Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Small Vesicles Carry Gluconeogenic Enzymes to the Vacuole for Degradation via the Vacuole Import and Degradation Pathway
3. Gluconeogenic Enzymes Are Secreted into the Periplasm during Glucose Starvation
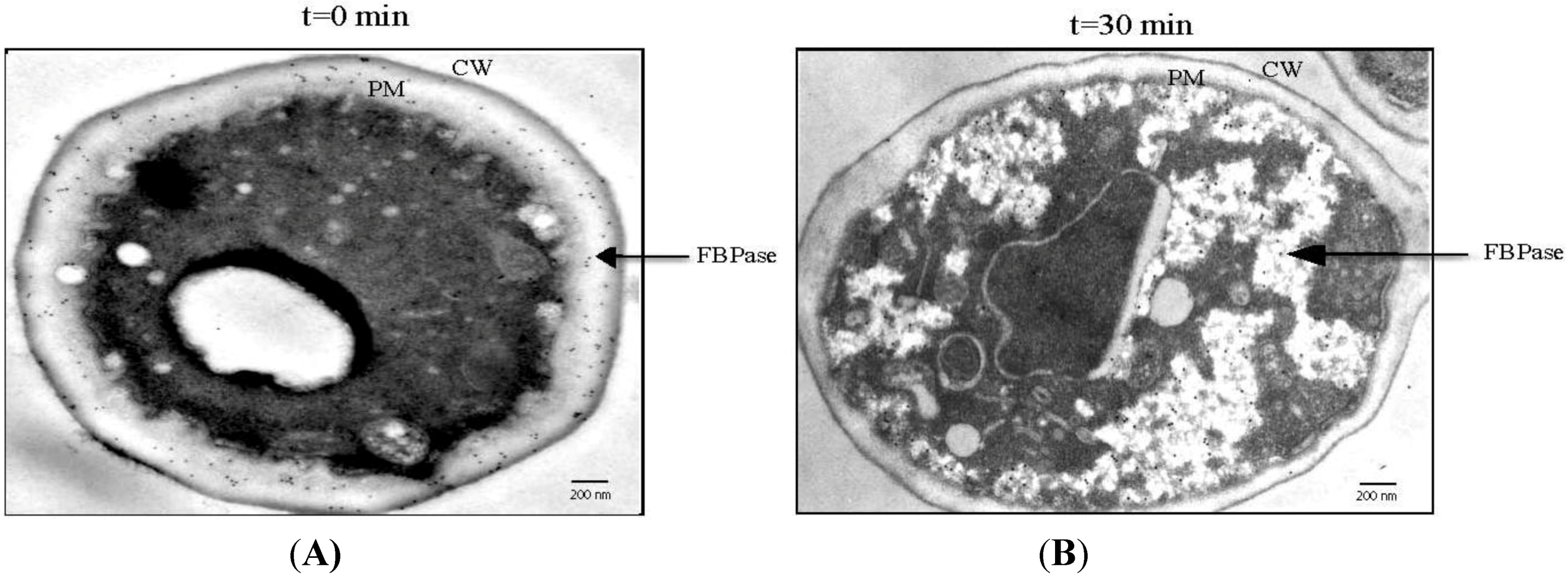
4. The Use of an Extraction Protocol to Detect Gluconeogenic Enzymes in the Extracellular Fraction in Glucose-Starved Cells
5. Proteomic Approach to Identify Extracellular Proteins in Glucose-Starved Cells
6. Vid Vesicles are Secreted as Extracellular Vesicles in Glucose-Starved Cells

7. Extracellular FBPase, MDH2, Icl1p, Pck1p, GAPDH, and Cpr1p are Associated with Membranes in the Vesicle-Enriched Fraction in Total Extracts
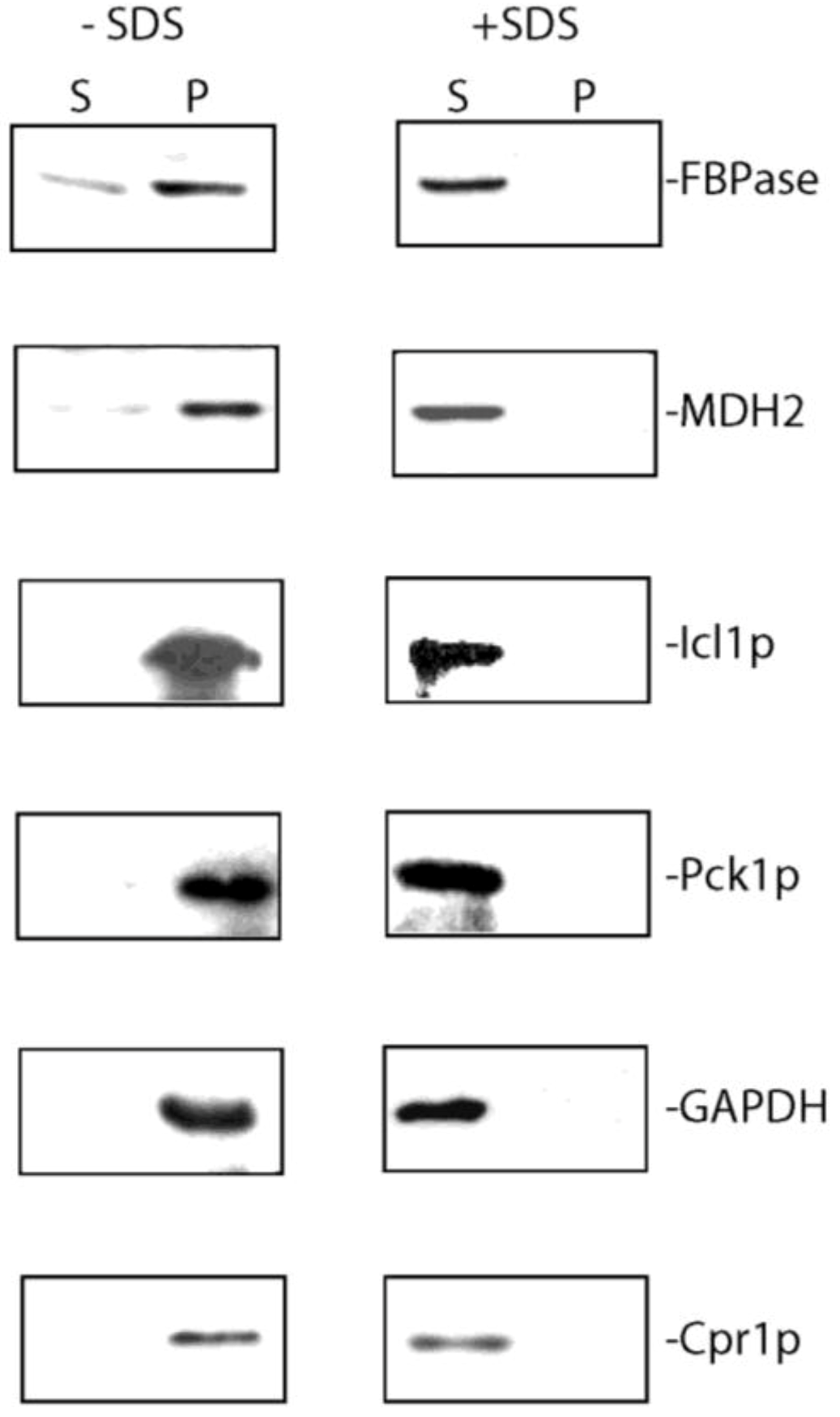
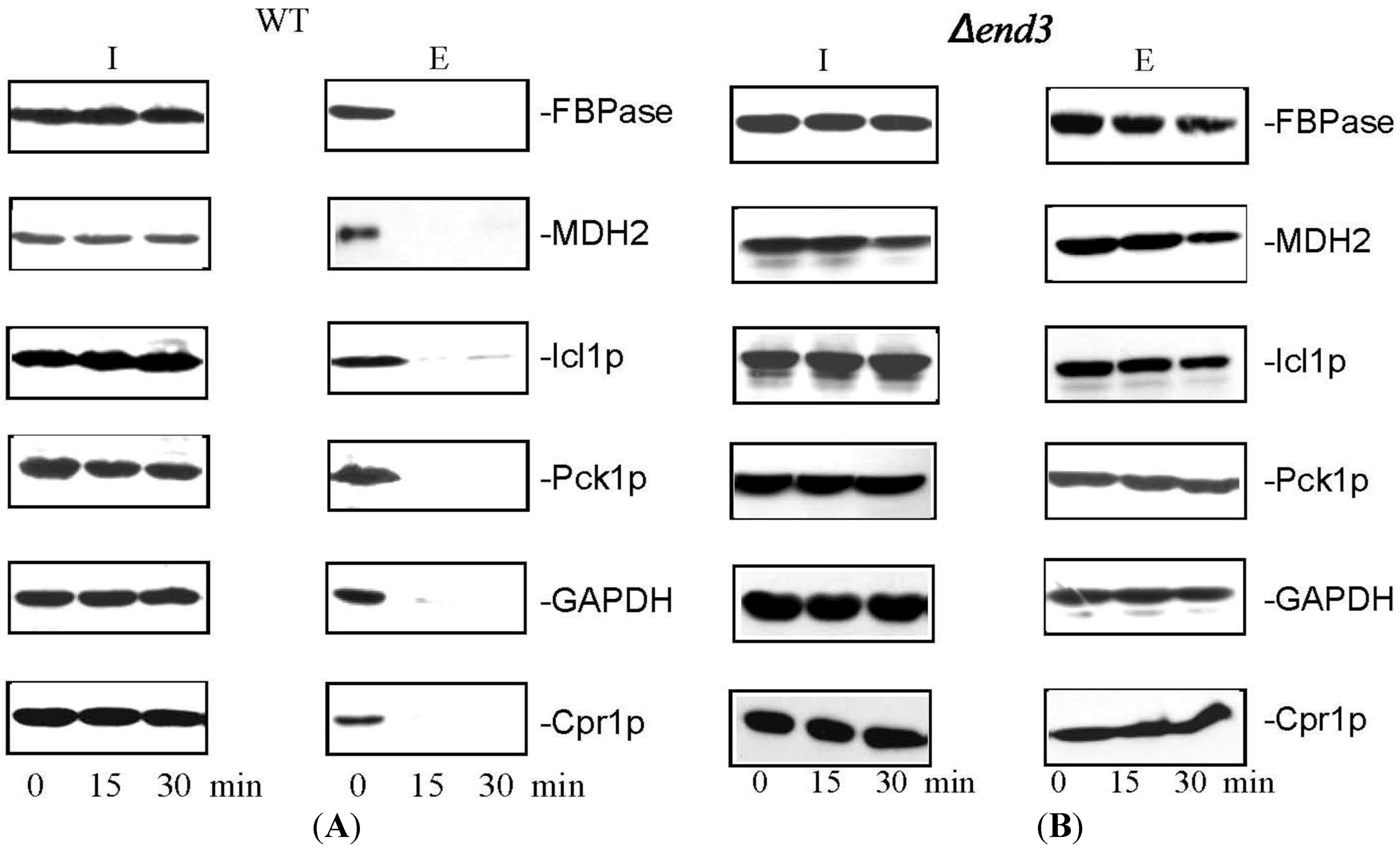
8. Glucose Induces a Rapid Clearance of Vid Vesicles and Vesicle-Associated Proteins from the Extracellular Fraction
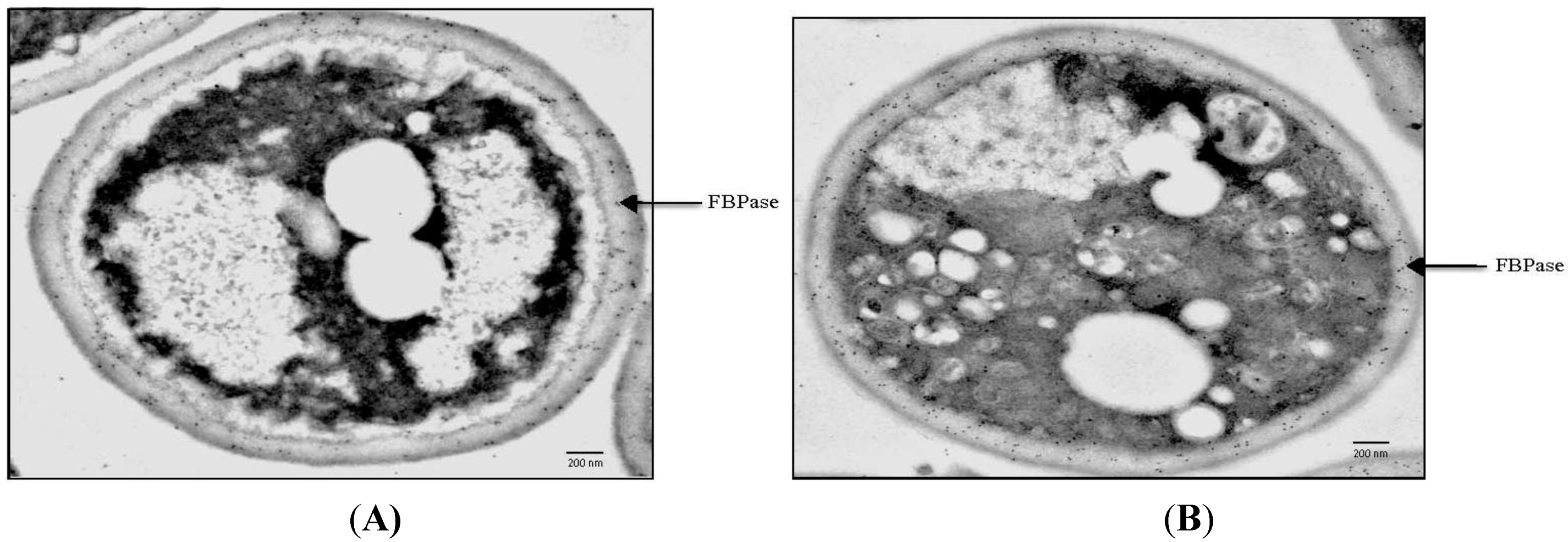
9. FBPase is Re-Distributed from the Periplasm to the Cytoplasm in Response to Glucose
10. VPS34 is Required for the Decline of Extracellular FBPase in Response to Glucose
| Strain | Glucose Starvation | Glucose Re-Feeding |
|---|---|---|
| WT | Secretion | Internalization |
| ∆vps34 | Normal secretion | Defective internalization |
| ∆end3 | Normal secretion | Defective internalization |
11. END3 is Essential for the Glucose-Induced Decrease of Extracellular Vesicles and Vesicle-Associated Proteins
12. Conclusions

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Carlson, M. Regulation of glucose utilization in yeast. Curr. Opin. Genet. Dev. 1998, 8, 560–564. [Google Scholar]
- Ludin, K.; Jiang, R.; Carlson, M. Glucose-regulated interaction of a regulatory subunit of protein phosphatase 1 with the Snf1 protein kinase in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1998, 95, 6245–6250. [Google Scholar]
- Fraenkel, D.G. The top genes: On the distance from transcript to function in yeast glycolysis. Curr. Opin. Microbiol. 2003, 6, 198–201. [Google Scholar]
- Gancedo, J.M. The early steps of glucose signalling in yeast. FEMS Microbiol. Rev. 2008, 32, 673–704. [Google Scholar]
- Gancedo, J.M. Yeast carbon catabolite repression. Microbiol. Mol. Biol. Rev. 1998, 62, 334–361. [Google Scholar]
- Zaman, S.; Lippman, S.I.; Zhao, X.; Broach, J.R. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 2008, 42, 27–81. [Google Scholar]
- Van den Brink, J.; Akeroyd, M.; van der Hoeven, R.; Pronk, J.T.; de Winde, J.H.; Daran-Lapujade, P. Energetic limits to metabolic flexibility: Responses of Saccharomyces cerevisiae to glucose-galactose transitions. Microbiology 2009, 155, 1340–1350. [Google Scholar]
- Haurie, V.; Sagliocco, F.; Boucherie, H. Dissecting regulatory networks by means of two-dimensional gel electrophoresis: Application to the study of the diauxic shift in the yeast Saccharomyces cerevisiae. Proteomics 2004, 4, 364–373. [Google Scholar]
- Kolkman, A.; Daran-Lapujade, P.; Fullaondo, A.; Olsthoorn, M.M.; Pronk, J.T.; Slijper, M.; Heck, A.J. Proteome analysis of yeast response to various nutrient limitations. Mol. Syst. Biol. 2006, 2. [Google Scholar] [CrossRef]
