Membranes in Lithium Ion Batteries
Abstract
:1. Introduction
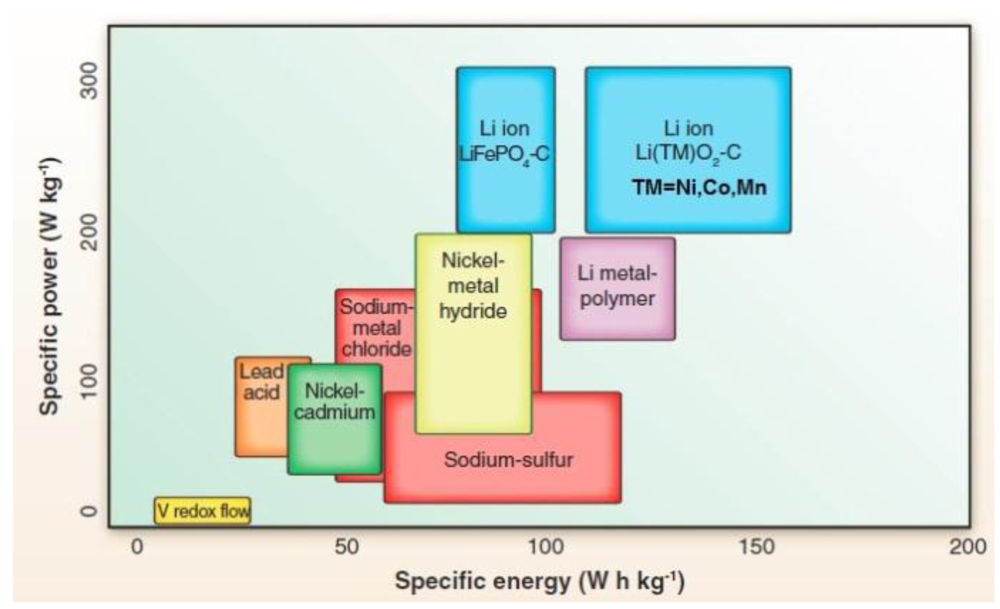
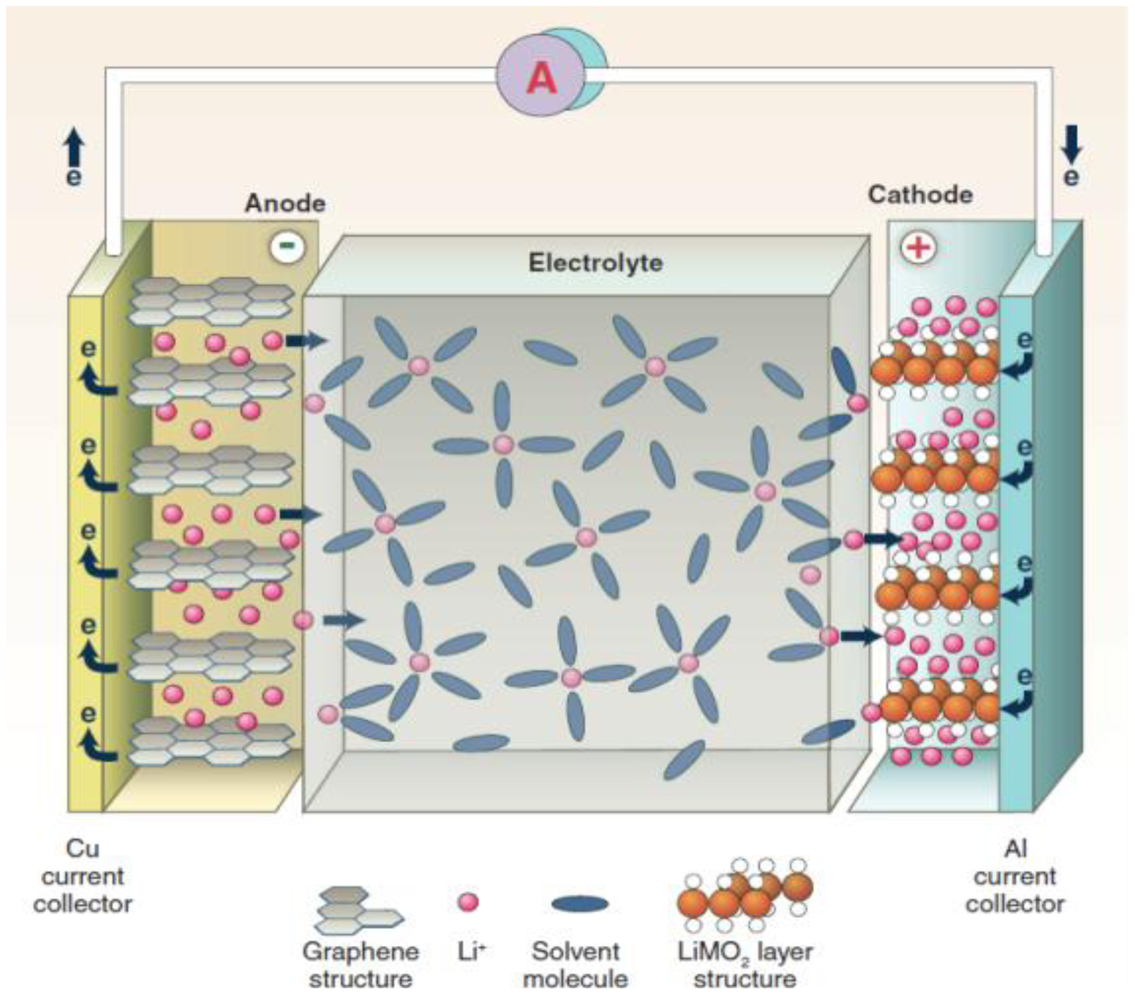
2. Solid Li Ion Conductors
2.1. Ceramic-Glass
2.1.1. Na Super-ionic Conductor (NASICON) Structure
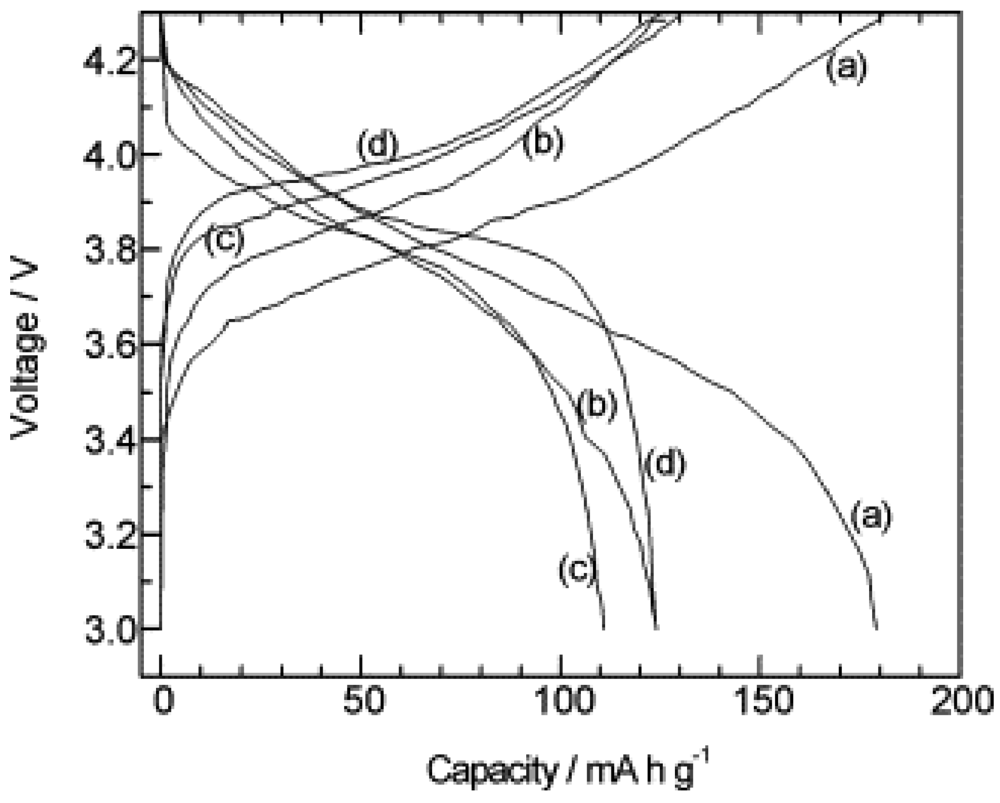
2.1.2. Garnet Structure
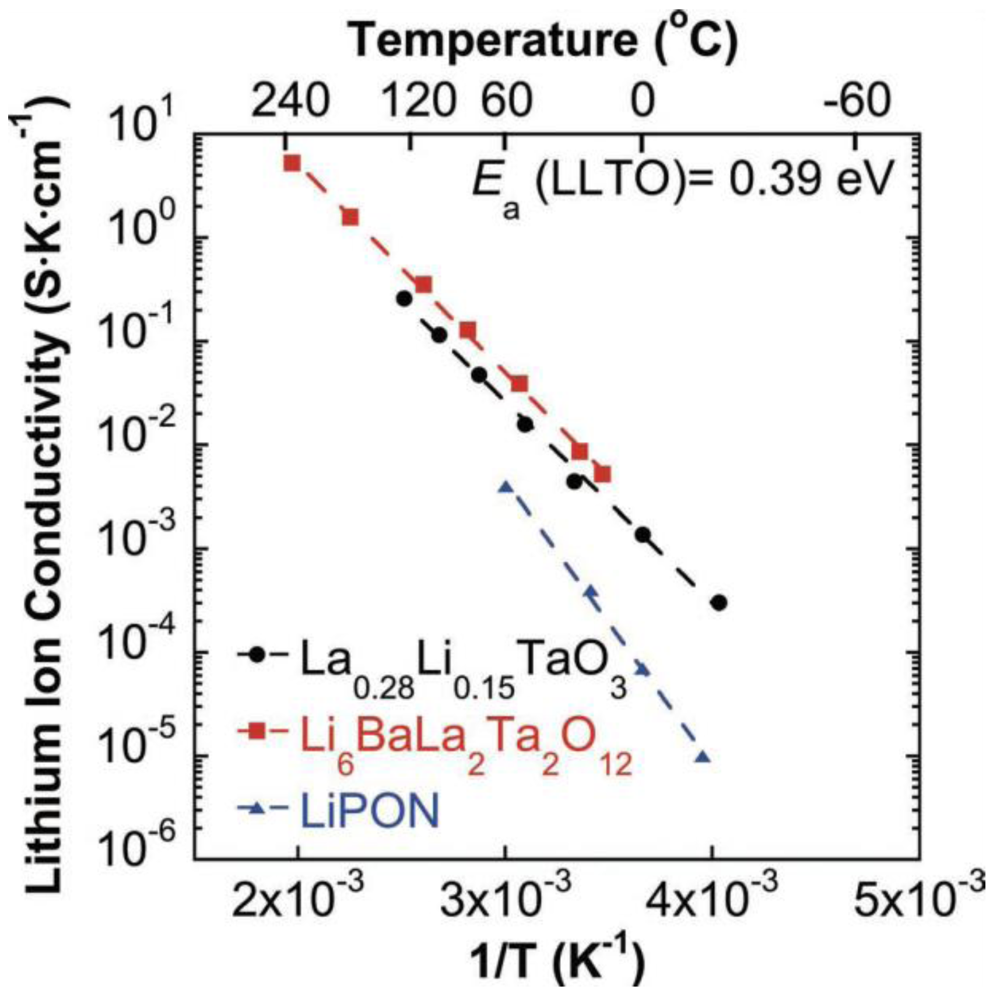
2.1.3. Perovskite Structure
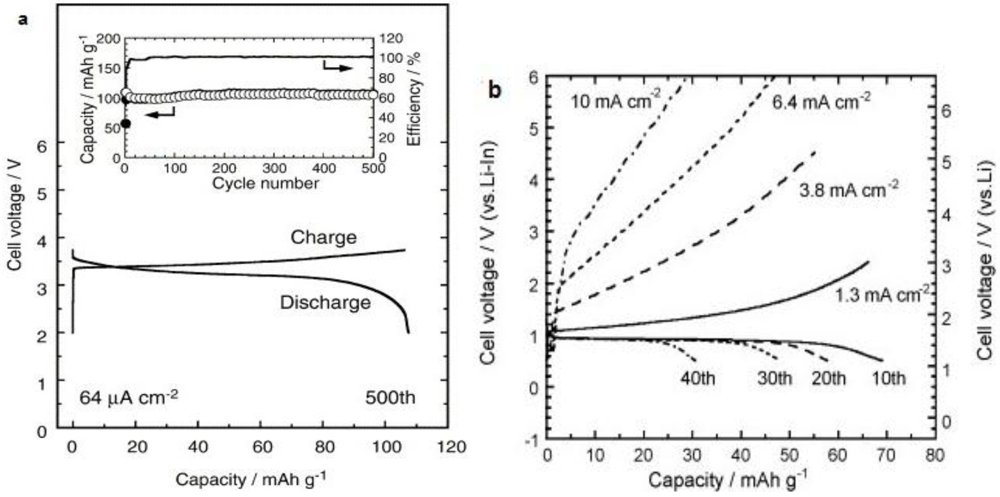
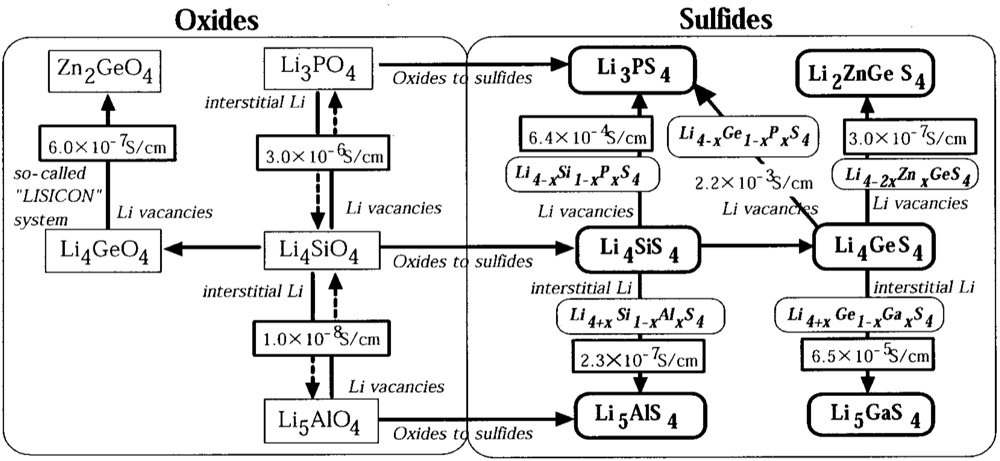
2.1.4. Sulfide Glass
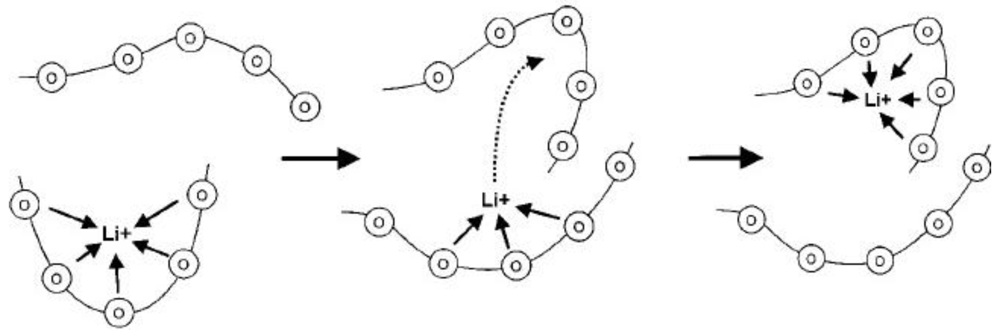
2.2. Solid Polymer Membranes
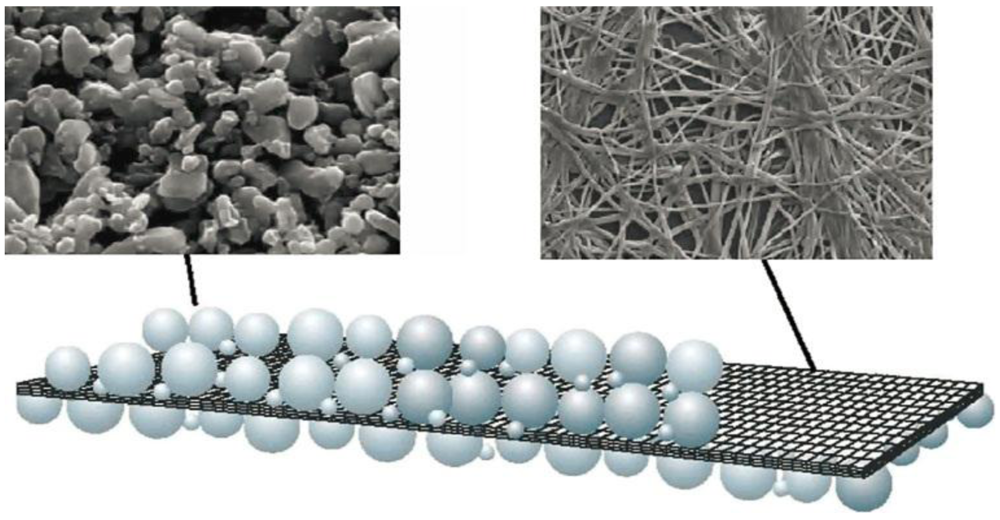
3. Microporous Separators
| Parameter | Target | Note |
|---|---|---|
| Thickness (µm) | <25 | – |
| MacMullin number | <8 | The ratio of the resistance of the separator filled with electrolyte to the resistance of the electrolyte alone |
| Gurley (s) | ~25/mil | The time required for air to pass through the separator |
| Pore size (µm) | <1 | – |
| Porosity (%) | ~40 | – |
| Puncture strength (g/mil) | >300 | – |
| Melt integrity (°C) | >150 | – |
| Chemical stability | Long enough time | – |
| Thermal stability | <5% shrinkage | – |
| Tensile strength | <2% offset at 1,000 psi | – |
| Skew (mm/m) | <2 | – |
3.1. Microporous Membranes
| Manufacturer | Material |
|---|---|
| Celgard LLC | PE, PP, PP/PE/PP |
| Asahi Kasei chemicals | PE, PP, and oxides-modified polyleffin |
| Entek membranes | PE, PP |
| SK energy | PE |
| Tonen | PE, PE-PP |

3.2. Nonwoven Films
| Technique | Material | Film properties |
|---|---|---|
| Paper-making process | Polyolefin, PA, PTFE, PVDF, PVC, polyester, etc. | High porosity (60%–80%), large pore size (20–50 µm) |
| Melt-blowing method | ||
| Electrospinning |
4. Gel Polymer Electrolytes
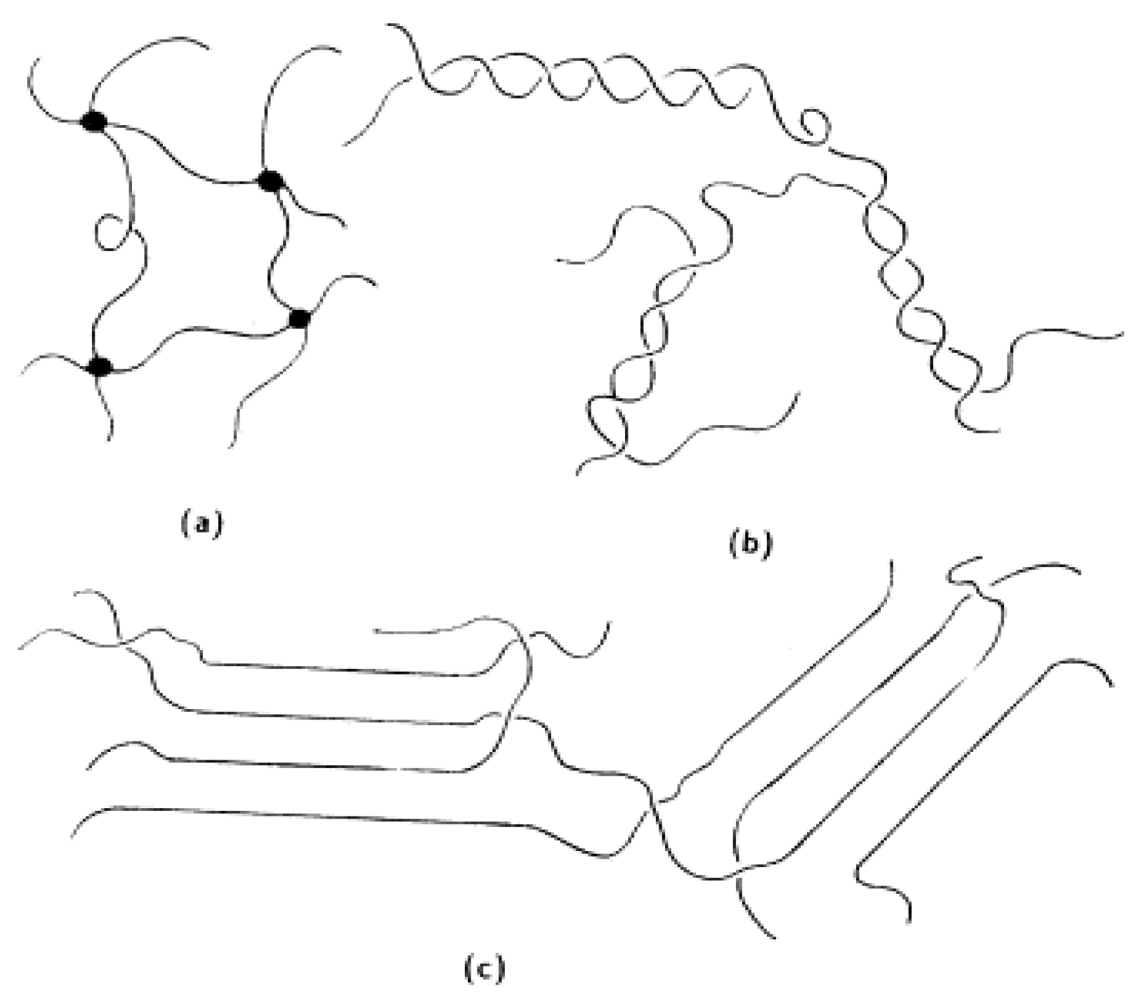

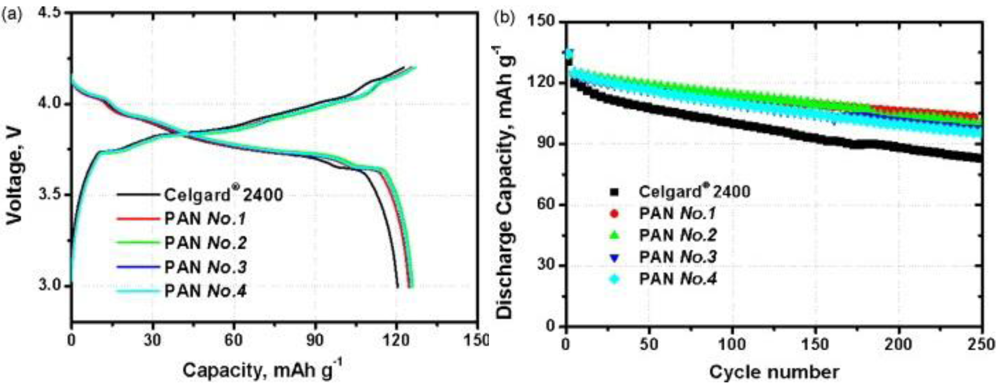
5. Conclusions
Acknowledgments
References
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef]
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Solid state lithium ion conductors: Design considerations by thermodynamic approach. Ionics 2002, 8, 281–292. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Kumar, B. Composite effect in superionically conducting lithium aluminium germanium phosphate based glass-ceramic. J. Power Sources 2008, 185, 480–485. [Google Scholar] [CrossRef]
