An Experimental Study of a Zeolite Membrane Reactor for Reverse Water Gas Shift
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Membrane Preparation
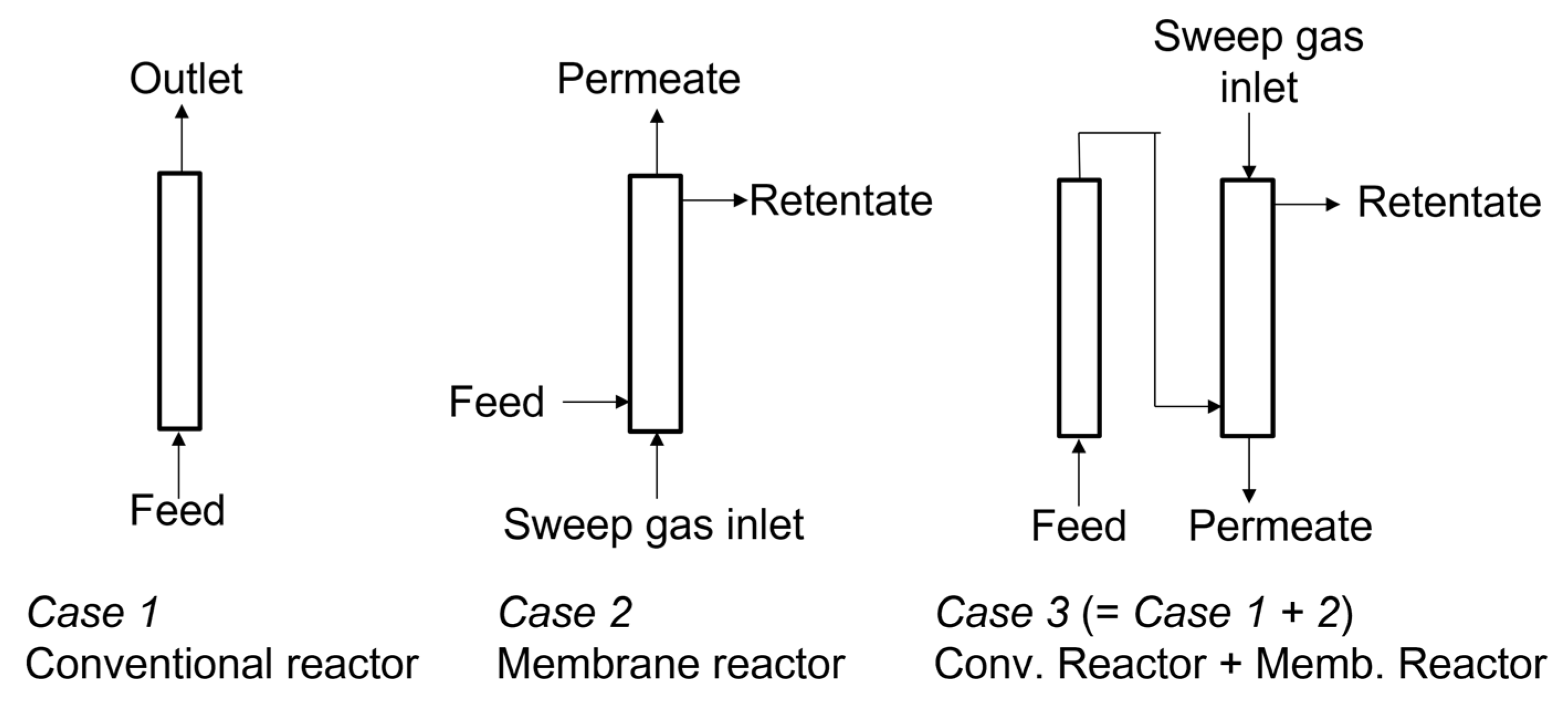
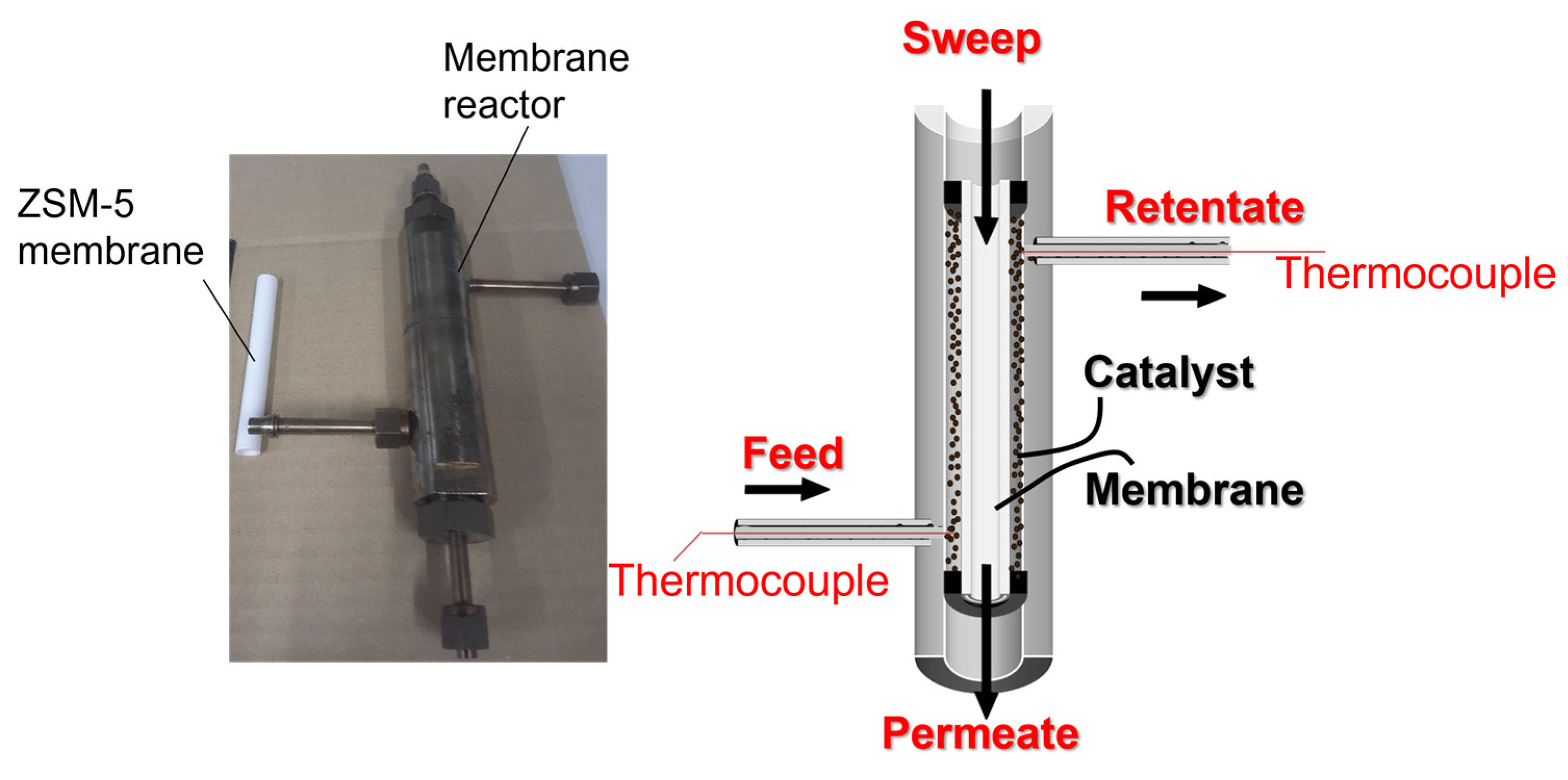
2.3. Membrane Reactor Test
3. Results and Discussion
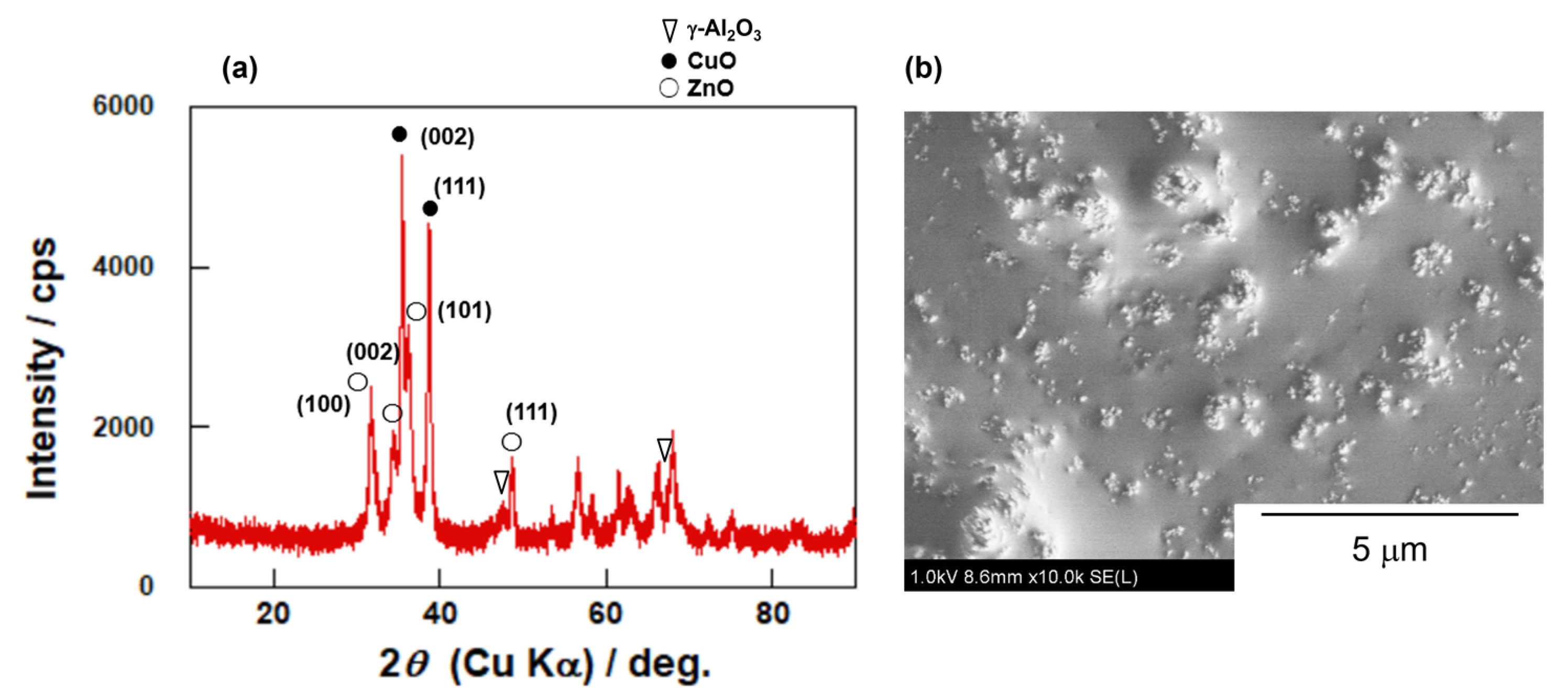
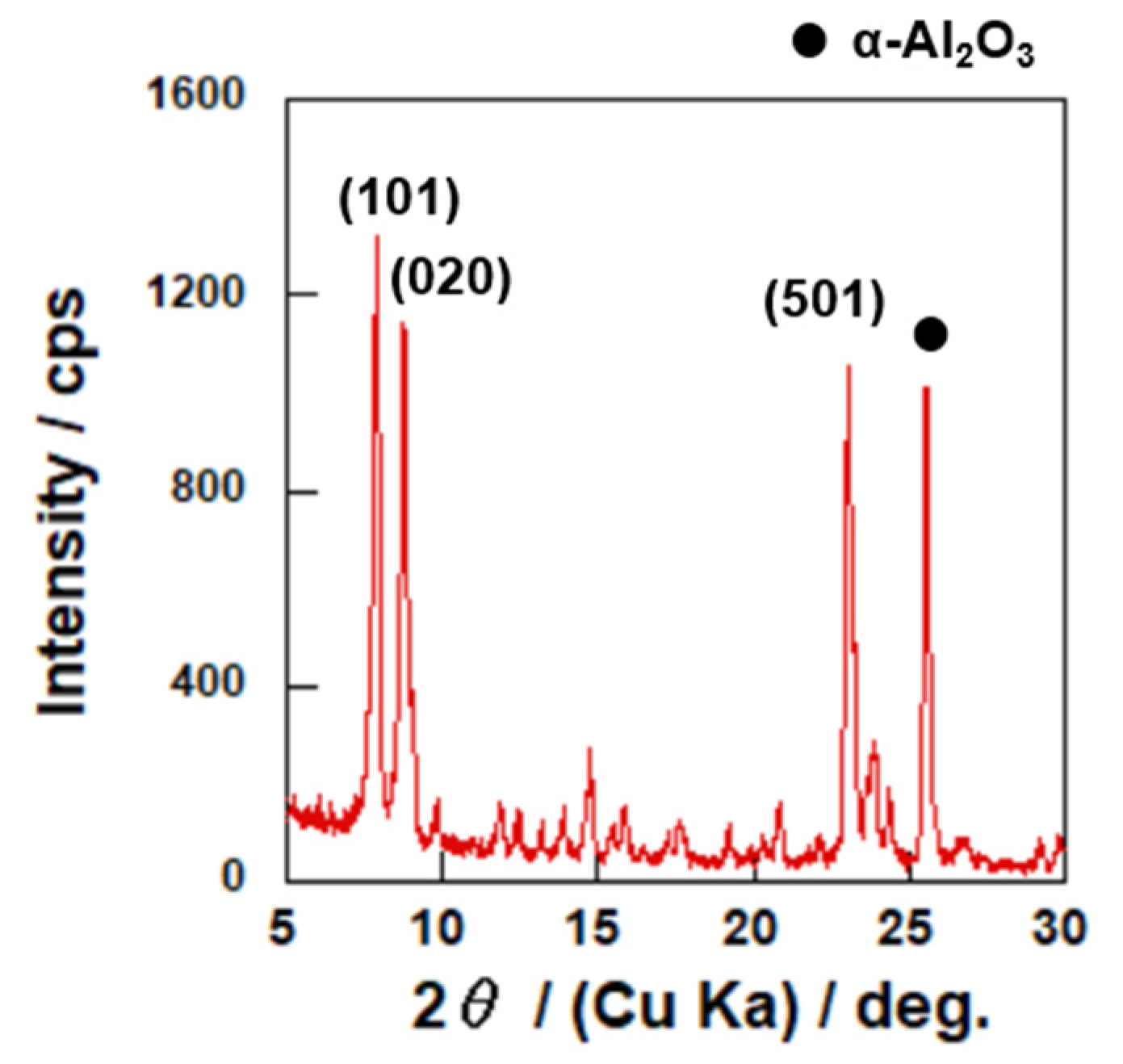
3.1. Characterizations of Catalyst and Membrane
3.2. Permeation and Separation Properties of MFI Zeolite for H2O/H2 and H2O/CO2
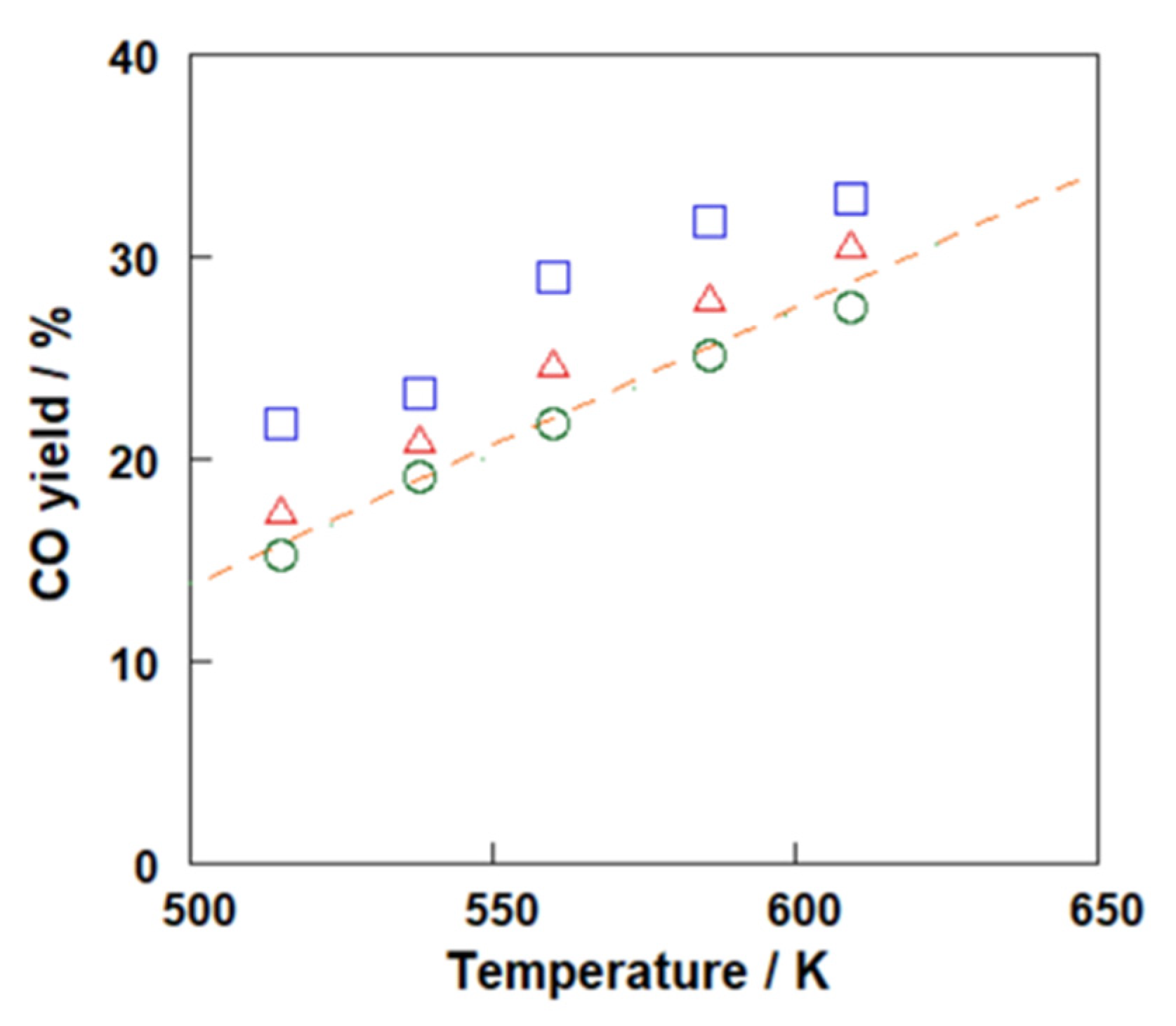
3.3. RWGS Membrane Reactor Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Castano, M.G.; Dorneanu, B.; Garcia, H.A. The reverse water gas shift reaction: A process system engineering perspective. React. Chem. Eng. 2021, 6, 954. [Google Scholar] [CrossRef]
- Dimitriou, I.; García-Gutiérrez, P.; Elder, R.H.; Cuéllar-Franca, R.M.; Azapagic, A.; Allen, R.W.K. Carbon dioxide utilisation for production of transport fuels: Process and economic analysis. Energy Environ. Sci. 2015, 8, 1775. [Google Scholar] [CrossRef] [Green Version]