- Francesca, G.; Francesca, M.; Tania, G.; Marina, B.; Maurizio, S.; Alessandra, M. Effect of different glucose concentrations on proteome of Saccharomyces cerevisiae. Biochim. Biophys. Acta 2010, 1804, 1516–1525. [Google Scholar]
- De Groot, M.J.; Daran-Lapujade, P.; van Breukelen, B.; Knijnenburg, T.A.; de Hulster, E.A.; Reinders, M.J.; Pronk, J.T.; Heck, A.J.; Slijper, M. Quantitative proteomics and transcriptomics of anaerobic and aerobic yeast cultures reveals post-transcriptional regulation of key cellular processes. Microbiology 2007, 153, 3864–3878. [Google Scholar]
- Usaite, R.; Wohlschlegel, J.; Venable, J.D.; Park, S.K.; Nielsen, J.; Olsson, L.; Yates Iii, J.R. Characterization of global yeast quantitative proteome data generated from the wild-type and glucose repression saccharomyces cerevisiae strains: The comparison of two quantitative methods. J. Proteome Res. 2008, 7, 266–275. [Google Scholar]
- Costenoble, R.; Picotti, P.; Reiter, L.; Stallmach, R.; Heinemann, M.; Sauer, U.; Aebersold, R. Comprehensive quantitative analysis of central carbon and amino-acid metabolism in Saccharomyces cerevisiae under multiple conditions by targeted proteomics. Mol. Syst. Biol. 2011, 7, 464. [Google Scholar]
- Kolkman, A.; Olsthoorn, M.M.; Heeremans, C.E.; Heck, A.J.; Slijper, M. Comparative proteome analysis of Saccharomyces cerevisiae grown in chemostat cultures limited for glucose or ethanol. Mol. Cell Proteomics 2005, 4, 1–11. [Google Scholar]
- Pham, T.K.; Chong, P.K.; Gan, C.S.; Wright, P.C. Proteomic analysis of Saccharomyces cerevisiae under high gravity fermentation conditions. J. Proteome Res. 2006, 5, 3411–3419. [Google Scholar]
- Klein, C.J.; Olsson, L.; Nielsen, J. Glucose control in Saccharomyces cerevisiae: The role of Mig1 in metabolic functions. Microbiology 1998, 144, 13–24. [Google Scholar]
- Yin, Z.; Wilson, S.; Hauser, N.C.; Tournu, H.; Hoheisel, J.D.; Brown, A.J. Glucose triggers different global responses in yeast, depending on the strength of the signal, and transiently stabilizes ribosomal protein mRNAs. Mol. Microbiol. 2003, 48, 713–724. [Google Scholar]
- Warner, J.R. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 1999, 24, 437–440. [Google Scholar]
- Venema, J.; Tollervey, D. Ribosome synthesis in Saccharomyces cerevisiae. Annu. Rev. Genet. 1999, 33, 261–311. [Google Scholar]
- Planta, R.J.; Goncalves, P.M.; Mager, W.H. Global regulators of ribosome biosynthesis in yeast. Biochem. Cell Biol. 1995, 73, 825–834. [Google Scholar]
- Goncalves, P.M.; Griffioen, G.; Minnee, R.; Bosma, M.; Kraakman, L.S.; Mager, W.H.; Planta, R.J. Transcription activation of yeast ribosomal protein genes requires additional elements apart from binding sites for Abf1p or Rap1p. Nucleic Acids Res. 1995, 23, 1475–1480. [Google Scholar]
- Newcomb, L.L.; Diderich, J.A.; Slattery, M.G.; Heideman, W. Glucose regulation of Saccharomyces cerevisiae cell cycle genes. Eukaryot. Cell 2003, 2, 143–149. [Google Scholar]
- Goncalves, P.M.; Griffioen, G.; Bebelman, J.P.; Planta, R.J. Signalling pathways leading to transcriptional regulation of genes involved in the activation of glycolysis in yeast. Mol. Microbiol. 1997, 25, 483–493. [Google Scholar]