- Kumar, B.; Thomas, D.; Kumar, J. Space-charge-mediated superionic transport in lithium ion conducting glass-ceramics. J. Electrochem. Soc. 2009, 156, A506–A513. [Google Scholar] [CrossRef]
- Xu, X.; Wen, Z.; Yang, X.; Zhang, J.; Gu, Z. High lithium ion conductivity glass-ceramics in Li2O-Al2O3-TiO2-P2O5 from nanoscaled glassy powders by mechanical milling. Solid State Ionics 2006, 177, 2611–2615. [Google Scholar] [CrossRef]
- Kosova, N.V.; Devyatkina, E.T.; Stepanov, A.P.; Buzlukov, A.L. Lithium conductivity and lithium diffusion in NASICON-type Li1+xTi2−xAlx(PO4)3 (x = 0; 0.3) prepared by mechanical activation. Ionics 2008, 14, 303–311. [Google Scholar] [CrossRef]
- Hasegawa, S.; Imanishi, N.; Zhang, T.; Xie, J.; Hirano, A.; Takeda, Y.; Yamamoto, O. Study on lithium/air secondary batteries—Stability of NASICON-type lithium ion conducting glass-ceramics with water. J. Power Sources 2009, 189, 371–377. [Google Scholar] [CrossRef]
- Takahashi, K.; Ohmura, J.; Im, D.; Lee, D.J.; Zhang, T.; Imanishi, N.; Hirano, A.; Phillipps, M.B.; Takeda, Y.; Yamamoto, O. A super high lithium ion conducting solid electrolyte of grain boundary modified Li1.4Ti1.6Al0.4(PO4)3. J. Electrochem. Soc. 2012, 159, A342–A348. [Google Scholar]
- Zhang, T.; Imanishi, N.; Hasegawa, S.; Hirano, A.; Xie, J.; Takeda, Y.; Yamamoto, O.; Sammes, N. Li/polymer electrolyte/water stable lithium-conducting glass ceramics composite for lithium—Air secondary batteries with an aqueous electrolyte. J. Electrochem. Soc. 2008, 155, A965–A969. [Google Scholar] [CrossRef]
- Xie, J.; Imanishi, N.; Zhang, T.; Hirano, A.; Takeda, Y.; Yamamoto, O. Li-ion transport in all-solid-state lithium batteries with LiCoO2 using NASICON-type glass ceramic electrolytes. J. Power Sources 2009, 189, 365–370. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Lithium ion conductivity of Li5+xBaxLa3−xTa2O12 (x=0-2) with garnet-related structure in dependence of the barium content. Ionics 2007, 13, 195–203. [Google Scholar] [CrossRef]