- Navarro, J.C.; Centeno, M.A.; Laguna, O.H.; Odriozola, J.A. Policies and Motivations for the CO2 Valorization through the Sabatier Reaction Using Structured Catalysts. A Review of the Most Recent Advances. Catalysts 2018, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, H.-G.; Hyeon, M.-H.; Kim, B.-G.; Kim, S.K.; Moon, S.-Y. Low-temperature CO2 hydrogenation overcoming equilibrium limitations with polyimide hollow fiber membrane reactor. Chem. Eng. J. 2021, 403, 126457. [Google Scholar] [CrossRef]
- Dzuryk, S.; Rezaei, E. Intensification of the Reverse Water Gas Shift Reaction by Water-Permeable Packed-Bed Membrane Reactors. Ind. Eng. Chem. Res. 2020, 59, 18907. [Google Scholar] [CrossRef]
- Matsukata, M.; Sawamura, K.-I.; Shirai, T.; Takada, M.; Sekine, Y.; Kikuchi, E. Controlled growth for synthesizing a compact mordenite membrane. J. Membr. Sci. 2008, 316, 18. [Google Scholar] [CrossRef]
- Gallucci, F.; Paturzo, L.; Basile, A. An experimental study of CO2 hydrogenation into methanol involving a zeolite membrane reactor. Chem. Eng. Proc. 2004, 43, 1029. [Google Scholar] [CrossRef]
- Gorbe, J.; Lasobras, J.; Francés, E.; Herguido, J.; Menéndez, M.; Kumakiri, I.; Kita, H. Preliminary study on the feasibility of using a zeolite A membrane in a membrane reactor for methanol production. Sep. Purif. Technol. 2018, 200, 164. [Google Scholar] [CrossRef] [Green Version]
- Seshimo, M.; Liu, B.; Lee, H.; Yogo, K.; Yamaguchi, Y.; Shigaki, N.; Mogi, Y.; Kita, H.; Nakao, S.-I. Membrane Reactor for Methanol Synthesis Using Si-Rich LTA Zeolite Membrane. Membranes 2021, 11, 505. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, K.; Izumi, T.; Kawasaki, K.; Daikohara, S.; Ohsuna, T.; Takada, M.; Sekine, Y.; Kikuchi, E.; Matsukata, M. Reverse-selective microporous membrane for gas separation. Chem. Asian. J. 2009, 4, 1070. [Google Scholar] [CrossRef] [PubMed]