- De Groot, E.; Bebelman, J.P.; Mager, W.H.; Planta, R.J. Very low amounts of glucose cause repression of the stress-responsive gene HSP12 in Saccharomyces cerevisiae. Microbiology 2000, 146, 367–375. [Google Scholar]
- Griffioen, G.; Mager, W.H.; Planta, R.J. Nutritional upshift response of ribosomal protein gene transcription in Saccharomyces cerevisiae. FEMS Microbiol. Lett. 1994, 123, 137–144. [Google Scholar]
- Sierkstra, L.N.; Sillje, H.H.; Verbakel, J.M.; Verrips, C.T. The glucose-6-phosphate-isomerase reaction is essential for normal glucose repression in Saccharomyces cerevisiae. Eur. J. Biochem. 1993, 214, 121–127. [Google Scholar]
- Moore, P.A.; Sagliocco, F.A.; Wood, R.M.; Brown, A.J. Yeast glycolytic mRNAs are differentially regulated. Mol. Cell Biol. 1991, 11, 5330–5337. [Google Scholar]
- Sierkstra, L.N.; Nouwen, N.P.; Verbakel, J.M.; Verrips, C.T. Regulation of glycolytic enzymes and the Crabtree effect in galactose-limited continuous cultures of Saccharomyces cerevisiae. Yeast 1993, 9, 787–795. [Google Scholar]
- Beullens, M.; Mbonyi, K.; Geerts, L.; Gladines, D.; Detremerie, K.; Jans, A.W.; Thevelein, J.M. Studies on the mechanism of the glucose-induced cAMP signal in glycolysis and glucose repression mutants of the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 1988, 172, 227–231. [Google Scholar]
- Lee, F.J.; Lin, L.W.; Smith, J.A. A glucose-repressible gene encodes acetyl-CoA hydrolase from Saccharomyces cerevisiae. J. Biol. Chem. 1990, 265, 7413–7418. [Google Scholar]
- Elbing, K.; Stahlberg, A.; Hohmann, S.; Gustafsson, L. Transcriptional responses to glucose at different glycolytic rates in Saccharomyces cerevisiae. Eur. J. Biochem. 2004, 271, 4855–4864. [Google Scholar]
- Cohen, R.; Holland, J.P.; Yokoi, T.; Holland, M.J. Identification of a regulatory region that mediates glucose-dependent induction of the Saccharomyces cerevisiae enolase gene ENO2. Mol. Cell Biol. 1986, 6, 2287–2297. [Google Scholar]
- Lascaris, R.; Piwowarski, J.; van der Spek, H.; Teixeira de Mattos, J.; Grivell, L.; Blom, J. Overexpression of HAP4 in glucose-derepressed yeast cells reveals respiratory control of glucose-regulated genes. Microbiology 2004, 150, 929–934. [Google Scholar]
- Scheffler, I.E.; de la Cruz, B.J.; Prieto, S. Control of mRNA turnover as a mechanism of glucose repression in Saccharomyces cerevisiae. Int. J. Biochem. Cell Biol. 1998, 30, 1175–1193. [Google Scholar]
- Gancedo, J.M. Carbon catabolite repression in yeast. Eur. J. Biochem. 1992, 206, 297–313. [Google Scholar] [Green Version]
- Yin, Z.; Hatton, L.; Brown, A.J. Differential post-transcriptional regulation of yeast mRNAs in response to high and low glucose concentrations. Mol. Microbiol. 2000, 35, 553–565. [Google Scholar]
- Regelmann, J.; Schule, T.; Josupeit, F.S.; Horak, J.; Rose, M.; Entian, K.D.; Thumm, M.; Wolf, D.H. Catabolite degradation of fructose-1,6-bisphosphatase in the yeast Saccharomyces cerevisiae: A genome-wide screen identifies eight novel GID genes and indicates the existence of two degradation pathways. Mol. Biol. Cell 2003, 14, 1652–1663. [Google Scholar]