- Awaka, J.; Kijima, N.; Takahashi, Y.; Hayakawa, H.; Akimoto, J. Synthesis and crystallographic studies of garnet-related lithium-ion conductors Li6CaLa2Ta2O12 and Li6BaLa2Ta2O12. Solid State Ionics 2009, 180, 602–606. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Lattice parameter and sintering temperature dependence of bulk and grain-boundary conduction of garnet-like solid Li-electrolytes. J. Electrochem. Soc. 2008, 155, A90–A101. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Effect of lithium ion content on the lithium ion conductivity of the garnet-like structure Li5+xBaLa2Ta2O11.5+0.5x (x = 0-2). Appl. Phys. A 2008, 91, 615–620. [Google Scholar] [CrossRef]
- Thangadurai, V.; Weppner, W. Li6ALa2Ta2O12 (A=Sr, Ba): Novel garnet-like oxides for fast lithium ion conduction. Adv. Func. Mater. 2005, 15, 107–112. [Google Scholar] [CrossRef]
- Thangadurai, V.; Adams, S.; Weppner, W. Crystal structure revision and identification of Li+-ion migration pathways in the garnet-like Li5La3M2O12 (M = Nb, Ta) oxides. Chem. Mater. 2004, 16, 2998–3006. [Google Scholar] [CrossRef]
- Mei, A.; Wang, X.; Feng, Y.; Zhao, S.; Li, G.; Geng, H.; Lin, Y.; Nan, C. Enhanced ionic transport in lithium lanthanum titanium oxide solid state electrolyte by introducing silica. Solid State Ionics 2008, 179, 2255–2259. [Google Scholar] [CrossRef]
- Jimenez, R.; Rivera, A.; Varez, A.; Sanz, J. Li mobility in Li0.5−xNaxLa0.5TiO3 perovskites (0 ≤ x ≤ 0.5): Influence of structural and compositional parameters. Solid State Ionics 2009, 180, 1362–1371. [Google Scholar] [CrossRef]
- Bohnke, O. The fast lithium-ion conducting oxides Li3xLa2/3−xTiO3 from fundamentals to application. Solid State Ionics 2008, 179, 9–15. [Google Scholar] [CrossRef]
- Zou, Y.; Inoue, N. Structure and lithium ionic conduction mechanism in La4/3−yLi3yTi2O6. Ionics 2005, 11, 333–342. [Google Scholar] [CrossRef]
- Stramare, S.; Thangadurai, V.; Weppner, W. Lithium lanthanum titanates: A review. Chem. Mater. 2003, 15, 3974–3990. [Google Scholar] [CrossRef]
- Ihlefeld, J.F.; Clem, P.; Doyle, B.; Kotula, P.; Fenton, K.; Apblett, C. Fast lithium-ion conducting thin-film electrolytes integrated directly on flexible substrates for high-power solid-state batteries. Adv. Mater. 2011, 23, 5663–5667. [Google Scholar] [CrossRef]