- Kakihana, M.; Yoshimura, M. Synthesis and Characteristics of Complex Multicomponent Oxides Prepared by Polymer Complex Method. Bull. Chem. Soc. Jpn. 1999, 72, 1427. [Google Scholar] [CrossRef]
- Sakai, M.; Seshimo, M.; Matsukata, M. Hydrophilic ZSM-5 membrane for forward osmosis operation. J. Water Process. Eng. 2019, 32, 100864. [Google Scholar] [CrossRef]
- Sakai, M.; Fujimaki, N.; Sasaki, Y.; Yasuda, N.; Seshimo, M.; Matsukata, M. Preferential Adsorption of Propylene over Propane on a Ag-Exchanged X-Type Zeolite Membrane. ACS Appl. Mater. Interfaces 2020, 12, 24086. [Google Scholar] [CrossRef] [PubMed]
- Sakai, M.; Tsuzuki, Y.; Fujimaki, N.; Matsukata, M. Olefin recovery by *BEA-type zeolite membrane: Affinity-based separation with olefin-Ag+ interaction. Chem. Asian. J. 2021, 16, 1105. [Google Scholar] [CrossRef] [PubMed]
- Hirota, Y.; Hayami, S.; Sasaki, F.; Matoba, S.; Yokoi, K.; Nishiyama, N. Effects of the Si-O-Si network structure on the per-meation properties of silylated ionic liquid-derived membranes. J. Chem. Eng. J. 2022, 55, 105. [Google Scholar] [CrossRef]
- Hirota, Y.; Nakai, T.; Hayami, S.; Sasaki, F.; Nishiyama, N. Evaluation of permeation mechanisms of silylated ionic liquid-derived organosilica membranes for toluene/methane separation. J. Jpn. Petrol. Inst. 2020, 63, 213. [Google Scholar] [CrossRef]
- Perry, R.H. Perry’s Chemical Engineers’ Handbook, 6th ed.; International Edition; McGraw-Hill: New York, NY, USA, 1984. [Google Scholar]


| System | Temperature/K | Permeance/10−7 mol m−2 s−1 Pa−1 | Separation Factor/- | ||
|---|---|---|---|---|---|
| H2O | H2 | CO2 | |||
| H2O | 523 | 3.89 | - | - | |
| 623 | 3.58 | - | - | ||
| H2 | 523 | - | 1.73 | - | |
| 623 | - | 2.68 | - | ||
| CO2 | 523 | - | - | 1.31 | |
| 623 | - | - | 1.60 | ||
| H2O/H2 | 523 | 2.99 | 0.154 | - | 17.2 |
| 623 | 1.78 | 0.204 | - | 8.11 | |