- Gancedo, J.M.; Gancedo, C. Inactivation of gluconeogenic enzymes in glycolytic mutants of Saccharomyces cerevisiae. Eur. J. Biochem. 1979, 101, 455–460. [Google Scholar]
- Entian, K.D.; Droll, L.; Mecke, D. Studies on rapid reversible and non-reversible inactivation of fructose-1,6-bisphosphatase and malate dehydrogenase in wild-type and glycolytic block mutants of Saccharomyces cerevisiae. Arch. Microbiol. 1983, 134, 187–192. [Google Scholar]
- Entian, K.D.; Frohlich, K.U.; Mecke, D. Regulation of enzymes and isoenzymes of carbohydrate metabolism in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1984, 799, 181–186. [Google Scholar]
- Holzer, H. Proteolytic catabolite inactivation in Saccharomyces cerevisiae. Revis. Biol. Cel. 1989, 21, 305–319. [Google Scholar]
- Brown, C.R.; Chiang, H.L. A selective autophagy pathway that degrades gluconeogenic enzymes during catabolite inactivation. Commun. Integr. Biol. 2009, 2, 177–183. [Google Scholar]
- Brown, C.R.; Cui, D.Y.; Hung, G.G.; Chiang, H.L. Cyclophilin A mediates Vid22p function in the import of fructose-1,6-bisphosphatase into Vid vesicles. J. Biol. Chem. 2001, 276, 48017–48026. [Google Scholar]
- Brown, C.R.; Dunton, D.; Chiang, H.L. The vacuole import and degradation pathway utilizes early steps of endocytosis and actin polymerization to deliver cargo proteins to the vacuole for degradation. J. Biol. Chem. 2010, 285, 1516–1528. [Google Scholar]
- Brown, C.R.; Hung, G.C.; Dunton, D.; Chiang, H.L. The TOR complex 1 is distributed in endosomes and in retrograde vesicles that form from the vacuole membrane and plays an important role in the vacuole import and degradation pathway. J. Biol. Chem. 2010, 285, 23359–23370. [Google Scholar]
- Brown, C.R.; McCann, J.A.; Chiang, H.L. The heat shock protein Ssa2p is required for import of fructose-1, 6-bisphosphatase into Vid vesicles. J. Cell Biol. 2000, 150, 65–76. [Google Scholar]
- Brown, C.R.; McCann, J.A.; Hung, G.G.; Elco, C.P.; Chiang, H.L. Vid22p, a novel plasma membrane protein, is required for the fructose-1,6-bisphosphatase degradation pathway. J. Cell Sci. 2002, 115, 655–666. [Google Scholar]
- Brown, C.R.; Wolfe, A.B.; Cui, D.; Chiang, H.L. The vacuolar import and degradation pathway merges with the endocytic pathway to deliver fructose-1,6-bisphosphatase to the vacuole for degradation. J. Biol. Chem. 2008, 283, 26116–26127. [Google Scholar]
- Hung, G.C.; Brown, C.R.; Wolfe, A.B.; Liu, J.; Chiang, H.L. Degradation of the gluconeogenic enzymes fructose-1,6-bisphosphatase and malate dehydrogenase is mediated by distinct proteolytic pathways and signaling events. J. Biol. Chem. 2004, 279, 49138–49150. [Google Scholar]
- Polakis, E.S.; Bartley, W. Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem. J. 1965, 97, 284–297. [Google Scholar]
- Polakis, E.S.; Bartley, W.; Meek, G.A. Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem. J. 1965, 97, 298–302. [Google Scholar]
- Satrustegui, J.; Machado, A. The synthesis of yeast matrix mitochondrial enzymes is regulated by different levels of mitochondrial function. Arch. Biochem. Biophys. 1977, 184, 355–363. [Google Scholar]
- Chiang, H.L.; Schekman, R. Regulated import and degradation of a cytosolic protein in the yeast vacuole. Nature 1991, 350, 313–318. [Google Scholar]