- Minami, T.; Hayashi, A.; Tatsumisago, M. Recent progress of glass and glass-ceramics as solid electrolytes for lithium secondary batteries. Solid State Ionics 2006, 177, 2715–2720. [Google Scholar] [CrossRef]
- Tatsumisago, M.; Mizuno, F.; Hayashi, A. All-solid-state lithium secondary batteries using sulfide-based glass-ceramic electrolytes. J. Power Sources 2006, 159, 193–199. [Google Scholar] [CrossRef]
- Minami, K.; Hayashi, A.; Ujiie, S.; Tatsumisago, M. Structure and properties of Li2S-P2S5-P2S3 glass and glass-ceramic electrolytes. J. Power Sources 2009, 189, 651–654. [Google Scholar] [CrossRef]
- Mizuno, F.; Ohtomo, T.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. Lithium ion conducting solid electrolytes prepared from Li2S, elemental P and S. Solid State Ionics 2006, 177, 2753–2757. [Google Scholar] [CrossRef]
- Okamoto, H.; Hikazudani, S.; Inazumi, C.; Takeuchi, T.; Tabuchi, M.; Tatsumi, K. Upper voltage and temperature dependencies for an all-solid-state In/LiCoO2 cell using sulfide glass electrolyte. Electrochem. Solid-State Lett. 2008, 11, A97–A100. [Google Scholar] [CrossRef]
- Yao, W.; Martin, S.W. Ionic conductivity of glasses in the MI + M2S + (0.1Ga2S3 + 0.9GeS2) system (M = Li, Na, K and Cs). Solid State Ionics 2008, 178, 1777–1784. [Google Scholar] [CrossRef]
- Nagamedianova, Z.; Hernández, A.; Sánchez, E. Conductivity studies on LiX-Li2S-Sb2S3-P2S5 (X=LiI or Li3PO4) glassy system. Ionics 2006, 12, 315–322. [Google Scholar] [CrossRef]
- Kitaura, H.; Hayashi, A.; Tadanaga, K.; Tatsumisago, M. High-rate performance of all-solid-state lithium secondary batteries using Li4Ti5O12 electrode. J. Power Sources 2009, 189, 145–148. [Google Scholar] [CrossRef]
- Kanno, R.; Murayama, M. Lithium ionic conductor thio-LISICON: The Li2S-GeS2-P2S5 system. J. Electrochem. Soc. 2001, 148, A742–A746. [Google Scholar] [CrossRef]
- Fenton, D.E.; Parker, J.M.; Wright, P.V. Complexes of alkali metal ions with poly(ethylene oxide). Polymer 1973, 14, 589. [Google Scholar]
- Appetecchi, G.B.; Passerini, S. Poly(ethylene oxide)-LiN(SO2CF2CF3)2 polymer electrolytes. J. Electrochem. Soc. 2002, 149, A891–A897. [Google Scholar] [CrossRef]
