66.26 | 6.78 | 18.77 | 0.28 | 0.41 | 0.34 | 0.55 | 4.31 | 0.15 | 0.29 | 0.21 | 0.53 | 1.12 |
| NaOH + HCl | 62.44 | 3.34 | 21.98 | 0.30 | 0.60 | 0.79 | 0.59 | 5.96 | 0.21 | 0.27 | 0.18 | 0.44 | 2.91 |
| Urea + HCl | 59.31 | 6.88 | 24.88 | 0.37 | 0.68 | 0.91 | 0.61 | 5.02 | 0.23 | 0.66 | 0.26 | 0.47 | 2.42 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanawar, H.; Bucs, S.S.; Pot, M.A.; Zlopasa, J.; Farhat, N.M.; Witkamp, G.-J.; Kruithof, J.C.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Pilot-Scale Assessment of Urea as a Chemical Cleaning Agent for Biofouling Control in Spiral-Wound Reverse Osmosis Membrane Elements. Membranes 2019, 9, 117. https://doi.org/10.3390/membranes9090117
Sanawar H, Bucs SS, Pot MA, Zlopasa J, Farhat NM, Witkamp G-J, Kruithof JC, van Loosdrecht MCM, Vrouwenvelder JS. Pilot-Scale Assessment of Urea as a Chemical Cleaning Agent for Biofouling Control in Spiral-Wound Reverse Osmosis Membrane Elements. Membranes. 2019; 9(9):117. https://doi.org/10.3390/membranes9090117
Chicago/Turabian StyleSanawar, Huma, Szilárd S. Bucs, Martin A. Pot, Jure Zlopasa, Nadia M. Farhat, Geert-Jan Witkamp, Joop C. Kruithof, Mark C. M. van Loosdrecht, and Johannes S. Vrouwenvelder. 2019. "Pilot-Scale Assessment of Urea as a Chemical Cleaning Agent for Biofouling Control in Spiral-Wound Reverse Osmosis Membrane Elements" Membranes 9, no. 9: 117. https://doi.org/10.3390/membranes9090117