- Hoffman, M.; Chiang, H.L. Isolation of degradation-deficient mutants defective in the targeting of fructose-1,6-bisphosphatase into the vacuole for degradation in Saccharomyces cerevisiae. Genetics 1996, 143, 1555–1566. [Google Scholar]
- Schork, S.M.; Thumm, M.; Wolf, D.H. Catabolite inactivation of fructose-1,6-bisphosphatase of Saccharomyces cerevisiae. Degradation occurs via the ubiquitin pathway. J. Biol. Chem. 1995, 270, 26446–26450. [Google Scholar]
- Schule, T.; Rose, M.; Entian, K.D.; Thumm, M.; Wolf, D.H. Ubc8p functions in catabolite degradation of fructose-1, 6-bisphosphatase in yeast. EMBO J. 2000, 19, 2161–2167. [Google Scholar]
- Gancedo, C. Inactivation of fructose-1,6-diphosphatase by glucose in yeast. J. Bacteriol. 1971, 107, 401–405. [Google Scholar]
- Pohlig, G.; Holzer, H. Phosphorylation and inactivation of yeast fructose-1,6-bisphosphatase by cyclic AMP-dependent protein kinase from yeast. J. Biol. Chem. 1985, 260, 13818–13823. [Google Scholar]
- Rittenhouse, J.; Moberly, L.; Marcus, F. Phosphorylation in vivo of yeast (Saccharomyces cerevisiae) fructose-1,6-bisphosphatase at the cyclic AMP-dependent site. J. Biol. Chem. 1987, 262, 10114–10119. [Google Scholar]
- Jiang, Y.; Davis, C.; Broach, J.R. Efficient transition to growth on fermentable carbon sources in Saccharomyces cerevisiae requires signaling through the Ras pathway. EMBO J. 1998, 17, 6942–6951. [Google Scholar]
- Gancedo, J.M.; Mazon, M.J.; Gancedo, C. Inactivation and phosphorylation of yeast fructose 1,6-bisphosphatase. Biochem. Soc. Trans. 1982, 10, 326–327. [Google Scholar]
- Lamponi, S.; Galassi, C.; Tortora, P.; Guerritore, A. Glucose-induced degradation of yeast fructose-1,6-bisphosphatase requires additional triggering events besides protein phosphorylation. FEBS Lett. 1987, 216, 265–269. [Google Scholar]
- Mazon, M.J.; Gancedo, J.M.; Gancedo, C. Inactivation of yeast fructose-1,6-bisphosphatase. In vivo phosphorylation of the enzyme. J. Biol. Chem. 1982, 257, 1128–1130. [Google Scholar]
- Toyoda, Y.; Fujii, H.; Miwa, I.; Okuda, J.; Sy, J. Anomeric specificity of glucose effect on cAMP, fructose 1,6-bisphosphatase, and trehalase in yeast. Biochem. Biophys. Res. Commun. 1987, 143, 212–217. [Google Scholar]
- Huang, P.H.; Chiang, H.L. Identification of novel vesicles in the cytosol to vacuole protein degradation pathway. J. Cell Biol. 1997, 136, 803–810. [Google Scholar]
- Shieh, H.L.; Chen, Y.; Brown, C.R.; Chiang, H.L. Biochemical analysis of fructose-1,6-bisphosphatase import into vacuole import and degradation vesicles reveals a role for UBC1 in vesicle biogenesis. J. Biol. Chem. 2001, 276, 10398–10406. [Google Scholar]
- Chiang, M.C.; Chiang, H.L. Vid24p, a novel protein localized to the fructose-1,6-bisphosphatase-containing vesicles, regulates targeting of fructose-1,6-bisphosphatase from the vesicles to the vacuole for degradation. J. Cell Biol. 1998, 140, 1347–1356. [Google Scholar]
- Giardina, B.J.; Dunton, D.; Chiang, H.L. Vid28 protein is required for the association of vacuole import and degradation (Vid) vesicles with actin patches and the retention of Vid vesicle proteins in the intracellular fraction. J. Biol. Chem. 2013, 288, 11636–11648. [Google Scholar]