- Villano, P.; Carewska, M.; Appetecchi, G.B.; Passerini, S. PEO-LiN(SO2CF2CF3)2 polymer electrolytes. J. Electrochem. Soc. 2002, 149, A1282–A1285. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Zane, D.; Scrosati, B. PEO-based electrolyte membranes based on LiBC4O8 salt. J. Electrochem. Soc. 2004, 151, A1369–A1374. [Google Scholar] [CrossRef]
- Shen, C.; Wang, J.; Tang, Z.; Wang, H.; Lian, H.; Zhang, J.; Cao, C. Physicochemical properties of poly(ethylene oxide)-based composite polymer electrolytes with a silane-modified mesoporous silica SBA-15. Electrochimi. Acta 2009, 54, 3490–3494. [Google Scholar]
- An, S.; Jeong, I.; Won, M.; Jeong, E.; Shim, Y. Effect of additives in PEO/PAA/PMAA composite solid polymer electrolytes on the ionic conductivity and Li ion battery performance. J. Appl. Electrochem. 2009, 39, 1573–1578. [Google Scholar] [CrossRef]
- Marzantowicz, M.; Dygas, J.R.; Krok, F.; Tomaszewska, A.; Florjańczyk, Z.; Zygadło-Monikowska, E.; Lapienis, G. Star-branched poly(ethylene oxide) LiN(CF3SO2)2: A promising polymer electrolyte. J. Power Sources 2009, 194, 51–57. [Google Scholar] [CrossRef]
- Fan, L.; Wang, X.; Long, F.; Wang, X. Enhanced ionic conductivities in composite polymer electrolytes by using succinonitrile as a plasticizer. Solid State Ionics 2008, 179, 1772–1775. [Google Scholar] [CrossRef]
- Meyer, W.H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 1998, 10, 439–448. [Google Scholar] [CrossRef]
- Ndeugueu, J.L.; Ikeda, M.; Aniya, M. Correlation between the temperature range of cooperativity and the fragility index in ion conducting polymers. Solid State Ionics 2010, 181, 16–19. [Google Scholar] [CrossRef]
- Chen-Yang, Y.W.; Wang, Y.; Chen, Y.; Li, Y.; Chen, H.; Chiu, H. Influence of silica aerogel on the properties of polyethylene oxide-based nanocomposite polymer electrolytes for lithium battery. J. Power Sources 2008, 182, 340–348. [Google Scholar] [CrossRef]
- Kim, G.T.; Appetecchi, G.; Carewska, M.; Joost, M.; Balducci, A.; Winter, M.; Passerini, S. UV cross-linked, lithium-conducting ternary polymer electrolytes containing ionic liquids. J. Power Sources 2010, 195, 6130–6137. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on the separators of liquid electrolyte Li-ion batteries. J. Power Sources 2007, 164, 351–364. [Google Scholar] [CrossRef]