| H2O/CO2 | 523 | 3.64 | - | 0.182 | 17.8 |
| 623 | 2.24 | - | 0.180 | 11.6 | |
| Configuration | Carbon Balance/% | ||||
|---|---|---|---|---|---|
| 515 K | 538 K | 560 K | 586 K | 609 K | |
| Case 1 | 101.0 | 100.6 | 99.8 | 99.8 | 99.7 |
| Case 2 | 100.7 | 100.4 | 103.4 | 100.1 | 102.1 |
| Case 3 | 98.1 | 99.6 | 99.4 | 98.0 | 99.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, M.; Tanaka, K.; Matsukata, M. An Experimental Study of a Zeolite Membrane Reactor for Reverse Water Gas Shift. Membranes 2022, 12, 1272. https://doi.org/10.3390/membranes12121272
Sakai M, Tanaka K, Matsukata M. An Experimental Study of a Zeolite Membrane Reactor for Reverse Water Gas Shift. Membranes. 2022; 12(12):1272. https://doi.org/10.3390/membranes12121272
Chicago/Turabian StyleSakai, Motomu, Kyoka Tanaka, and Masahiko Matsukata. 2022. "An Experimental Study of a Zeolite Membrane Reactor for Reverse Water Gas Shift" Membranes 12, no. 12: 1272. https://doi.org/10.3390/membranes12121272