- Alibhoy, A.A.; Giardina, B.J.; Dunton, D.D.; Chiang, H.L. Vid30 is required for the association of Vid vesicles and actin patches in the vacuole import and degradation pathway. Autophagy 2012, 8, 29–46. [Google Scholar]
- Giardina, B.J.; Chiang, H.L. The key gluconeogenic enzyme fructose-1,6-bisphosphatase is secreted during prolonged glucose starvation and is internalized following glucose re-feeding via the non-classical secretory and internalizing pathways in Saccharomyces cerevisiae. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef]
- Giardina, B.J.; Chiang, H.L. Fructose-1,6-bisphosphatase, Malate Dehydrogenase, Isocitrate Lyase, Phosphoenolpyruvate Carboxykinase, Glyceraldehyde-3-phosphate Dehydrogenase, and Cyclophilin A are secreted in Saccharomyces cerevisiae grown in low glucose. Commun. Integr. Biol. 2013, 6, e27216. [Google Scholar]
- Alibhoy, A.A.; Giardina, B.J.; Dunton, D.D.; Chiang, H.L. Vps34p is required for the decline of extracellular fructose-1,6-bisphosphatase in the vacuole import and degradation pathway. J. Biol. Chem. 2012, 287, 33080–33093. [Google Scholar]
- Giardina, B.J.; Stanley, B.A.; Chiang, H.L. Glucose induces rapid changes in the secretome of Saccharomyces cerevisiae. Proteome Sci. 2014, 12. [Google Scholar] [CrossRef]
- Lamonica, J.M.; Wagner, M.; Eschenbrenner, M.; Williams, L.E.; Miller, T.L.; Patra, G.; DelVecchio, V.G. Comparative secretome analyses of three Bacillus anthracis strains with variant plasmid contents. Infect. Immun. 2005, 73, 3646–3658. [Google Scholar]
- Schaumburg, J.; Diekmann, O.; Hagendorff, P.; Bergmann, S.; Rohde, M.; Hammerschmidt, S.; Jansch, L.; Wehland, J.; Karst, U. The cell wall subproteome of Listeria monocytogenes. Proteomics 2004, 4, 2991–3006. [Google Scholar]
- Cass, C.L.; Johnson, J.R.; Califf, L.L.; Xu, T.; Hernandez, H.J.; Stadecker, M.J.; Yates, J.R., 3rd; Williams, D.L. Proteomic analysis of Schistosoma mansoni egg secretions. Mol. Biochem. Parasitol. 2007, 155, 84–93. [Google Scholar]
- Sotillo, J.; Valero, M.L.; Sanchez Del Pino, M.M.; Fried, B.; Esteban, J.G.; Marcilla, A.; Toledo, R. Excretory/secretory proteome of the adult stage of Echinostoma caproni. Parasitol. Res. 2010, 107, 691–697. [Google Scholar]
- Zheng, M.; Hu, K.; Liu, W.; Hu, X.; Hu, F.; Huang, L.; Wang, P.; Hu, Y.; Huang, Y.; Li, W.; et al. Proteomic analysis of excretory secretory products from Clonorchis sinensis adult worms: Molecular characterization and serological reactivity of a excretory-secretory antigen-fructose-1,6-bisphosphatase. Parasitol. Res. 2011, 109, 737–744. [Google Scholar]
- Albuquerque, P.C.; Nakayasu, E.S.; Rodrigues, M.L.; Frases, S.; Casadevall, A.; Zancope-Oliveira, R.M.; Almeida, I.C.; Nosanchuk, J.D. Vesicular transport in Histoplasma capsulatum: An effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008, 10, 1695–1710. [Google Scholar]
- Lee, H.S.; Jeong, J.; Lee, K.J. Characterization of vesicles secreted from insulinoma NIT-1 cells. J. Proteome Res. 2009, 8, 2851–2862. [Google Scholar]