- Venugopal, G.; Moore, J.; Howard, J.; Pendalwar, S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources 1999, 77, 34–41. [Google Scholar] [CrossRef]
- Yao, Z.P.; Rånby, B. Surface modification by continuous graft copolymerization. IV. Photoinitiated graft copolymerization onto polypropylene fiber surface. J. Appl. Poly. Sci. 1990, 41, 1469–1478. [Google Scholar] [CrossRef]
- Ko, J.M.; Min, B.G.; Kim, D.W.; Ryu, K.S.; Kim, K.M.; Lee, Y.G.; Chang, S.H. Thin-film type Li-ion battery, using a polyethylene separator grafted with glycidyl methacrylate. Electrochimi. Acta 2004, 50, 367–370. [Google Scholar] [CrossRef]
- Augustin, S.; Hennige, V.; Hörpel, G.; Hying, C. Ceramic but flexible: New ceramic membrane foils for fuel cells and batteries. Desalination 2002, 146, 23–28. [Google Scholar] [CrossRef]
- Song, J.Y.; Wang, Y.Y.; Wan, C.C. Review of gel-type polymer electrolytes for lithium-ion batteries. J. Power Sources 1999, 77, 183–197. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Gozdz, A.S.; Schmutz, C.; Shokoohi, F.; Warren, P.C. Performance of Bellcore’s plastic rechargeable Li-ion batteries. Solid State Ionics 1996, 86-88, 49–54. [Google Scholar] [CrossRef]
- Gentili, V.; Panero, S.; Reale, P.; Scrosati, B. Role of the polymer matrix in determining the chemical-physical and electrochemical properties of gel polymer electrolytes for lithium batteries. Ionics 2007, 13, 111–116. [Google Scholar] [CrossRef]
- Gentili, V.; Panero, S.; Reale, P.; Scrosati, B. Composite gel-type polymer electrolytes for advanced, rechargeable lithium batteries. J. Power Sources 2007, 170, 185–190. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, Y.; Sun, K.; Zhou, X.; Zhang, N. A microporous gel electrolyte based on poly(vinylidene fluoride-co-hexafluoropropylene)/fully cyanoethylated cellulose derivative blend for lithium-ion battery. Electrochimi. Acta 2009, 54, 1888–1892. [Google Scholar]
- Raghavan, P.; Zhao, X.; Kim, J.; Manuel, J.; Chauhan, G.; Ahn, J.; Nah, C. Ionic conductivity and electrochemical properties of nanocomposite polymer electrolytes based on electrospun poly(vinylidene fluoride-co-hexafluoropropylene) with nano-sized ceramic fillers. Electrochimi. Acta 2008, 54, 228–234. [Google Scholar] [CrossRef]