- Rowe, J.D.; Harbertson, J.F.; Osborne, J.P.; Freitag, M.; Lim, J.; Bakalinsky, A.T. Systematic identification of yeast proteins extracted into model wine during aging on the yeast lees. J. Agric. Food Chem. 2010, 58, 2337–2346. [Google Scholar]
- Oliveira, D.L.; Nakayasu, E.S.; Joffe, L.S.; Guimaraes, A.J.; Sobreira, T.J.; Nosanchuk, J.D.; Cordero, R.J.; Frases, S.; Casadevall, A.; Almeida, I.C.; Nimrichter, L.; Rodrigues, M.L. Characterization of yeast extracellular vesicles: Evidence for the participation of different pathways of cellular traffic in vesicle biogenesis. PLoS One 2010, 5, e11113. [Google Scholar] [CrossRef]
- Shin, Y.K.; Yoo, B.C.; Hong, Y.S.; Chang, H.J.; Jung, K.H.; Jeong, S.Y.; Park, J.G. Upregulation of glycolytic enzymes in proteins secreted from human colon cancer cells with 5-fluorouracil resistance. Electrophoresis 2009, 30, 2182–2192. [Google Scholar]
- Yamashita, R.; Fujiwara, Y.; Ikari, K.; Hamada, K.; Otomo, A.; Yasuda, K.; Noda, M.; Kaburagi, Y. Extracellular proteome of human hepatoma cell, HepG2 analyzed using two-dimensional liquid chromatography coupled with tandem mass spectrometry. Mol. Cell Biochem. 2007, 298, 83–92. [Google Scholar]
- Pavlou, M.P.; Diamandis, E.P. The cancer cell secretome: A good source for discovering biomarkers? J. Proteomics 2010, 73, 1896–1906. [Google Scholar]
- Martinez-Gomariz, M.; Perumal, P.; Mekala, S.; Nombela, C.; Chaffin, W.L.; Gil, C. Proteomic analysis of cytoplasmic and surface proteins from yeast cells, hyphae, and biofilms of Candida albicans. Proteomics 2009, 9, 2230–2252. [Google Scholar]
- Giardina, B.J.; Stanley, B.A.; Chiang, H.L. Comparative Proteomic Analysis of Transition of Saccharomyces cerevisiae from Glucose-Deficient Medium to Glucose-Rich Medium. Proteome Sci. 2012, 10, 40:1–40:21. [Google Scholar]
- Cleves, A.E.; Cooper, D.N.; Barondes, S.H.; Kelly, R.B. A new pathway for protein export in Saccharomyces cerevisiae. J. Cell Biol. 1996, 133, 1017–1026. [Google Scholar]
- Chaffin, W.L.; Lopez-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martinez, J.P. Cell wall and secreted proteins of Candida albicans: Identification, function, and expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar]
- Nombela, C.; Gil, C.; Chaffin, W.L. Non-conventional protein secretion in yeast. Trends Microbiol. 2006, 14, 15–21. [Google Scholar]
- Mrsa, V.; Seidl, T.; Gentzsch, M.; Tanner, W. Specific labelling of cell wall proteins by biotinylation. Identification of four covalently linked O-mannosylated proteins of Saccharomyces cerevisiae. Yeast 1997, 13, 1145–1154. [Google Scholar] [CrossRef]
- Klis, F.M.; de Jong, M.; Brul, S.; de Groot, P.W. Extraction of cell surface-associated proteins from living yeast cells. Yeast 2007, 24, 253–258. [Google Scholar]
- Giardina, B.J.; Stein, K.; Chiang, H.L. The endocytosis gene END3 is essential for the glucose-induced rapid decline of small vesicles in the extracellular fraction in Saccharomyces cerevisiae. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Insenser, M.R.; Hernaez, M.L.; Nombela, C.; Molina, M.; Molero, G.; Gil, C. Gel and gel-free proteomics to identify Saccharomyces cerevisiae cell surface proteins. J. Proteomics 2010, 73, 1183–1195. [Google Scholar] [CrossRef]
- Mathivanan, S.; Ji, H.; Simpson, R.J. Exosomes: Extracellular organelles important in intercellular communication. J. Proteomics 2010, 73, 1907–1920. [Google Scholar] [CrossRef]
- Mathivanan, S.; Simpson, R.J. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics 2009, 9, 4997–5000. [Google Scholar]
- Simpson, R.J.; Lim, J.W.; Moritz, R.L.; Mathivanan, S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev. Proteomics 2009, 6, 267–283. [Google Scholar] [CrossRef]
- Cui, D.Y.; Brown, C.R.; Chiang, H.L. The type 1 phosphatase Reg1p-Glc7p is required for the glucose-induced degradation of fructose-1,6-bisphosphatase in the vacuole. J. Biol. Chem. 2004, 279, 9713–9724. [Google Scholar]
- Liu, J.; Brown, C.R.; Chiang, H.L. Degradation of the gluconeogenic enzyme fructose-1,6-bisphosphatase is dependent on the vacuolar ATPase. Autophagy 2005, 1, 146–156. [Google Scholar] [CrossRef]
- Brown, C.R.; Liu, J.; Hung, G.C.; Carter, D.; Cui, D.; Chiang, H.L. The Vid vesicle to vacuole trafficking event requires components of the SNARE membrane fusion machinery. J. Biol. Chem. 2003, 278, 25688–25699. [Google Scholar]
- Atay, S.; Godwin, A.K. Tumor-derived exosomes: A message delivery system for tumor progression. Commun. Integr. Biol. 2014, 7. [Google Scholar] [CrossRef]
- Christianson, H.C.; Svensson, K.J.; Belting, M. Exosome and microvesicle mediated phene transfer in mammalian cells. Semin. Cancer Biol. 2014. [Google Scholar] [CrossRef]
- Khalyfa, A.; Gozal, D. Exosomal miRNAs as potential biomarkers of cardiovascular risk in children. J. Transl. Med. 2014, 12, 162:1–162:12. [Google Scholar]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29C, 116–125. [Google Scholar]
- Sun, Y.; Liu, J. Potential of Cancer Cell-Derived Exosomes in Clinical Application: A Review of Recent Research Advances. Clin. Ther. 2014, 36, 863–872. [Google Scholar]
- Tickner, J.A.; Urquhart, A.J.; Stephenson, S.A.; Richard, D.J.; O’Byrne, K.J. Functions and therapeutic roles of exosomes in cancer. Front. Oncol. 2014, 4. [Google Scholar] [CrossRef]
- Ye, S.B.; Li, Z.L.; Luo, D.H.; Huang, B.J.; Chen, Y.S.; Zhang, X.S.; Cui, J.; Zeng, Y.X.; Li, J. Tumor-derived exosomes promote tumor progression and T-cell dysfunction through the regulation of enriched exosomal microRNAs in human nasopharyngeal carcinoma. Oncotarget 2014, 5, 5439–5452. [Google Scholar]
- Tian, T.; Zhu, Y.L.; Zhou, Y.Y.; Liang, G.F.; Wang, Y.Y.; Hu, F.H.; Xiao, Z.D. Exosome Uptake through Clathrin-mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery. J. Biol. Chem. 2014, 289, 22258–22267. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stein, K.; Chiang, H.-L. Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces cerevisiae. Membranes 2014, 4, 608-629. https://doi.org/10.3390/membranes4030608
Stein K, Chiang H-L. Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces cerevisiae. Membranes. 2014; 4(3):608-629. https://doi.org/10.3390/membranes4030608
Chicago/Turabian StyleStein, Kathryn, and Hui-Ling Chiang. 2014. "Exocytosis and Endocytosis of Small Vesicles across the Plasma Membrane in Saccharomyces cerevisiae" Membranes 4, no. 3: 608-629. https://doi.org/10.3390/membranes4030608