- Ren, Z.; Sun, K.; Liu, Y.; Zhou, X.; Zhang, N.; Zhu, X. Polymer electrolytes based on poly(vinylidene fluoride-co-hexafluoropropylene) with crosslinked poly(ethylene glycol) for lithium batteries. Solid State Ionics 2009, 180, 693–697. [Google Scholar] [CrossRef]
- Ye, H.; Huang, J.; Xu, J.; Khalfan, A.; Greenbaum, S. Li ion conducting polymer gel electrolytes based on ionic liquid/PVDF-HFP blends. J. Electrochem. Soc. 2007, 154, A1048–A1057. [Google Scholar]
- Ferrari, S.; Quartarone, E.; Mustarelli, P.; Magistris, A.; Fagnoni, M.; Protti, S.; Gerbaldi, C.; Spinella, A. Lithium ion conducting PVDF-HFP composite gel electrolytes based on N-methoxyethyl-N-methylpyrrolidinium bis(trifluoromethanesulfonyl)-imide ionic liquid. J. PowerSources 2010, 195, 559–566. [Google Scholar]
- Kim, D.W.; Oh, B.K.; Choi, Y.M. Electrochemical performance of lithium-ion polymer cell using gel polymer electrolyte based on acrylonitrile-methyl methacrylate-styrene terpolymer. Solid State Ionics 1999, 123, 243–249. [Google Scholar] [CrossRef]
- Rao, M.; Geng, X.; Liao, Y.; Hu, S.; Li, W. Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery. J. Membr. Sci. 2012, 399-400, 37–42. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, X.; Li, W.; Li, Z.; Zhang, T.; Zhang, H. Macroporous polymer electrolytes based on PVDF/PEO-b-PMMA block copolymer blends for rechargeable lithium ion battery. J. Membr. Sci. 2009, 334, 117–122. [Google Scholar] [CrossRef]
- Zhang, H.P.; Zhang, P.; Li, Z.H.; Sun, M.; Wu, Y.P.; Wu, H.Q. A novel sandwiched membrane as polymer electrolyte for lithium ion battery. Electrochem. Comm. 2007, 9, 1700–1703. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, Z.; Gao, D.; Zhang, H. A novel sandwiched membrane as polymer electrolyte for application in lithium-ion battery. J. Membr. Sci. 2009, 326, 260–264. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, J.; Kim, J.; Lee, S. Facile fabrication of nanoporous composite separator membranes for lithium-ion batteries: Poly(methyl methacrylate) colloidal particles-embedded nonwoven poly(ethylene terephthalate). J. Mater. Chem. 2011, 21, 8192–8198. [Google Scholar] [CrossRef]
- Dautzenberg, G.; Croce, F.; Passerini, S.; Scrosati, B. Characterization of PAN-based gel electrolytes. electrochemical stability and lithium cyclability. Chem. Mater. 1994, 6, 538–542. [Google Scholar] [CrossRef]
- Alamgir, M.; Abraham, K.M. Room temperature rechargeable polymer electrolyte batteries. J. Power Sources 1995, 54, 40–45. [Google Scholar] [CrossRef]
- Abraham, K.M. Li+-conductive solid polymer electrolytes with liquid-like conductivity. J. Electrochem. Soc. 1990, 137, 1657–1658. [Google Scholar] [CrossRef]
- Abraham, K.M.; Choe, H.S.; Pasquariello, D.M. Polyacrylonitrile electrolyte-based Li ion batteries. Electrochimi. Acta 1998, 43, 2399–2412. [Google Scholar] [CrossRef]
- Peramunage, D.; Abraham, K.M. The Li4Ti5O12/PAN electrolyte/LiMn2O4 rechargeable battery with passivation-free electrodes. J. Electrochem. Soc. 1998, 145, 2615–2622. [Google Scholar] [CrossRef]
- Appetecchi, G.B.; Romagnoli, P.; Scrosati, B. Composite gel membranes: A new class of improved polymer electrolytes for lithium batteries. Electrochem. Comm. 2001, 3, 281–284. [Google Scholar] [CrossRef]
- Cho, T.H.; Sakai, T.; Tanase, S.; Kimura, K.; Kondo, Y.; Tarao, T.; Tanaka, M. Electrochemical performances of polyacrylonitrile nanofiber-based nonwoven separator for lithium-ion battery. Electrochem. Solid-State Lett. 2007, 10, A159–A162. [Google Scholar] [CrossRef]
- Liang, Y.; Ji, L.; Guo, B.; Lin, Z.; Yao, Y.; Li, Y.; Alcoutlabi, M.; Qiu, Y.; Zhang, X. Preparation and electrochemical characterization of ionic-conducting lithium lanthanum titanate oxide/polyacrylonitrile submicron composite fiber-based lithium-ion battery separators. J. Power Sources 2011, 196, 436–441. [Google Scholar]
- Cho, T.H.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yang, M.; Hou, J. Membranes in Lithium Ion Batteries. Membranes 2012, 2, 367-383. https://doi.org/10.3390/membranes2030367
Yang M, Hou J. Membranes in Lithium Ion Batteries. Membranes. 2012; 2(3):367-383. https://doi.org/10.3390/membranes2030367
Chicago/Turabian StyleYang, Min, and Junbo Hou. 2012. "Membranes in Lithium Ion Batteries" Membranes 2, no. 3: 367-383. https://doi.org/10.3390/membranes2030367




